Published online Apr 14, 2014. doi: 10.3748/wjg.v20.i14.4050
Revised: November 6, 2013
Accepted: January 2, 2014
Published online: April 14, 2014
Processing time: 220 Days and 1.3 Hours
AIM: To verify the performance of a lesion size measurement system through a clinical study.
METHODS: Our proposed system, which consists of a conventional endoscope, an optical device, an optical probe, and a personal computer, generates a grid scale to measure the lesion size from an endoscopic image. The width of the grid scale is constantly adjusted according to the distance between the tip of the endoscope and lesion because the lesion size on an endoscopic image changes according to the distance. The shape of the grid scale was corrected to match the distortion of the endoscopic image. The distance was calculated using the amount of laser light reflected from the lesion through an optical probe inserted into the instrument channel of the endoscope. The endoscopist can thus measure the lesion size without contact by comparing the lesion with the size of the grid scale on the endoscopic image. (1) A basic test was performed to verify the relationship between the measurement error eM and the tilt angle of the endoscope; and (2) The sizes of three colon polyps were measured using our system during endoscopy. These sizes were immediately measured by scale after their removal.
RESULTS: There was no error at α = 0°. In addition, the values of eM (mean ± SD) were 0.24 ± 0.11 mm (α = 10°), 0.90 ± 0.58 mm (α = 20°) and 2.31 ± 1.41 mm (α = 30°). According to these results, our system has been confirmed to measure accurately when the tilt angle is less than 20°. The measurement error was approximately 1 mm in the clinical study. Therefore, it was concluded that our proposed measurement system was also effective in clinical examinations.
CONCLUSION: By combining simple optical equipment with a conventional endoscope, a quick and accurate system for measuring lesion size was established.
Core tip: Our suggested system, which combines a conventional endoscope, several optical devices and a personal computer, can measure a lesion size by superimposing a grid scale on an image of the lesion and adjusting the width adequately according to the distance between the tip of the endoscope and the target lesion. Endoscopists can measure lesions by comparing the size of the grid scale with the size of the lesion on the monitor. After a basic performance test, the first clinical study for 3 colon polyps was performed. In the clinical study, we confirmed that our measurement system obtained a measurement accuracy of ± 1 mm when the tilt angle of the endoscope was less than 20°.
- Citation: Oka K, Seki T, Akatsu T, Wakabayashi T, Inui K, Yoshino J. Clinical study using novel endoscopic system for measuring size of gastrointestinal lesion. World J Gastroenterol 2014; 20(14): 4050-4058
- URL: https://www.wjgnet.com/1007-9327/full/v20/i14/4050.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i14.4050
In recent years, the rapid progression of endoscopic technology has enabled minimally invasive and precise screening/treatments using endoscopes in various regions of the body. Concurrently, many techniques for measuring the size of a lesion during endoscopy have been proposed. An endoscopic measurement method is strongly expected to not only improve endoscopic technology but to also assess the healing progress of lesions, to monitor organs in order to devise treatment strategies, and to analyze pathophysiology[1-6]. However, there has been no reliable measurement method used thus far. Thus, even though a lesion is discovered using an endoscope, endoscopists use only their own eyes to estimate its size[7,8]. The research and development of measurement systems has tended to focus on improving the accuracy, which increases the complexity and cost of the system. Therefore, a measurement system has yet to appear on the market.
To solve these problems, we developed a novel system for measuring lesion size. We aimed to establish and disseminate a technique based on the following five concepts: (1) simple and easy measurement; (2) inexpensive installation; (3) retention of conventional endoscopic operations; (4) accuracy on a practical level; and (5) disseminative system.
As mentioned above, we added a simplified function for measurement to the endoscope, making our system’s configuration as simple as possible. We also attempted to reduce the cost of the entire system by using an inexpensive optical device that can be directly combined with a conventional endoscope. Due to the simplicity of the measuring method and low cost of apparatus installation, our measuring system will be useful, improve current technologies, and should spread widely. The measurement of polyps/lesions is an important process because size changes constitute useful information for determining an endoscopic treatment indication. Using this simple and easy method of measurement, the information mentioned above can be readily acquired.
The aim of this paper is to introduce our newly developed lesion size measurement system, which can be combined with a conventional endoscope, to present fundamental verification regarding the distortion of an endoscopic image, and to report the first clinical study observing and measuring the polyp sizes in the colons of several patients as a comprehensive evaluation.
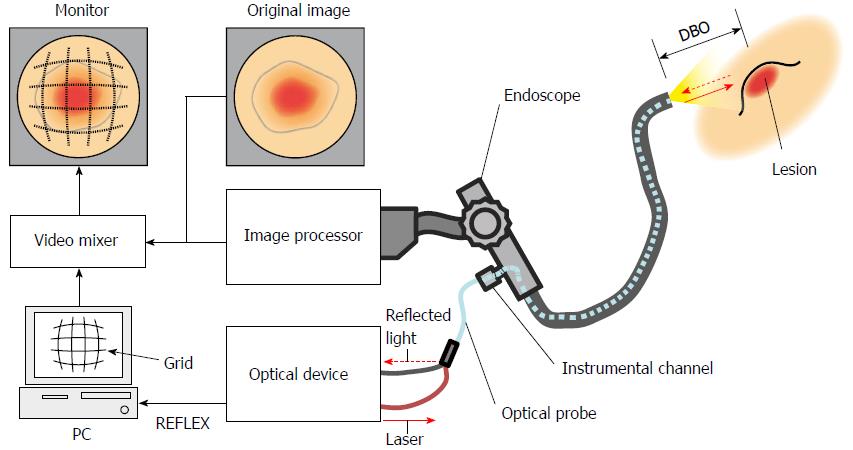
Our proposed system measures the size of target lesions by displaying a grid scale (grid) on the endoscopic image. The width of the grid is automatically adjusted in real time, depending on the distance between the tip of the endoscope and the target lesion (herein referred to as the distance between objects: DBO). The shape of the grid is distorted to match the aberration of the endoscopic image. The DBO is measured without contact between the endoscope and the lesion using a laser.
This system consists of a conventional endoscope with an instrument channel, an image processor, an optical device, an optical probe, a personal computer (PC), a video mixer and a monitor (Figure 1). A laser Doppler-type blood flow meter (FLO-N1, OMEGAWAVE.INC, Tokyo, Japan) was chosen as the optical device for our system. The optical probe (diameter: φ1.8 mm) consists of two optical fibers connected to the optical device and inserted into the endoscope tip through the instrument channel. The low level laser (wavelength: 780 nm, output: 3 mW) emitted from the optical device is illuminated on a target lesion through an optical fiber inside the probe (shown as a continuous line arrow in Figure 1). The light reflected from the lesion is received by the optical device through the other optical fiber in the probe (shown as a dashed line arrow in Figure 1). The received light is converted into an electric signal inside the optical device, which is output to a PC as an analog voltage (herein referred to as REFLEX). Because the amount of received light changes according to the DBO and the optical device outputs a REFLEX equivalent to the amount of received light, there is a relationship between the REFLEX and DBO. Utilizing the characteristic that the REFLEX changes in relation to the DBO, the PC calculates the DBO on the basis of the REFLEX and then generates an adequate grid width. The shape of the grid is simultaneously distorted, corresponding to the aberration of the endoscope lens. The adjusted grid is superimposed on an original image by a video mixer and displayed on the monitor.
To assess the efficacy of our system, we examined (1) the measurement accuracy of the DBO and the displayed grid; (2) the distortion correction; (3) the size adjustment; and (4) the measurement accuracy as basic verification tests. Thereafter, a clinical test was performed in which (5) the polyp sizes in the colons of several patients were measured instead of lesion sizes.
The endoscope was fixed at right angles with a sheet of white paper as a target object. The DBO was adjusted every 5 mm between 10 and 40 mm. The mean REFLEX was obtained at each distance for 10 s (50 ms sampling period) in air. Then, we assumed that the relationship between the DBO value LDBO and the REFLEX value VR could be represented by Eq. (1) The values of α, calculated using power approximations, were constants that can be obtained from the data of the aforementioned experiment. LDBO = αVRβ. Furthermore, the estimated DBO error εD was obtained from Eq. (2), where LT is the actual DBO. εD = |LT - LDBO|/LT× 100.
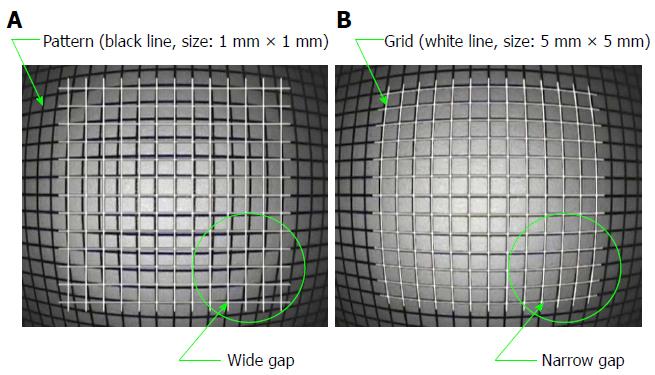
When an unbent grid was superimposed on the endoscopic image that was distorted by the objective lens, a measurement error occurred. Therefore, the grid shape was adjusted to match the distortion of the endoscopic image. This adjustment was accomplished by displaying 1 mm square patterns vertically on the digestive endoscope and adjusting the grid shape by superimposing the distorted patterns on the endoscopic image.
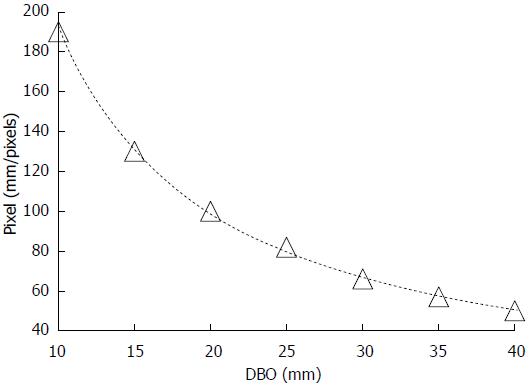
Because the size of the lesion on the endoscopic image changes in relation to the DBO, it is necessary to adjust the width of the grid in real time during an endoscopic examination. Therefore, relationships between the unit pixel number corresponding to the width of the grid and the DBO were derived by displaying 1 mm square patterns vertically on the digestive endoscope, adjusting the DBO every 5 mm between 10 and 40 mm, and measuring each of the actual grid sizes on the endoscopic image.
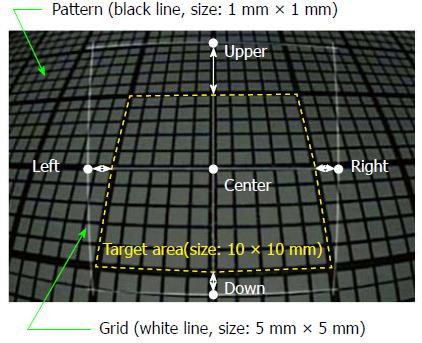
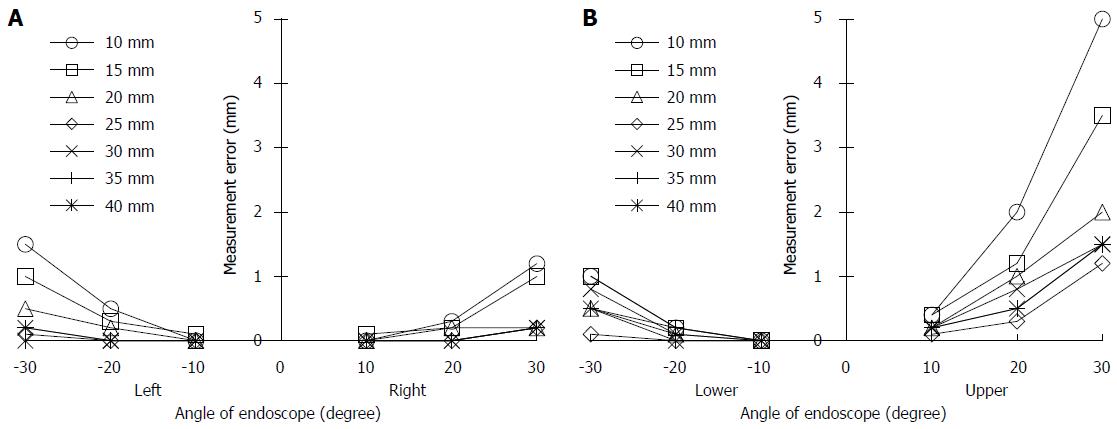
The accuracy of the grid measurement was validated by displaying 1 mm patterns on an endoscope, superimposing the displayed pattern on a grid that was adjusted according to the DBO, and measuring the differences. The DBO was adjusted every 5 mm between 10 and 40 mm, and the tilt angle of the endoscope was adjusted every 10° between 0° and 30°. The endoscopic image in Figure 2 shows a corrected 5 mm square grid of white lines superimposed on 1 mm square patterns (DBO: 10 mm, tilt angle: 30°). The measurement error of the grid, εM, was obtained by comparing the target area (size: 10 mm × 10 mm, dashed yellow line) and four points (upper, lower, right, left), each of which was 5 mm away from the center of the grid. Under these conditions, the measurement errors between the grid and the pattern are shown as white arrows.
This study, which included a gastrointestinal clinical study, was approved by the Investigational Review Board of Fujita Health University (Aichi, Japan), and all patients gave written informed consent prior to participation. Before the clinical study, the 3 patients with colon polyps received magnesium citrate and sennoside for bowel preparation. The colon conditions of the patients did not affect the lesion size measurement because the bowel preparation resulted in a good, cleansed condition of the colon. The conventional endoscope (OLYMPUS) was used, and the endoscope and the optical probe were sterilized after each use by the same method as that used for a general endoscope.
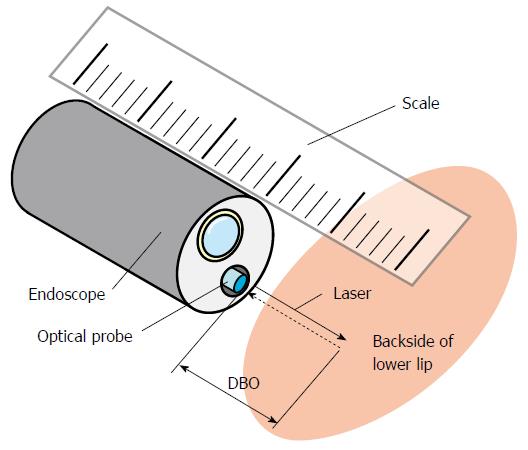
The calibration of the measuring system was performed just before the clinical study as follows. The optical probe, which can emit a laser for DBO measurements, was inserted into the instrument channel of the endoscope. Then, the DBO was adjusted every 5 mm between 5 and 20 mm using a scale, and the REFLEX was obtained at each distance to derive Eq. (1). The shape of the grid was distorted to match the aberration of the endoscopic image. The inside of the lower lip was used as a calibration target (Figure 3).
In the clinical study, endoscopy was conducted on 3 patients to validate whether this system was able to measure the sizes of colon polyps. The endoscopists measured the polyp size, which they could observe from the front using our system. After the clinical study, 3 colon polyps were removed by hot snare and immediately measured on a scale.
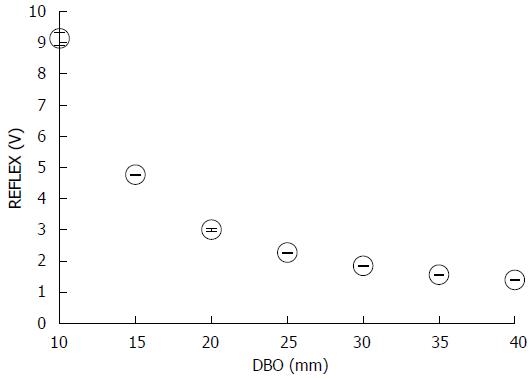
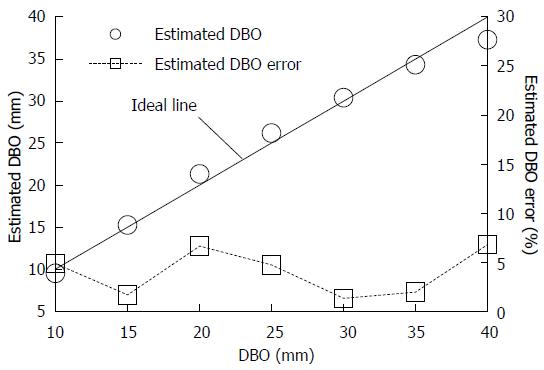
Figure 4 shows the mean REFLEX with the error range (n = 3); the figure shows that the REFLEX decreased exponentially with increasing DBO. Figure 5 shows the ideal line as a solid line using the estimated DBO (circle plots) derived from Eq. (1) (α = 47.3555, β = 0.7259) and the estimated DBO error εD (square plots) derived from Eq. (2). The value of εD (mean ± SD) was 4.0% ± 2.3%. Furthermore, the fact that the actual endoscopic examinations and treatments were performed using a sidelong view was taken into consideration. The results of our error verification of the measurements when the endoscope was tilted toward an object were as follows: 5.6% ± 1.6% at 5°, 16.6% ± 1.5% at 10°, and 48.4% ± 1.7% at 15°.
Figure 6 shows a grid shape (white lines) that was distorted to match the pattern (black lines) of the 1 mm square, as photographed by an endoscope. Images from before and after the distortion correction are shown in Figure 6A and B, respectively. The further away the grid was from the center, the more gaps occurred before the distortion correction. After performing the distortion correction, even the grids far from the center matched the distortion of the endoscopic image.
Figure 7 shows the relationship between the DBO and the pixel number, which defines the size of the grid. The pixel number decreased exponentially with increasing DBO. Accordingly, when the endoscope tip approached the target lesion, the width of the grid increased, and when the tip was retracted, the width of the grid decreased. These relationships made it possible to adjust the grid width according to the DBO computed from the REFLEX in real time.
The relationships between the tilt angle of the endoscope and the εM values were obtained at points left and right (Figure 8A) and lower and upper (Figure 8B). There was no error at the tilt angle of 0°; therefore, the results at this angle were omitted. The value of εM increased with increasing tilt angle and DBO. These results also showed that the εM value for Upper were the largest.
For Upper, the values of εM (mean ± SD) were 0.24 ± 0.11 mm at 10°, 0.90 ± 0.58 mm at 20° and 2.31 ± 1.41 mm at 30°. If the tilt angle was 20° or less, the εM accordingly became less than 1 mm, which suggests that our system has sufficient accuracy. Therefore, in the clinical study, lesions that could be seen from a frontal view were chosen for size measurement.
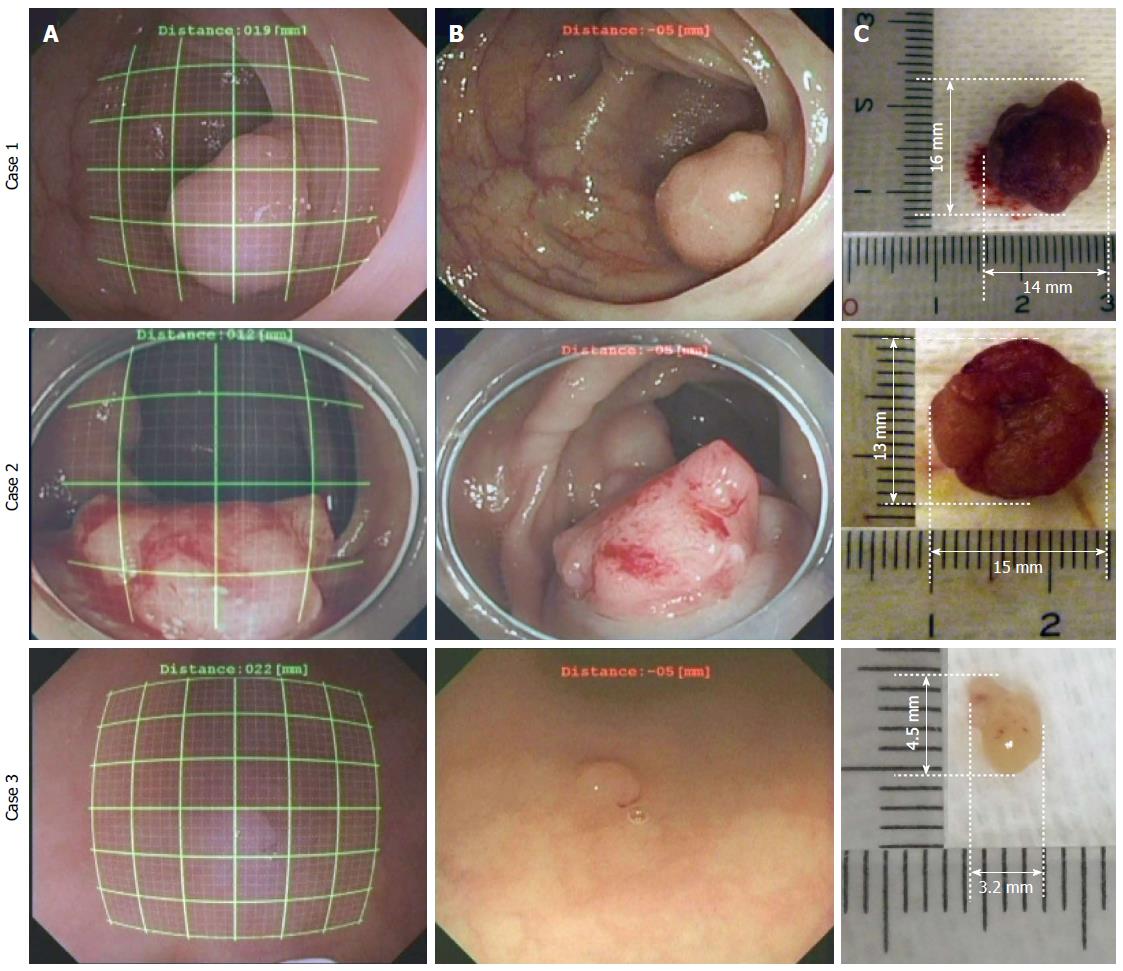
The results of the polyp size measurements in the colon are shown in Figure 9. The left line represents the endoscopic image during the measurement using the grids (thick line every 5 mm and thin line every 1 mm) in situ; the center line is the original image, and the right line is the polyp, which is removed after the measurement using a scale in vitro. The measurement errors in the clinical study, εC, were derived from the results measured using the grid Dg and the results measured using the scale Ds and are listed in Table 1. Regarding the results, εC were all within 1 mm. In conclusion, even if an endoscopic image was distorted and the DBO value changed, the sizes of the lesions were accurately measured using our system.
| Case No. | Measurement results (mm2) | Measurement error (mm) εc | |
| Using a grid in situ Dg | Using a scale in vitro Ds | ||
| 1 | 13.0 × 16.0 | 14.0 × 16.0 | 1.0 |
| 2 | 14.0 | 13.0 × 15.0 | 1.0 |
| 3 | 4 × 4 | 3.2 × 4.5 | 0.8 |
Various methods for measuring lesion size have been proposed as endoscopic techniques have been developed. These methods can be classified into the following five categories: (1) comparative measurement; (2) image processing; (3) optical rules; (4) laser diffraction grating techniques; and (5) stereo endoscopy.
Method (1) is a comparison technique in which the size of the lesion is compared to an object of known size placed near the lesion as the measuring alternative[9-11]. It is one of the simplest measuring techniques. However, because it is necessary to place the object right next to the target lesion, there is a possibility of perforation by the object, such as biopsy forceps, of measurement instability if the object used is similar to a rubber disc, and of an unnecessary prolongation of the examination time[12].
Method (2) is an image processing technique used to correct the distortion on an endoscopic image caused by wide-angled lenses for accurate measurement[13-16]. The calibration for the measurement is easy, but this method needs further refinement to correct for the tilt angles[17].
Method (3) is based on the enlargement ratio of the image and uses the focusing mechanism of the endoscope[18]. Its operability on the endoscope is good, but the variations in focus affect the measurement’s accuracy.
Method (4) is a technique that projects optical patterns toward the target using a diffraction grating or a scanning mirror, and then, the size is measured by processing the image on the basis of interferometry or triangulation[19-23]. Forthright measurement is unnecessary, and this method has the advantage of diameter and depth measurements. However, the measurement error increases in cases when the ulcer diameter is smaller than 5 mm. There is also a concern about the initial cost of this method.
Method (5) is a technique that applies the parallax of stereo endoscope lenses and measures the three-dimensional shape of the lesion with image processing by triangulation[24,25]. This method enables the measurement of the distance and the volume of lesions; however, the initial costs and measurement times are high.
These complicated tasks slow image processing, and the high costs of implementing the methods stated above need to be improved. These problems inhibit the widespread use of these techniques in clinical practice.
However, the characteristics of our novel lesion measurement system are as follows: (1) The measurement error caused by a change in the DBO or by distortion in the endoscopic image is small; (2) Non-contact measurement is possible in real time; (3) The diameter of the probe is only 1.8 mm, and all GI endoscopes except choledochoscopes have several channels greater than 2.0 mm in diameter. Therefore, the probe can be combined with most conventional GI endoscopes[26]; (4) Special procedures for measurement are not required; and (5) Our system is feasible by simply attaching a laser for measurements to a conventional endoscope. A basic test and a clinical study were performed to verify the effectiveness of our system.
From the basic test, it was confirmed that an accurate measurement on an endoscopic image was possible in cases when the tilt angle of the endoscope tip was less than 20°. Furthermore, the measurement error decreased with decreasing tilt angle or increasing DBO.
From the clinical study, colon polyps were measured with an error of less than 1 mm. As shown in Figure 9A and B, our system worked accurately because the endoscope could be used to observe the front of a polyp that protruded from the colon wall. Furthermore, it successfully controlled the increase in measurement error due to the endoscopic tilt angle by increasing the DBO. As shown in Figure 9B, the tilt angle of the endoscope might be wider than in the case of Figure 9A. However, the polyp size was obtained at the lower part of the grid with a small error, as shown in Figure 8B. Therefore, a small error was obtained in Figure 9A.
Small polyps and a polyp spread on tissue surfaces, as shown in Figure 9C, were also measured. Because the polyps were observed from the front with the endoscope, the measurement error was small (εC < 0.8 mm). The measurement error increased in proportion to the increasing tilt angle, as shown in Figure 8. Therefore, when observing small lesions at close range, it is desirable to observe them from the front as directly as possible. It is difficult to measure or observe small lesions with a large tilt angle of the endoscope at close range in actual clinical settings. Consequently, lesions are most often observed under conditions that ensure a small measurement error. Thus, the sizes of lesions are inevitably measured under conditions that provide good accuracy. When the lesion is in a narrow place, such as an esophagus, where the tilt angle for grid measurement cannot be controlled, we plan on attaining grid measurement by the image processing of an endoscope image and the modification of the grid form. Although the size of the removed lesion is not the same as that of the lesion before removal, we considered that there is almost no difference between the size of a polyp that is immediately measured after removal and the size of a polyp before removal. In addition, our measurement system provides useful information for determining the lesion area and indications for endoscopic treatment, such as colon polypectomy, endoscopic mucosal resection, and endoscopic submucosal dissection.
In conclusion, our proposed system requires no special operation for the measurement of lesion size. Therefore, endoscopists can easily obtain accurate results by conventional endoscopic techniques. In addition, the necessary equipment for these measurements includes an optical device, a PC, and software, which reduces the startup costs. A measurement error of less than 1 mm is within a reasonable and permissible tolerance because the error is equivalent to or less than the errors obtained in previous studies[8,9,13,15,21,27].
In this study, the following results were obtained: (1) Our novel system for measuring lesion sizes can be combined with conventional endoscopes. Keeping the configuration of the equipment simple reduces the initial cost of the system. Although the laser Doppler blood-flow meter was used in this research, this arrangement can apply to many simple/cheap pieces of equipment in combination with an optical device; (2) Using a laser light, the measurement accuracy of the DBO was confirmed to be 4.0% ± 2.3%; (3) To improve measurement accuracy, the width of the grid had to be adjusted in real time and associated with the change in the DBO while the shape of the grid had to be distorted to match the characteristic aberrations of the endoscopic images; (4) From the verification test for the grid availability, measurement errors occurred in the range of a DBO between 10 and 40 mm and a tilt angle between 0° and 30°. The mean measurement errors for the target area (10 mm × 10 mm) were 0.24 ± 0.11 mm at a tilt angle of 10°, 0.90 ± 0.58 mm at 20° and 2.31 ± 1.41 mm at 30°. There was no error at a tilt angle of 0°. According to these results, it was confirmed that our system was able to measure accurately when the tilt angle was less than 20°. Therefore, to measure accurately, it is necessary to observe the lesions from the front as much as possible; (5) A clinical study was performed to measure the sizes of 3 colon polyps using the grid and to then compare them with the actual sizes of the polyps after removal. The measurement error was less than 1 mm. Therefore, it was concluded that our proposed measurement system was also effective in clinical examinations; (6) This system is applicable in the following conditions: a lesion size of 16 mm (the maximum size of the results of the clinical study in case 1) or less and lesions observed from the front (tilt angle < 20°); and (7) The future aim for this technology is to improve the reliability of the measurement accuracy of our system by implementing its use in various clinical examinations.
Lesion size is traditionally measured by comparing the lesion to objects of known size (e.g., biopsy forceps) under an endoscope. However, this method is an onerous task for a doctor as he or she must place an object near or in contact with the lesion. This new system that can measure the lesion size simply without contact has been developed, and the basic performance of our system has been verified.
An endoscopic measurement method is highly expected to help in not only improving endoscopic technology but also in assessing the healing process of lesions, in monitoring organs to devise treatment strategies, and in analyzing pathophysiology. Several techniques for measuring the size of a lesion during endoscopy have been proposed, such as the comparison technique and the image processing technique. However, these techniques are not simply or easily performed by endoscopists.
A lesion size measurement system was achieved by combining a conventional endoscope with a simple/cheap optical device. Keeping the configuration of the equipment simple reduces the initial cost of the system. In addition, the system does not require any special operation.
The study’s results suggest that by combining simple optical equipment with a conventional endoscope, this lesion size measurement system requires no special operation for the measurement of lesion size. Further, endoscopists can easily obtain accurate results using conventional endoscopic techniques.
The authors have developed a new measurement system using a laser. Using this system, it is possible to measure accurately, with an error of less than 1 mm for observations from the front. Introducing this system contributes to the decision-making process during treatment and to elucidating the pathogenesis of a disease.
P- Reviewers: Chiu KW, Ding XW, Muscarella LF S- Editor: Cui XM L- Editor: A E- Editor: Ma S
| 1. | Read TE, Read JD, Butterly LF. Importance of adenomas 5 mm or less in diameter that are detected by sigmoidoscopy. N Engl J Med. 1997;336:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 114] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Butterly LF, Chase MP, Pohl H, Fiarman GS. Prevalence of clinically important histology in small adenomas. Clin Gastroenterol Hepatol. 2006;4:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 3. | Matsuda T, Saito Y, Fujii T, Uraoka T, Nakajima T, Kobayashi N, Emura F, Ono A, Shimoda T, Ikematsu H. Size does not determine the grade of malignancy of early invasive colorectal cancer. World J Gastroenterol. 2009;15:2708-2713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 4. | Lowenfels AB, Williams JL, Holub JL, Maisonneuve P, Lieberman DA. Determinants of polyp size in patients undergoing screening colonoscopy. BMC Gastroenterol. 2011;11:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Wang HM, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Lu J. Tumor size as a prognostic factor in patients with advanced gastric cancer in the lower third of the stomach. World J Gastroenterol. 2012;18:5470-5475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Longcroft-Wheaton G, Duku M, Mead R, Basford P, Bhandari P. Risk stratification system for evaluation of complex polyps can predict outcomes of endoscopic mucosal resection. Dis Colon Rectum. 2013;56:960-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Schoen RE, Gerber LD, Margulies C. The pathologic measurement of polyp size is preferable to the endoscopic estimate. Gastrointest Endosc. 1997;46:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 145] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Choi J, Kim SG, Im JP, Kim JS, Jung HC. Endoscopic estimation of tumor size in early gastric cancer. Dig Dis Sci. 2013;58:2329-2336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Dancygier H, Wurbs D, Classen M. A new method for the endoscopic determination of gastrointestinal ulcer area. Endoscopy. 1981;13:214-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Okabe H, Ohida M, Okada N, Mitsuhashi T, Katsumata T, Saigengi K, Nakahashi K. A new disk method for the endoscopic determination of gastric ulcer area. Gastrointest Endosc. 1986;32:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Hensley HH, Merkel CE, Chang WC, Devarajan K, Cooper HS, Clapper ML. Endoscopic imaging and size estimation of colorectal adenomas in the multiple intestinal neoplasia mouse. Gastrointest Endosc. 2009;69:742-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Sonnenberg A, Giger M, Kern L, Noll C, Study K, Weber KB, Blum AL. How reliable is determination of ulcer size by endoscopy. Br Med J. 1979;2:1322-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 41] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Vakil N, Smith W, Bourgeois K, Everbach EC, Knyrim K. Endoscopic measurement of lesion size: improved accuracy with image processing. Gastrointest Endosc. 1994;40:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Margulies C, Krevsky B, Catalano MF. How accurate are endoscopic estimates of size. Gastrointest Endosc. 1994;40:174-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Smith WE, Vakil N, Maislin SA. Correction of distortion in endoscope images. IEEE Trans Med Imaging. 1992;11:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Hofstad B, Vatn M, Larsen S, Huitfeldt HS, Osnes M. In situ measurement of colorectal polyps to compare video and fiberoptic endoscopes. Endoscopy. 1994;26:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Vakil N. Measurement of lesions by endoscopy: an overview. Endoscopy. 1995;27:694-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Maruyama M, Ichioka S, Takemoto T, Kondo T, Yamagata S. A new type of fibergastroscope (FGS-BLG) for measurement of gastric lesions. Tohoku J Exp Med. 1972;107:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Haneishi H, Ogura T, Miyake Y. Profilometry of a gastrointestinal surface by an endoscope with laser beam projection. Opt Lett. 1994;19:601-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Yamaguchi M, Okazaki Y, Yanai H, Takemoto T. Three-dimensional determination of gastric ulcer size with laser endoscopy. Endoscopy. 1988;20:263-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Hasegawa K, Noda K, Sato Y. Electronic Endoscope System for Shape Measurement. Pattern Recognition, Proceedings. 16th International Conference on. 2002;792-795. [DOI] [Full Text] |
| 22. | Seibel EJ, Smithwick QY. Unique features of optical scanning, single fiber endoscopy. Lasers Surg Med. 2002;30:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Chan M, Lin W, Zhou C, Qu JY. Miniaturized three-dimensional endoscopic imaging system based on active stereovision. Appl Opt. 2003;42:1888-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Catalano MF, Van Dam J, Bedford R, Cothren RM, Sivak MV. Preliminary evaluation of the prototype stereoscopic endoscope: precise three-dimensional measurement system. Gastrointest Endosc. 1993;39:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Yao K, Matsui T, Furukawa H, Yao T, Sakurai T, Mitsuyasu T. A new stereoscopic endoscopy system: accurate 3-dimensional measurement in vitro and in vivo with distortion-correction function. Gastrointest Endosc. 2002;55:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Varadarajulu S, Banerjee S, Barth BA, Desilets DJ, Kaul V, Kethu SR, Pedrosa MC, Pfau PR, Tokar JL, Wang A. GI endoscopes. Gastrointest Endosc. 2011;74:1-6.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Gopalswamy N, Shenoy VN, Choudhry U, Markert RJ, Peace N, Bhutani MS, Barde CJ. Is in vivo measurement of size of polyps during colonoscopy accurate. Gastrointest Endosc. 1997;46:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 134] [Article Influence: 4.8] [Reference Citation Analysis (0)] |