Published online Apr 14, 2014. doi: 10.3748/wjg.v20.i14.4011
Revised: May 10, 2013
Accepted: July 4, 2013
Published online: April 14, 2014
Processing time: 411 Days and 8.4 Hours
AIM: To assess NY-ESO-1 expression in a cohort of esophageal adenocarcinomas.
METHODS: A retrospective search of our tissue archive for esophageal resection specimens containing esophageal adenocarcinoma was performed, for cases which had previously been reported for diagnostic purposes, using the systematised nomenclature of human and veterinary medicine coding system. Original haematoxylin and eosin stained sections were reviewed, using light microscopy, to confirm classification and tumour differentiation. A total of 27 adenocarcinoma resection specimens were then assessed using immunohistochemistry for NY-ESO-1 expression: 4 well differentiated, 14 moderately differentiated, 4 moderate-poorly differentiated, and 5 poorly differentiated.
RESULTS: Four out of a total of 27 cases of esophageal adenocarcinoma examined (15%) displayed diffuse cytoplasmic and nuclear expression for NY-ESO-1. They displayed a heterogeneous and mosaic-type pattern of diffuse staining. Diffuse cytoplasmic staining was not identified in any of these structures: stroma, normal squamous epithelium, normal submucosal gland and duct, Barrett’s esophagus (goblet cell), Barrett’s esophagus (non-goblet cell) and high grade glandular dysplasia. All adenocarcinomas showed an unexpected dot-type pattern of staining at nuclear, paranuclear and cytoplasmic locations. Similar dot-type staining, with varying frequency and size of dots, was observed on examination of Barrett’s metaplasia, esophageal submucosal gland acini and the large bowel negative control, predominantly at the crypt base. Furthermore, a prominent pattern of apical (luminal) cytoplasmic dot-type staining was observed in some cases of Barrett’s metaplasia and also adenocarcinoma. A further morphological finding of interest was noted on examination of haematoxylin and eosin stained sections, as aggregates of lymphocytes were consistently noted to surround submucosal glands.
CONCLUSION: We have demonstrated for the first time NY-ESO-1 expression by esophageal adenocarcinomas, Barrett’s metaplasia and normal tissues other than germ cells.
Core tip: NY-ESO-1 is a cancer-testis antigen of particular interest, as it displays exceptional immunogenicity - hence, it is an attractive candidate for cancer vaccine therapy. To our knowledge, we have demonstrated for the first time, using immunohistochemistry, strong and diffuse nuclear and cytoplasmic expression for NY-ESO-1 in a cohort of esophageal adenocarcinoma cases. We have also demonstrated NY-ESO-1 expression in normal tissues other than germ cells, albeit as dot-positivity, indicating shared protein expression and association between primitive germ cells and somatic cells. We further relate our findings to proposed locations of stem cells in the esophagus and large bowel.
- Citation: Hayes SJ, Hng KN, Clark P, Thistlethwaite F, Hawkins RE, Ang Y. Immunohistochemical assessment of NY-ESO-1 expression in esophageal adenocarcinoma resection specimens. World J Gastroenterol 2014; 20(14): 4011-4016
- URL: https://www.wjgnet.com/1007-9327/full/v20/i14/4011.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i14.4011
Cancer-testis antigens (CT Ag) are a subset of cancer antigens which are widely expressed by a variety of malignant tumours. NY-ESO-1 is a CT Ag of particular interest, as it displays exceptional immunogenicity, making it an attractive candidate for cancer vaccine therapy[1]. Previous studies have reported it to be expressed by spermatogonia, oogonia and placenta.
NY-ESO-1 is expressed in varying frequency by many types of malignant tumour: immunohistochemical (IHC) studies for example have shown expression in 7/130 (6%) of endometrial carcinomas; 13/52 (25%) of non small cell carcinomas of the lung; and 18/22 (82%) of neuroblastomas. Two separate IHC studies of squamous carcinoma of the esophagus have shown expression in 44/213 (21%) and 18/56 (32%) of cases[1].
NY-ESO-1 has also been shown to be expressed by mesenchymal stem cells, using immunofluorescence performed on cultured cells derived from bone marrow aspirates and fetuses[2].
To our knowledge, its expression in esophageal adenocarcinoma (EAC) has not been investigated. Hence, further study is warranted, as this malignancy represents an attractive candidate for cancer vaccine treatment.
EAC has shown an increasing incidence over the past three decades, and it has a poor prognosis, with a 5 year survival rate of less than 10%[3,4]. Barrett’s esophagus (BE) is a metaplastic pre-malignant lesion for EAC, and both BE and EAC are considered of stem cell derivation, in accordance with the cancer stem cell theory[3,5-7].
In this study, we investigate NY-ESO-1 expression by IHC in a cohort of 27 EAC resection specimens; these specimens contained other esophageal tissue structures, including putative stem cell compartments, and this provides an additional opportunity to explore the proposition made by Cronwright et al[2], of NY-ESO-1 representing a stem cell marker.
The location of esophageal stem cells remains open to debate, but an emerging theory proposes a location within the submucosal gland/duct unit[8-11]. A further suggested location is the basal compartment of the interpapillary layer of the squamous epithelium[12]. Indeed, it may be the case that more than one population of progenitor cells exists within the esophagus[6].
The study was approved by the National Research Ethics Service (NRES Committee North West-Cheshire, Rec reference: 11/NW/0632). A retrospective search was made of the tissue archive at Salford Royal NHS Foundation Trust for esophageal resection specimens containing EAC. This search was made over a 7 month period, using the systematised nomenclature of human and veterinary medicine coding system, for cases which had previously been reported for diagnostic purposes. This search revealed a total of 27 EAC cases: 4 well differentiated; 14 moderately differentiated; 4 moderate-poorly differentiated and 5 poorly differentiated. Original sections, stained with haematoxylin and eosin (HE), were reviewed using light microscopy, by a histopathologist with a gastrointestinal interest (Hayes SJ), to confirm classification and tumour differentiation. One representative section of each tumour was selected for IHC, and these sections were noted to include the following additional components: 27/27 stroma; 22/27 normal squamous epithelium; 7/27 normal esophageal duct; 10/27 normal submucosal gland; 9/27 BE (goblet cell); 3/27 BE (non-goblet cell, cardiac type); 1/27 BE (non-goblet cell, fundic-type); 1/27 high grade glandular dysplasia.
All tissues had been fixed in neutral buffered formaldehyde and processed into paraffin wax by standard histological methods. Further 3 μm thick sections were cut and placed on adhesive coated slides (Snowcoat, Surgipath Europe). Sections were dried overnight at 60 °C.
IHC staining was performed using a Leica Bond Max immunostainer. Antigen retrieval was done for 25 min at high pH using Bond epitope retrieval solution 2, (Leica AR9640). NY-ESO-1 was identified using anti-NY-ESO-1, clone E978 (Invitrogen), diluted 1 + 400 for 30 min. Antibody binding was visualised using a Bond polymer refine detection kit (Leica DS9800) according to the manufacturer recommendations. The sections were then dehydrated, cleared and cover-slipped.
Sections of normal adult testis were used as a positive control. Normal colon, thyroid gland and omentum were selected as negative control sections.
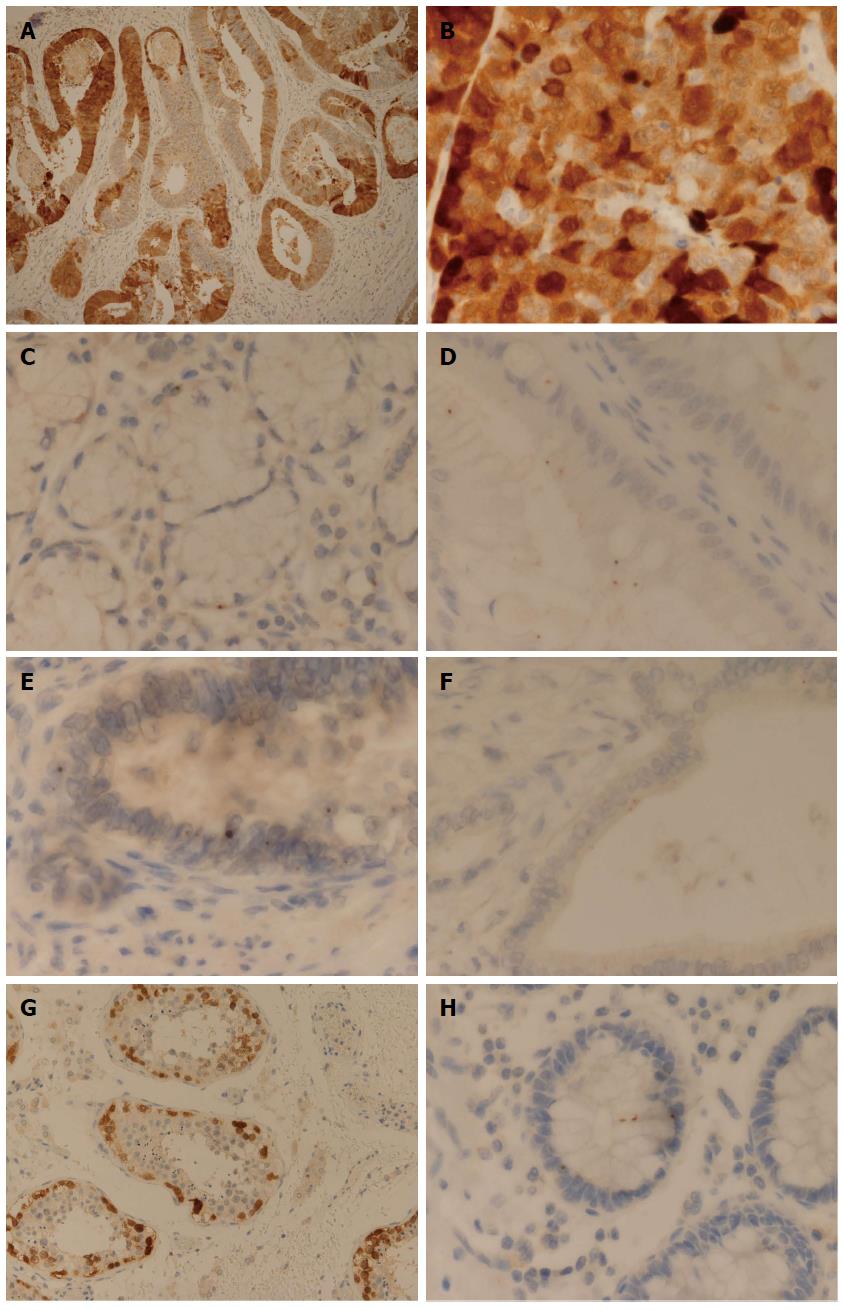
Out of a total of 27 cases of EAC examined, 4 cases (15%) displayed diffuse cytoplasmic and nuclear expression for NY-ESO-1: 2 moderately differentiated; 1 moderately-poorly differentiated; 1 poorly differentiated. They displayed a heterogeneous and mosaic-type pattern of diffuse staining (Figure 1A). Furthermore, the pattern of staining seen in all 4 cases was noted to have a rather granular pattern (Figure 1B).
As noted in the methodology, the cancer cases selected for assessment of NY-ESO-1 expression also contained the following tissue components: stroma; normal squamous epithelium; normal submucosal gland/duct; BE (goblet cell); BE (non-goblet cell); and high grade glandular dysplasia.
Diffuse cytoplasmic staining was not identified in any of these structures (P value < 0.005, χ2 test). However, an interesting dot-type pattern of expression for NY-ESO-1 was detected in all of these structures, with varying frequency and sizes of dots. Individual cells contained single or multiple dots, which were present at different cellular locations (nuclear, paranuclear and cytoplasmic).
Occasional extremely small and barely discernible speckles of NY-ESO-1 expression were identified at the following locations and frequency: stroma (17/27); basal aspect of squamous epithelium (12/22); normal duct (5/7); BE, non-goblet cell-fundic type (1/1).
A dot-type pattern of staining comprising of more frequent and predominantly larger (medium sized dots), was seen at the following locations: normal submucosal gland (7/10, Figure 1C); BE, goblet cell (9/9); BE, non-goblet cell-antral type (3/3). These larger dots were also located at nuclear, paranuclear and cytoplasmic locations.
A further interesting pattern of staining was seen on examination of 8/9 BE cases (goblet cell type) and in 1/3 of the cases of BE (antral type); these cases demonstrated a prominent pattern of apical (luminal) cytoplasmic dot-type staining (Figure 1D).
The largest and most frequent dots were identified in cases of high grade glandular dysplasia (HGD) (1/1) and EAC (27/27) (Figure 1E). Of the 4 cases of EAC demonstrating a diffuse heterogeneous pattern of expression, the areas of tumour with an absence of diffuse cytoplasmic expression did show a pattern of dot-type expression. One case of EAC which appeared moderately differentiated showed prominent cytoplasmic dot-type staining, with dots seen to be closely located and coalescing in places (perhaps representing incipient diffuse staining). Two cases of EAC (one well differentiated and one moderately-poorly differentiated) showed a prominent apical cytoplasmic pattern of staining, similar to that seen in BE (Figure 1F).
Some variation in dot size was identified in EAC (i.e., as well as large dots being present, some medium sized dots were also identified). Variation was also demonstrated in the frequency of dots seen; most cases of EAC showed a high frequency of dot-type staining, but 2 cases (one well differentiated and the other poorly differentiated) showed only a scanty pattern of dot-type expression.
A further morphological finding of interest was noted on examination of HE stained sections; aggregates of lymphocytes were consistently seen to surround submucosal glands in the tissue sections examined.
The positive control sections of adult testis showed cytoplasmic and nuclear basal staining of spermatogonia and primary spermatocytes, in accordance with that reported by previous studies[2] (Figure 1G).
Thyroid gland and omentum, used as negative control sections consistently showed an absence of staining for NY-ESO-1. However, sections of large bowel used as a negative control displayed occasional unexpected medium-sized dot-type staining. A total of 9/11 large bowel negative control sections showed dot-expression for NY-ESO-1 (nuclear, paranuclear and cytoplasmic locations). The total number of dots observed was 26, of which 19 (73%) were located at the crypt base and 7 (27%) were located at a mid-crypt location.
It was noted that one basal crypt showed a particularly interesting pattern of dot-expression, containing a nuclear dot, a paranuclear dot and two cytoplasmic dots (both of which were located in alignment with and apparently corresponding to the paranuclear dot) (Figure 1H).
To our knowledge, we have demonstrated for the first time, using IHC methodology, strong and diffuse nuclear and cytoplasmic expression for NY-ESO-1 in a cohort of EAC cases (15%); this is comparable to the frequencies of expression reported in esophageal squamous carcinoma (21% and 32%)[1]. Our findings indicate that at least a sub-set of EACs may be responsive to cancer vaccine treatment.
In this study, diffuse expression was noted in tumours that appeared moderately, moderately-poorly and poorly differentiated. None of the well differentiated tumours examined showed evidence of diffuse staining. Therefore, these findings suggest an association between NY-ESO-1 expression in EAC and a more poorly differentiated phenotype.
Furthermore, all of the tumours demonstrating diffuse expression showed evidence of heterogeneity, with an interesting mosaic-type pattern (Figure 1A). We speculate this to relate to a general signalling defect, perceived by localised groups of cells over-expressing NY-ESO-1, or alternatively, localised amplification of stem cell activity. As cancer-testis antigen expression is thought to be regulated epigenetically, it is uncertain whether the heterogeneous pattern of NY-ESO-1 expression observed in this and previous studies are a stable trait, or whether it varies over time (i.e., those cells not expressing the protein at one time point may demonstrate expression at a separate time point)[11]. As such, it is at present uncertain whether heterogeneity of expression would affect response to cancer vaccine therapy.
This study describes a varying frequency and size of dot-type staining (nuclear, paranuclear and cytoplasmic) within normal esophageal tissue compartments, BE, HGD and EAC. Occasional dot-type expression for NY-ESO-1, involving extremely small speckles of expression, were identified by the stroma, esophageal squamous epithelium (towards its base), submucosal gland ducts and BE (fundic-type). These findings are of uncertain significance, as these speckles were barely identifiable using light microscopy.
Larger and more frequent dot-type expression was identified by submucosal glands, BE (goblet cell-type) and BE (antral-type). The largest dots were seen in HGD and EAC.
A pattern of dot-type staining on examination of IHC markers at multiple cellular locations is highly unusual. The possibility has been considered of it representing a staining artefact. However, NY-ESO-1 expression was noted to be strong and crisp in the tissue sections examined, including the positive control, and there was an absence of background staining; in our experience, these features indicate the experimental technique to have worked appropriately. As such, we consider the possibility of staining artefact to be unlikely and the unusual pattern of expression seen suggests a biological process of great interest. It is more typical in histopathological practice, for instance, to observe dot-type staining for IHC markers at a single location, such as paranuclear staining for CK20 in Merkel cell carcinoma.
It is uncertain as to what these dots actually represent, and this will require further investigation. They do not with any certainty represent the structure of an organelle, but perhaps more likely relate to aggregates of protein.
A further purpose of this study was to investigate putative esophageal stem cell compartments. Stem cells within the gastrointestinal tract have proven histologically elusive, making it necessary to define IHC markers to identify them. Some previous success has been achieved in defining candidate stem cell markers in the large bowel, such as LgR5 and CD133[5,13]; at this location, for example, investigation using LgR5 has helped to clarify the presence of multiple stem cells, residing at the crypt base[13]. In relation to these findings, we have described unexpected dot-type staining for NY-ESO-1, predominantly located at the bases of crypts, on examination of colonic tissue used as a negative control; this adds further support to the proposition made by Cronwright et al[2], of NY-ESO-1 representing a stem cell marker.
Stem cell expression for NY-ESO-1 could be perceived as a potential handicap, considering that NY-ESO-1 is currently being targeted by cancer vaccine treatment; however, vaccine treatment targeting NY-ESO-1 has been reported to be well tolerated, and this may relate to an important distinction between the dot expression and diffuse expression for NY-ESO-1[1].
The only normal tissue compartment examined which contained a larger and more frequent dot-type pattern of NY-ESO-1 expression was the esophageal submucosal gland unit. As NY-ESO-1 has been proposed to represent a stem cell marker, this would support the emerging theory, postulating the submucosal gland to represent an esophageal stem cell compartment[2]. Of further interest, Cronwright et al[2] described NY-ESO-1 to localise to nucleoli-like structures in mesenchymal stem cells, representing a similar pattern to the nuclear dot-type staining observed in our study.
Furthermore, we have described the presence of lymphoid aggregates, probably related to a process of gastric reflux, which were consistently noted to show tropism for submucosal gland units; this inflammatory cell infiltrate may have a role in activation of stem cells within the submucosal gland. Additional supportive evidence for NY-ESO-1 representing a stem cell marker is provided by the presence of significantly sized dot-type staining for NY-ESO-1 involving BE, HGD and EAC, which are all considered of stem cell derivation[5-7].
Although BE showed expression for NY-ESO-1 at different cellular locations, expression was prominently demonstrated within the cytoplasm, often at an apical and luminal aspect. A similar pattern of expression was also noted on examination of EAC cases; a possible explanation for this is that NY-ESO-1 may be involved in a process of vesicle trafficking in BE and EAC, with paracrine function associated with exocytosis into the esophageal lumen. This process is thought to be involved in cellular communication, whereby microvesicles have been suggested to cause epigenetic reprogramming of target cells[14]. Such a process could potentially contribute to the documented phenomenon of field cancerization associated with BE and EAC[15]. Alternatively, vesicles in this instance may have a role in protein storage, or transport to another part of the cell, such as the nucleus, depending on their function,
A limitation of this study is related to the rather difficult and subjective assessment of dot size; this was addressed by the involvement of a pathologist in this study (Hayes SJ) who has experience in morphological assessment. Further studies should aim for further refinement in the accuracy of dot size assessment.
In conclusion, this study demonstrates, using IHC methodology, diffuse expression for NY-ESO-1 in a proportion of EAC cases. Furthermore, it demonstrates for the first time, NY-ESO-1 to be expressed in normal tissues other than germ cells, albeit as dot-positivity. If further study could demonstrate dot-type expression in other normal tissues, then this would provide correlation with previously described, but unexplained, low levels of NY-ESO-1 mRNA expression observed in normal tissues[16,17]. Our findings provide supportive evidence that NY-ESO-1 represents a stem cell marker, which in turn indicates shared protein expression and association between primitive germ cells and somatic stem cells. This study also provides supportive evidence for both the esophageal submucosal gland unit and the crypt base of large bowel to represent stem cell compartments.
Finally, we advocate the merit of future studies with an aim to further clarify the precise nature and function of NY-ESO-1 expression in normal, metaplastic and neoplastic tissues.
We are grateful to Professor Andrew Sharrocks, University of Manchester, for his critical comments on review of this manuscript.
Many genes which are expressed in the testis are also expressed in cancer, and they are commonly termed cancer-testis antigens. NY-ESO-1 is a cancer-testis antigen of particular interest, as it displays exceptional immunogenicity, making it an attractive candidate for cancer vaccine therapy.
Esophageal adenocarcinoma is recognised as a difficult to treat malignancy with a poor prognosis and, as such, it represents an attractive candidate for cancer vaccine therapy. To our knowledge, NY-ESO-1 expression in esophageal adenocarcinoma (EAC) has not been investigated. Hence, further study is warranted, as this malignancy represents an attractive candidate for cancer vaccine treatment.
Authors have demonstrated for the first time, using immunohistochemistry, expression for NY-ESO-1 in a cohort of esophageal adenocarcinoma cases. They have also described a novel dot-type pattern of expression for NY-ESO-1 in normal, metaplastic and neoplastic tissues. Their findings provide supportive evidence that NY-ESO-1 represents a stem cell marker, which in turn indicates shared protein expression and association between primitive germ cells and somatic stem cells. This work and other previous studies also provide supportive evidence for both the esophageal submucosal gland unit and the crypt base of large bowel to represent stem cell compartments.
Esophageal adenocarcinoma expressing NY-ESO-1 represents a candidate for cancer vaccine therapy.
Cancer-testis antigens: a subset of cancer antigens which are widely expressed by a variety of malignant tumours. NY-ESO-1: a CT Ag of particular interest which was initially discovered using serological identification of antigens by recombinant expression cloning (SEREX) methodology. Immunohistochemistry: a standardized method of using specific epitope(s) and antibody binding technology, to localize and study expression of particular antigen(s)/marker(s). Stem cells: pluripotent cell lineage capable of potential differentiations or transdifferentiations.
This is a novel study, demonstrating NY-ESO-1 expression in esophageal adenocarcinomas, which has utility with regard to further immunotherapy treatment. Further studies are advocated in order to clarify the significance of the described dot-type expression in normal, metaplastic and neoplastic tissues.
P- Reviewers: Nagahara H, Trifan A S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Nicholaou T, Ebert L, Davis ID, Robson N, Klein O, Maraskovsky E, Chen W, Cebon J. Directions in the immune targeting of cancer: lessons learned from the cancer-testis Ag NY-ESO-1. Immunol Cell Biol. 2006;84:303-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Cronwright G, Le Blanc K, Götherström C, Darcy P, Ehnman M, Brodin B. Cancer/testis antigen expression in human mesenchymal stem cells: down-regulation of SSX impairs cell migration and matrix metalloproteinase 2 expression. Cancer Res. 2005;65:2207-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Souza RF, Krishnan K, Spechler SJ. Acid, bile, and CDX: the ABCs of making Barrett’s metaplasia. Am J Physiol Gastrointest Liver Physiol. 2008;295:G211-G218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Coupland VH, Allum W, Blazeby JM, Mendall MA, Hardwick RH, Linklater KM, Møller H, Davies EA. Incidence and survival of oesophageal and gastric cancer in England between 1998 and 2007, a population-based study. BMC Cancer. 2012;12:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Zeki SS, Graham TA, Wright NA. Stem cells and their implications for colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Barbera M, Fitzgerald RC. Cellular origin of Barrett’s metaplasia and oesophageal stem cells. Biochem Soc Trans. 2010;38:370-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Fitzgerald RC. Molecular basis of Barrett’s oesophagus and oesophageal adenocarcinoma. Gut. 2006;55:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Paulson TG, Xu L, Sanchez C, Blount PL, Ayub K, Odze RD, Reid BJ. Neosquamous epithelium does not typically arise from Barrett’s epithelium. Clin Cancer Res. 2006;12:1701-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Leedham SJ, Preston SL, McDonald SA, Elia G, Bhandari P, Poller D, Harrison R, Novelli MR, Jankowski JA, Wright NA. Individual crypt genetic heterogeneity and the origin of metaplastic glandular epithelium in human Barrett’s oesophagus. Gut. 2008;57:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Coad RA, Woodman AC, Warner PJ, Barr H, Wright NA, Shepherd NA. On the histogenesis of Barrett’s oesophagus and its associated squamous islands: a three-dimensional study of their morphological relationship with native oesophageal gland ducts. J Pathol. 2005;206:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Nicholson AM, Graham TA, Simpson A, Humphries A, Burch N, Rodriguez-Justo M, Novelli M, Harrison R, Wright NA, McDonald SA. Barrett’s metaplasia glands are clonal, contain multiple stem cells and share a common squamous progenitor. Gut. 2012;61:1380-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Seery JP. Stem cells of the oesophageal epithelium. J Cell Sci. 2002;115:1783-1789. [PubMed] |
| 13. | Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4336] [Article Influence: 240.9] [Reference Citation Analysis (0)] |
| 14. | Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1092] [Cited by in RCA: 1048] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 15. | Graham TA, McDonald SA, Wright NA. Field cancerization in the GI tract. Future Oncol. 2011;7:981-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Jungbluth AA, Chen YT, Stockert E, Busam KJ, Kolb D, Iversen K, Coplan K, Williamson B, Altorki N, Old LJ. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92:856-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 273] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 17. | Sugita Y, Wada H, Fujita S, Nakata T, Sato S, Noguchi Y, Jungbluth AA, Yamaguchi M, Chen YT, Stockert E. NY-ESO-1 expression and immunogenicity in malignant and benign breast tumors. Cancer Res. 2004;64:2199-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |