Published online Apr 14, 2014. doi: 10.3748/wjg.v20.i14.3835
Revised: November 27, 2013
Accepted: January 3, 2014
Published online: April 14, 2014
Processing time: 194 Days and 6.8 Hours
Recent epidemiological studies, basic research and clinical trials on colorectal cancer (CRC) prevention have helped identify candidates for effective chemopreventive drugs. However, because of the conflicting results of clinical trials or side effects, the effective use of chemopreventive drugs has not been generalized, except for patients with a high-risk for developing hereditary CRC. Advances in genetic and molecular technologies have highlighted the greater complexity of carcinogenesis, especially the heterogeneity of tumors. We need to target cells and processes that are critical to carcinogenesis for chemoprevention and treatment of advanced cancer. Recent research has shown that intestinal stem cells may serve an important role in tumor initiation and formation of cancer stem cells. Moreover, studies have shown that the tumor microenvironment may play additional roles in dedifferentiation, to enable tumor cells to take on stem cell features and promote the formation of tumorigenic stem cells. Therefore, early tumorigenic changes of stem cells and signals for dedifferentiation may be good targets for chemoprevention. In this review, I focus on cancer stem cells in colorectal carcinogenesis and the effect of major chemopreventive drugs on stem cell-related pathways.
Core tip: To develop optimal chemopreventive agents, we need to target cells and pathways that are essential and critical to carcinogenesis: early tumorigenic changes of stem cells and signals for dedifferentiation may be good targets for chemoprevention. Major chemopreventive drugs, such as non-steroidal anti-inflammatory drugs, statins, proliferator-activated receptor γ agonists and metformin, have cancer stem cell (CSC)-suppressing effects via regulation of stem cell-regulating pathways, stem cell niche in the tumor microenvironment, and altered tumor metabolism. These stem cell-related steps in tumorigenesis could be critical targets for chemoprevention and CSC-targeted adjunctive treatment of colorectal cancer.
-
Citation: Kim TI. Chemopreventive drugs: Mechanisms
via inhibition of cancer stem cells in colorectal cancer. World J Gastroenterol 2014; 20(14): 3835-3846 - URL: https://www.wjgnet.com/1007-9327/full/v20/i14/3835.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i14.3835
Colorectal cancer (CRC) is one of the most commonly diagnosed cancers, and is a major cause of cancer morbidity and mortality worldwide[1]. Although there have been improvements in surgical and oncological therapies, the data have shown limited survival improvements in advanced CRC[2]. Therefore, prevention strategies remain the most promising avenue for reducing both the incidence and mortality of CRC.
CRC development is a multi-step process that occurs over a span of about 10 years, thereby providing an opportunity for prevention and early detection[3]. CRC screening and polyp removal are effective interventions for CRC prevention[3,4]. However, along with screening efforts, we need a specific prevention strategy for patients at high-risk for developing CRC. Chemoprevention involves the use of a variety of agents that can prevent, delay, or even reverse the development of pre-malignant lesions by suppressing the multi-step carcinogenic process. Many studies have demonstrated that pre-malignant lesions can be reversed and prevented pharmacologically[5]. This effect is of particular importance to high-risk individuals with a hereditary predisposition for or susceptibility to the environmental causes of CRC. Chemoprevention shows great promise in this regard and the ideal chemopreventive agent, with an excellent safety profile, remains to be discovered.
Until now, there have been several major candidates for CRC chemopreventive drugs, including aspirin and non-steroidal anti-inflammatory drugs (NSAIDs), statins, peroxisome proliferator-activated receptor (PPAR) γ agonist, and metformin, which exhibit chemopreventive effects in epidemiologic studies, in in vitro and in vivo experiments, and in some clinical trials.
NSAIDs have received the most attention as chemoprevention agents in CRC, and experimental and clinical studies have consistently shown that NSAIDs may reduce the risk of colorectal adenoma or cancer[6,7]. In experimental models, either nonselective or cyclooxygenase-2 (COX2)-selective NSAIDs have been shown to suppress CRC growth through COX2-dependent and -independent mechanisms, such as activation of apoptotic and anti-inflammatory signals[8,9].
Many clinical trials have addressed the cancer-preventive effect of aspirin, using colorectal adenomas as a surrogate primary end point for cancer, and the data support its benefits in reducing the risk of CRC. In patients with a history of a previous CRC[10] or a history of colorectal adenomas[6,11], the recurrence of adenoma was reduced in patients who received aspirin vs those who did not. In addition, in patients with hereditary non-polyposis CRC, the long-term use of aspirin reduced the incidence of CRC, with an HR of 0.63 (95%CI: 0.35-1.13)[12].
In addition to aspirin, other NSAIDs have also shown efficacy in CRC prevention trials. For example, in one clinical trial, in which patients with a history of resected adenomas were randomized to receive either sulindac plus difluoromethylornithine or matched placebos, promising results were seen, in that the risk ratio was 0.30 (95%CI: 0.18-0.49) for recurrent adenomas and 0.085 (95%CI: 0.011-0.650) for advanced adenomas in the intervention arm relative to the placebo arm[13]. In addition, recent long-term follow-up studies have reported that NSAIDs may also reduce the recurrence and mortality of CRC[14-16]. Meanwhile, celecoxib, a selective COX2 inhibitor, showed promise in inhibiting adenoma occurrence in familial adenomatous polyposis patients[17] and in patients with a history of colorectal polyps[18,19]. However, COX2 selective inhibitors are no longer considered for prevention of CRC because of their cardiovascular toxicities[20-22].
Statins, widely used cholesterol-lowering drugs, inhibit cholesterol synthesis via inhibition of 3-hydroxy-3-methylglutaryl coenzyme A (HMGCoA) reductase, the rate-limiting enzyme in the mevalonate and cholesterol-synthesis pathway. Many of the downstream products of this pathway are required for critical cellular functions, such as maintenance of membrane integrity, signaling, protein synthesis and cell cycle progression[23,24]. However, clinical studies examining the relationship between statins and CRC incidence have yielded mixed results. Although some case-control and cohort studies and a meta-analysis study[25-31] demonstrated a protective effect against CRC in statin users, other studies failed to do so[32-37]. Siddiqui et al[38] showed reduced recurrence of polyps (OR = 0.51, 95%CI: 0.43-0.60) and high-risk polyps (OR = 0.74, 95%CI: 0.52-0.96), and diminished polyp size and number in patients who used statins continuously for up to 5 years. Moreover, statins have an excellent safety profile. To overcome the possible discrepancies in the results from studies of statins in CRC chemoprevention, a thorough analysis of the underlying CRC risk, methodologies and exposure time are needed, along with data from well-designed large-scale clinical trials and epidemiological studies in the future. The exact role of statins in chemoprevention remains to be elucidated. In addition, recent data have suggested that statins may have beneficial effects on disease progression and survival, indicating that long-term use of statins may be associated with a less-advanced tumor stage and a better survival rate[34,39].
PPARγ agonists, such as thiazolidinedione (TZD), an insulin-sensitizing diabetes drug, also have anti-cancer activities, involving inhibition of cell growth and induction of apoptosis and terminal cellular differentiation[40-43]. PPARs have central roles in the regulation of glucose and lipid homeostasis, and also regulate cell proliferation, differentiation, and inflammation[44]. Recently, several studies have reported that the use of TZDs may be associated with a decreased risk of CRC in patients with diabetes[45,46], and in some cases, PPARγ agonists have also shown modest efficacy for chemoprevention in clinical trials[47,48]. In addition, PPARγ expression in CRC primary tumors correlates well with overall survival of CRC patients[49], which is consistent with animal experiments showing that intestinal tumors are exacerbated in APC min/+ mice with genetic ablation of Pparg, compared with control APC min/+ mice[50]. However, controversy regarding the anti-tumor effect of PPARγ agonists persists because some studies indicated that activation of PPARγ promotes tumorigenesis[51-54]. Furthermore, clinical studies show that TZD may be associated with an increased risk of heart failure[55], bone fractures[56-58] and possibly bladder cancer[59]. Whether these are PPARγ-mediated side effects or off-target effects remain uncertain. Although PPARγ is currently considered a potential target for chemoprevention, previous results are based mainly on observational and preclinical studies, and rigorous clinical trials are needed to address the utility of PPARγ agonists in CRC chemoprevention.
Metformin is a classic biguanide drug that has been used as first-line therapy for type 2 diabetes mellitus (DM). Metformin inhibits hepatic gluconeogenesis and reduces insulin resistance. It is a safe and economical drug that has been used for more than 50 years. Most CRC-specific observational studies and meta-analyses reported that patients with type 2 DM who were taking metformin have a lower risk of CRC and better outcomes compared with patients not taking the drug[60-64]. Moreover, metformin showed a protective effect for colorectal adenoma recurrence in colonoscopic surveillance of CRC patients with diabetes[65]. Preclinical studies in animal models support these findings, showing that metformin induced AMP-activated protein kinase (AMPK) activation and inhibited tumor development and growth, including colon tumorigenesis[66,67]. In terms of its molecular mechanism, metformin regulates insulin/insulin-like growth factor-related pathways, inflammatory activity, and the AMPK/mammalian target of the rapamycin (mTOR) pathway[68]. Activated AMPK inhibits the mTOR-mediated synthesis of key proteins responsible for malignancy and growth of cancer cells[69], and is thought to be a main mediator of the potential anti-cancer mechanism of metformin. Despite the promising results in these studies, data pertaining to metformin from well-designed clinical trials for CRC and its precancerous lesions are lacking. Furthermore, the anti-tumor effect of metformin on non-diabetic patients should also be assessed, because the safety of metformin is well known and it has no glucose-lowering effects in non-diabetic patients. One clinical trial demonstrated an inhibitory effect of metformin in aberrant crypt foci formation of the rectum in patients who did not have diabetes[70]. However, this study showed a short-term effect of metformin in a small number of subjects. Therefore, large-scale randomized controlled trials are required to confirm the chemopreventive and therapeutic effects of metformin, especially for non-diabetic patients[71].
Among the most-promising chemopreventive drugs currently being studied, NSAIDs have consistently shown a protective effect against CRC; however, they are generally not recommended for widespread chemoprevention because of the increased risk of bleeding[72]. In addition, COX2-selective inhibitors showed increased cardiovascular morbidity[73]. However, statins, PPARγ agonists and metformin have a relatively good safety profile, and these drugs show similarities in their abilities to improve metabolic disorders that are known to be associated with increased cancer risk, such as diabetes, obesity, dyslipidemias, and chronic inflammation. For widespread acceptance of these chemopreventive drugs, more definite chemopreventive effects in large-scale well-designed clinical trials need to be demonstrated, along with a more acceptable safety profile for PPARγ agonists.
Future directions in CRC chemoprevention will include genetic and molecular approaches for identifying pathways that are associated with cancer initiation and development, and personalized approaches to predict risk, drug susceptibility and toxicity. In addition, mechanism-based combinations of agents will also maximize effectiveness, while limiting drug toxicity. From the perspective of identifying new targets for chemoprevention, besides the traditional targeting of the multi-step process in colorectal carcinogenesis, new evidence has demonstrated that targeting essential cell types and critical signaling pathways, such as tumor-initiating stem cells and stem cell-related pathways, could be another effective strategy for preventing colorectal tumorigenesis.
Much evidence has shown that genomic instability, including chromosomal and microsatellite instability, and epigenetic changes are important mechanisms for multi-step tumorigenesis of CRC[74]. However, we have come to understand that tumors show inter- and intra-tumoral heterogeneity, even in the same patient, and more complex intercellular interactions. Therefore, we need to identify the cells and cellular interactions that are critical in tumors to eradicate cancer cells and prevent cancer development. Recent evidence revealed that stem cells, the tumor microenvironment and metabolic alterations are closely related and critical steps in colorectal carcinogenesis. With recent advances in our understanding of the homeostatic control of intestinal crypts and microenvironments (niches), we are able to delineate the steps in early carcinogenesis of CRC, which could lead to development of new targets for chemoprevention of CRC.
Within the crypts of the intestinal mucosa, the intestinal epithelium is a permanently renewing tissue, the architecture of which is maintained by the ability of the intestinal stem cells to self-renew and generate a hierarchy of proliferative and differentiated cells[75]. The balance between proliferation and cell death is important for homeostasis of the intestinal epithelium. Using genetic lineage-tracing methods for stem cell markers, several markers of intestinal stem cells, including B-lymphoma Mo-MLV insertion region 1 (BMI1), telomerase reverse transcriptase, leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5), leucine-rich repeats and immunoglobulin-like domains 1 (Lrig1), olfactomedin 4 and achaete-scute complex homolog 2 (ASCL2), have been identified[76-80].
As for the stem cell niche or microenvironment, subepithelial mesenchymal cells, their secreted growth factors, and basement membrane factors that regulate epithelial cell function support the intestinal crypts. Pericryptal myofibroblasts (PCMFs), one of the microenvironment or niche components, have crucial functions and roles in intestinal organogenesis, regulation of epithelial cell proliferation and differentiation, mucosal protection, wound healing and extracellular matrix (ECM) metabolism that affects the growth of the basement membrane[81,82]. In addition, many inflammatory cells, such as macrophages and lymphocytes, are also important components of the microenvironment in both normal and pathological states. PCMFs and inflammatory cells are located immediately beneath the basement membrane, just under the epithelial cells, and function through the secretion of growth factors, cytokines and basement membrane/ECM proteins; they become activated through direct and indirect interactions[82]. Several studies showed that interstitial myofibroblasts and inflammatory cells increase in neoplastic lesions, suggesting that myofibroblasts and inflammatory cells play critical roles in colorectal neoplasia, as well as under normal conditions[83-85].
The stem/progenitor cells, transient amplifying cells, and, finally, the terminally differentiated cells in intestinal crypts are under homeostatic control through important signals, such as the Wnt, bone morphogenic protein (BMP), NOTCH, Hh and phosphoinositol 3-kinase (PI3K)/mTOR pathways[86]. The regulation of these signals occurs via the tight control of interactions between stem/progenitor cells and the niche microenvironment, such as the PCMFs, smooth muscle cells and inflammatory cells[86]. The dysregulation of cryptal homeostasis can induce tumorigenesis and the micro environmental factors secreted by inflammatory cells and myofibroblasts in tumors, as well as accumulation of epithelial changes, have important roles in early tumor progression[86].
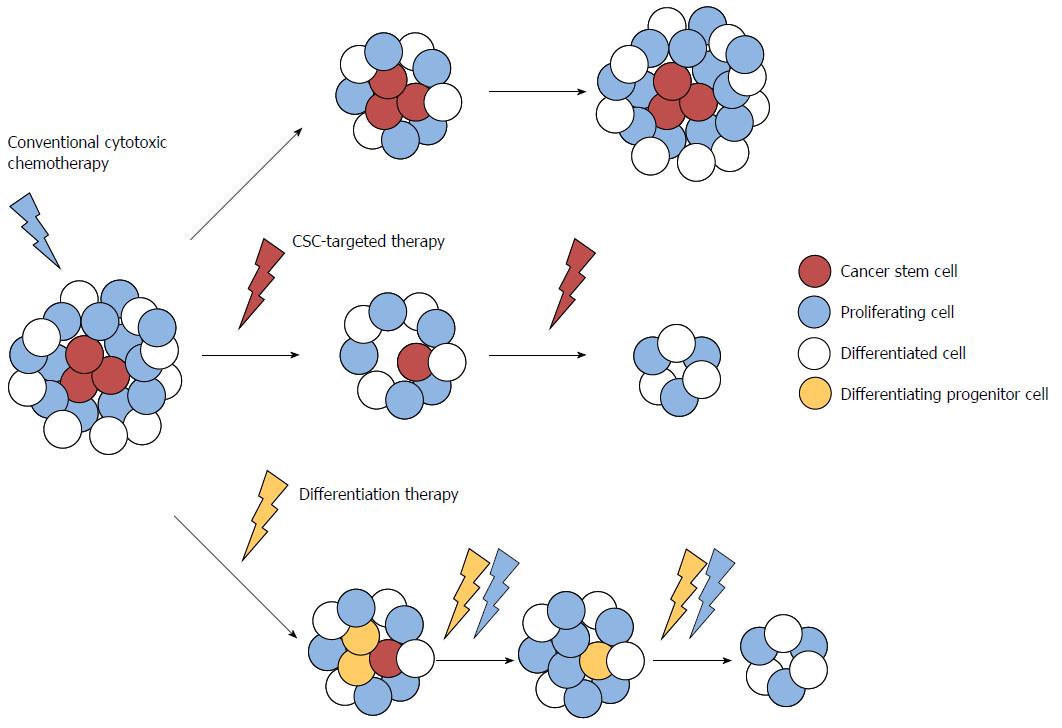
Recent evidence suggests that CRC may arise from mutated colorectal stem or progenitor cells that have been termed colorectal cancer stem cells (CSCs) or initiating cells because of their exclusive ability to sustain tumor formation[87,88]. Colorectal CSCs have been identified based on the expression of specific cell surface markers, such as cluster of differentiation (CD)133, CD44, CD166, aldehyde dehydrogenase, doublecortin-like kinase 1 (Dclk1), Lgr5 and Eph receptor B2, and these cells demonstrated stem/progenitor cell properties, in terms of their ability to self-renew, differentiate, and proliferate indefinitely to drive continuous expansion of the malignant cell population[89-93]. These data emphasize the importance of better characterization of CSCs, because the limited numbers of CSCs within the bulk of the tumor may account for their capability to escape conventional therapies, leading to relapse and metastasis. CSCs are now recognized as a specific target for the complete elimination of CRC (Figure 1). In addition, because these CSCs appear in the very early stages of colorectal carcinogenesis, the early changes that occur in normal and cancer stem cells during carcinogenesis might be an effective target for chemoprevention, as well as treatment of advanced CRC.
Recent evidence suggests interesting similarities in the effects of the above-mentioned major chemopreventive drugs, such as aspirin, NSAIDs, statins, PPARγ agonists, and metformin; these include their CSC-suppressing effect, anti-inflammatory action, and regulation of altered tumor metabolism. In addition, both anti-inflammatory effects and regulation of altered tumor metabolism are also associated with the CSC-suppressing effects of these drugs.
CSCs are involved in tumor initiation, growth, recurrence, and metastasis; therefore, these data suggest that the preventive and survival-improving effects of chemopreventive drugs on CRC might be related to their CSC-suppressing ability. Therefore, in this section, I focus on the relationship between normal/cancer stem cell-related pathways and the mechanism of the chemopreventive drugs.
The anticarcinogenic activity of NSAIDs in CRC may depend mostly on the inhibition of COX2 activity, because prostaglandins (PGs) play an important role in tumorigenesis in CRC[94-97]. COX2 is reported to be overexpressed in 85% of human CRC cases and in about 50% of colorectal adenomas[94], and was also identified in an animal model[97], in which a COX2 null mutation significantly reduced the number and size of polyps in Apc∆716 mice[98]. An earlier study reported that COX2 over-expression leads to the production of PGE2, which ultimately stimulates β-catenin-mediated transcription in colon cancer[99]. The WNT/β-catenin pathway is thought to be involved in the regulation of CSCs, and is one of the most interesting therapeutic targets in CSCs[100]. In terms of the anti-CSC effect of NSAIDs, Moon et al[101] showed that the anti-CSC effects of NSAIDs were related to both COX2-dependent and -independent pathways.
As traditional NSAIDs exert anticancer effects via COX2-independent mechanisms[102], the COX2-independent pathways of NSAIDs could be involved in their anti-CSC activity. In several previous reports, NSAIDs were shown to inhibit NOTCH/hairy and enhancer of split 1 (HES1) signaling pathway as a γ-secretase inhibitor[103,104] and activate the PPARγ expression as a PPARγ agonist[105,106]. NOTCH/HES1 signaling has been shown to be oncogenic in CRCs, inhibiting the terminal differentiation of epithelial cells[107], and the dysregulation of the NOTCH/HES1 signaling was implicated in the self-renewal and maintenance of CSCs in CRC[108]. Meanwhile, PPARγ activation resulted in growth arrest and induced differentiation of colon cancer cells[45]. In addition, the CSC-inhibitory effect of PPARγ agonists through the inhibition of the Janus kinase-signal transducer and activator of transcription (STAT) pathway was demonstrated in brain CSCs[109]. In this context, Moon et al[101] showed that the NOTCH pathway and PPARγ could be related to CSCs in CRC and be down- and up-regulated by NSAIDs, respectively, suggesting that NSAIDs suppress colon CSCs via COX2-independent pathways.
In addition, Qiu et al[110] demonstrated that sulindac induces apoptosis to remove the intestinal stem cells with aberrant Wnt signaling, and that diablo IAP-binding mitochondrial protein (also referred to as SMAC), a mitochondrial apoptogenic protein, has a central role in this tumor-suppressive effect of sulindac. These results suggested that the chemopreventive effect of NSAIDs is mediated through the elimination of stem cells that are inappropriately activated by oncogenic events.
Some natural chemopreventive dietary compounds, such as curcumin, sulforaphane and piperine, also have been shown to suppress CSCs through inhibition of WNT/β-catenin signaling[111-113].
Statins also have anti-CSC activity. Kodach et al[114] showed that tumor-suppressive BMP signaling was silenced by promoter hypermethylation of BMP2 in CRC, and downregulation of DNA methyltransferase activity by statin led to BMP2 promoter demethylation and upregulation of BMP2 expression, culminating in the differentiation of CRC cells and reduction of “stemness”.
Therefore, the suppression of Wnt and NOTCH signaling, and activation of BMP and PPARγ signaling that is induced by NSAIDs, statins, PPARγ agonists, and some natural chemopreventive dietary compounds might have potential effects on the fate of stem cells, inducing cell differentiation, cell cycle arrest, and apoptosis.
Recent studies show that bidirectional conversion between CSCs and non-CSCs can be triggered by stromal factors secreted by inflammatory cells or myofibroblasts in the tumor microenvironment[115,116]. These factors enhance Wnt activation, induce dedifferentiation of non-stem cells and expand stem cell properties during tumorigenesis. In this regard, the anti-inflammatory effect of chemopreventive drugs can retard this de-differentiating effect.
As an important component of the tumor microenvironment, chronic inflammation is also a key factor in the progression of many cancers. Nuclear factor (NF)-κB represents a key transcription factor within the inflammatory tumor microenvironment. Schwitalla et al[116] demonstrated NF-κB’s function in CSCs, showing that elevated NF-κB signaling enhances Wnt activation and induces de-differentiation of non-stem cells that have acquired a tumor-initiating capacity. Subsequently, epithelial cell-specific ablation of the RelA/p65 subunit of NF-κB retards crypt stem cell expansion; these data support the concept of bidirectional conversion and the importance of inflammatory signaling for de-differentiation and generation of CSCs[116].
Metformin inhibits initial cellular transformation and selectively suppresses CSCs by inhibition of NF-κB and STAT3[117]. In addition, metformin reduces inflammatory responses via inhibition of tumor necrosis factor (TNF)-production in human monocytes[118], and metformin-induced AMPK signaling inhibits the NF-κB-mediated inflammatory responses[119]. Thus, metformin may target the inflammatory processes in the microenvironment of most neoplastic tissues and cancer cells[117,119].
Similarly, PPARγ agonists can attenuate NF-κB-dependent signaling and induce downregulation of pro-inflammatory target genes, such as TNF and interleukin-6[120], and statins also reduce colon tumorigenesis via their potential anti-inflammatory and immunomodulatory properties[121,122]. In addition, the anti-inflammatory action of NSAIDs is already well established. All the major chemopreventive drugs discussed in this review have anti-inflammatory properties, which suggest their association with anti-CSC activity through an anti-inflammatory action on the tumor microenvironment, along with their direct effects on CSCs.
Vermeulen et al[115] demonstrated that myofibroblast-secreted factors, specifically hepatocyte growth factor, activate β-catenin-dependent transcription and subsequently CSC clonogenicity, indicating that Wnt activity and cancer stemness are regulated by microenvironmental factors. They also showed that myofibroblast-secreted factors restore the CSC phenotype in more differentiated cells, suggesting the dynamic bidirectional conversion of stemness of colon cancer cells[115].
Currently, the regulation of microenvironmetal factors has become one of the major targets for development of anti-CSC drugs, and they could be important targets for chemoprevention as well, because the stem cell niche is involved in the very early stages of tumorigenesis.
In terms of cancer metabolism, recent evidence reveals that metabolic alterations and reprogramming of cancer cells are not indirect responses to cell proliferation, but altered metabolism itself can be tumorigenic by changing cell signaling and blocking cellular differentiation[123].
The AMPK/mTOR pathway is a central cellular energy sensor[124]. Liver kinase B1 (LKB1), the upstream activator of AMPK, is a tumor suppressor, and the major pathway controlled by LKB1-AMPK activation is the mTOR signaling pathway, which regulates cell growth and proliferation[124,125]. Activation of AMPK led to inhibition of the mTOR through phosphorylation and subsequent activation of the tumor suppressor tuberous sclerosis complex 2. mTOR is a key regulator of growth factor and nutrient signals, as well as a critical mediator of the PI3K/protein kinase B/Akt pathway, one of the most frequently dysregulated signaling pathways in human cancer[124,126].
In preclinical studies, metformin induced tumor suppression through mTOR inhibition by AMPK activation[127]. In addition, activation of the Akt/mTOR pathway has been associated with malignant progression, resistance to many types of cancer therapy and poor prognosis[126]. The PI3K/Akt/mTOR pathway has, therefore, recently been identified as a target for novel cancer therapy, and the inhibition of mTOR signaling is thought to be one of the major mechanisms involving the anti-cancer effect of metformin. Activation of AMPK also induces cell cycle arrest by inhibiting the expression of cyclin D1 and activating p21/p27, resulting in cellular senescence, quiescence, and apoptosis[128,129].
Recent studies have demonstrated that metformin could selectively suppress cancer stem cells using in vitro experiments and in vivo xenograft models[117,130,131]. Metformin has also been shown to improve tumor response to chemotherapy, by activating a cytotoxic effect on CSCs that exhibit chemoresistant features[117,130,132]. The PI3K/Akt/mTOR pathway is activated for maintenance and proliferation of CSCs[133-135]; therefore, the mechanism of action of metformin-induced CSC suppression involves the activation of AMPK and the consequent inactivation of mTOR. CSCs are known to be resistant to conventional chemotherapy, and are a cause of cancer recurrence and metastasis; therefore, metformin’s effect of eliminating cancer stem cells suggest the possibility of metformin as an adjunctive agent combined with conventional chemotherapy, as well as a chemopreventive drug.
Moreover, aspirin, statin, and PPARγ agonists also induce the activation of AMPK, targeting regulators of intracellular energy homeostasis and metabolism[136-140]. These could contribute to their protective effects against development of CRC.
Candidates for effective CRC chemopreventive drugs have been identified through epidemiological studies, basic research and clinical trials. However, to develop more effective chemopreventive drugs with good safety profiles, we need to target cells and pathways that are essential and critical to carcinogenesis, along with targeting the traditional multi-step process of CRC tumorigenesis.
With recent advances in our understanding of intestinal crypt homeostasis and its dysregulation, mutated stem cells and CSCs in early carcinogenesis are likely to be promising targets for chemoprevention of CRC. However, to target the CSCs and stem cell-specific signaling pathways in early carcinogenesis, a detailed understanding of the mechanisms of stem cell maintenance and differentiation, and their relationship with the carcinogenic pathways is needed.
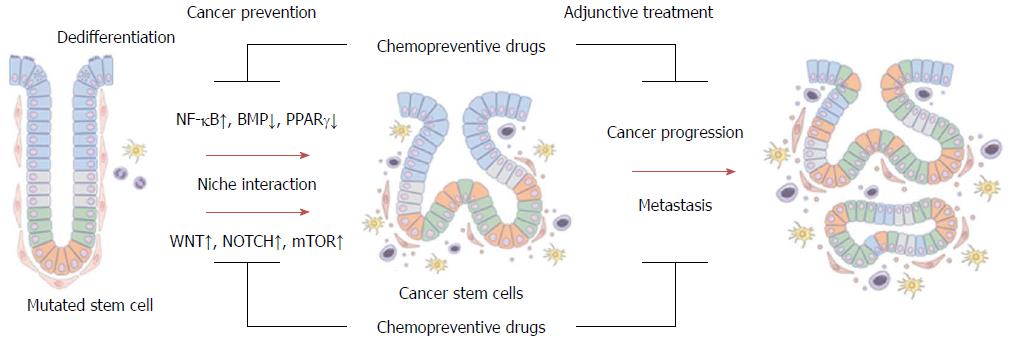
Several recent reports indicate that major chemopreventive drugs like aspirin, NSAIDs, statins, PPARγ agonists, and metformin have CSC-suppressing effects via regulation of stem cell-regulating pathways (Wnt, NOTCH, and BMP), stem cell niche or tumor microenvironment (inflammatory NF-κB and stromal factor-induced Wnt pathways) and altered tumor metabolism (AMPK/mTOR pathway) (Figure 2).
Changes in the stem cells, microenvironment, and metabolism are closely related, underlying essential steps in early carcinogenesis and tumor progression, and could be critical targets for chemoprevention and treatment of CRC (Figure 2). In addition to being more effective anti-neoplastic and chemopreventive drugs, either alone or in combination with other agents, these chemopreventive drugs could also be the basis for development of chemically modified drugs with better chemopreventive activity and a more desirable safety profile.
P- Reviewers: Anant S, Minana MD, Tanabe S S- Editor: Qi Y L- Editor: Stewart GJ E- Editor: Zhang DN
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11831] [Article Influence: 845.1] [Reference Citation Analysis (4)] |
| 2. | Cersosimo RJ. Management of advanced colorectal cancer, Part 1. Am J Health Syst Pharm. 2013;70:395-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124:544-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1501] [Cited by in RCA: 1437] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 4. | Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1442] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 5. | Umar A, Dunn BK, Greenwald P. Future directions in cancer prevention. Nat Rev Cancer. 2012;12:835-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 6. | Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1021] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 7. | Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA. 2005;294:914-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 354] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 8. | Jacoby RF, Seibert K, Cole CE, Kelloff G, Lubet RA. The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the min mouse model of adenomatous polyposis. Cancer Res. 2000;60:5040-5044. [PubMed] |
| 9. | Beazer-Barclay Y, Levy DB, Moser AR, Dove WF, Hamilton SR, Vogelstein B, Kinzler KW. Sulindac suppresses tumorigenesis in the Min mouse. Carcinogenesis. 1996;17:1757-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, Petrelli N, Pipas JM, Karp DD, Loprinzi CL. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 816] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 11. | Benamouzig R, Deyra J, Martin A, Girard B, Jullian E, Piednoir B, Couturier D, Coste T, Little J, Chaussade S. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 274] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 12. | Burn J, Gerdes AM, Macrae F, Mecklin JP, Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081-2087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 698] [Cited by in RCA: 698] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 13. | Meyskens FL, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, Lawson MJ, Kidao J, McCracken J. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila). 2008;1:32-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 412] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 14. | Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, Imamura Y, Qian ZR, Baba Y, Shima K. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 667] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 15. | Chia WK, Ali R, Toh HC. Aspirin as adjuvant therapy for colorectal cancer--reinterpreting paradigms. Nat Rev Clin Oncol. 2012;9:561-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Langley RE, Rothwell PM. Potential biomarker for aspirin use in colorectal cancer therapy. Nat Rev Clin Oncol. 2013;10:8-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1815] [Cited by in RCA: 1715] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 18. | Arber N, Eagle CJ, Spicak J, Rácz I, Dite P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 725] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 19. | Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, Tang J, Rosenstein RB, Wittes J, Corle D. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 783] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 20. | Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1849] [Cited by in RCA: 1724] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 21. | Kerr DJ, Dunn JA, Langman MJ, Smith JL, Midgley RS, Stanley A, Stokes JC, Julier P, Iveson C, Duvvuri R. Rofecoxib and cardiovascular adverse events in adjuvant treatment of colorectal cancer. N Engl J Med. 2007;357:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Solomon DH, Avorn J, Stürmer T, Glynn RJ, Mogun H, Schneeweiss S. Cardiovascular outcomes in new users of coxibs and nonsteroidal antiinflammatory drugs: high-risk subgroups and time course of risk. Arthritis Rheum. 2006;54:1378-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003;9:10-19. [PubMed] |
| 24. | Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16:508-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 453] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 25. | Taylor ML, Wells BJ, Smolak MJ. Statins and cancer: a meta-analysis of case-control studies. Eur J Cancer Prev. 2008;17:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Friedman GD, Flick ED, Udaltsova N, Chan J, Quesenberry CP, Habel LA. Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiol Drug Saf. 2008;17:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 27. | Shadman M, Newcomb PA, Hampton JM, Wernli KJ, Trentham-Dietz A. Non-steroidal anti-inflammatory drugs and statins in relation to colorectal cancer risk. World J Gastroenterol. 2009;15:2336-2339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Lipkin SM, Chao EC, Moreno V, Rozek LS, Rennert H, Pinchev M, Dizon D, Rennert G, Kopelovich L, Gruber SB. Genetic variation in 3-hydroxy-3-methylglutaryl CoA reductase modifies the chemopreventive activity of statins for colorectal cancer. Cancer Prev Res (Phila). 2010;3:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Hachem C, Morgan R, Johnson M, Kuebeler M, El-Serag H. Statins and the risk of colorectal carcinoma: a nested case-control study in veterans with diabetes. Am J Gastroenterol. 2009;104:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Broughton T, Sington J, Beales IL. Statin use is associated with a reduced incidence of colorectal cancer: a colonoscopy-controlled case-control study. BMC Gastroenterol. 2012;12:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Poynter JN, Gruber SB, Higgins PD, Almog R, Bonner JD, Rennert HS, Low M, Greenson JK, Rennert G. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 595] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 32. | Singh H, Mahmud SM, Turner D, Xue L, Demers AA, Bernstein CN. Long-term use of statins and risk of colorectal cancer: a population-based study. Am J Gastroenterol. 2009;104:3015-3023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Haukka J, Sankila R, Klaukka T, Lonnqvist J, Niskanen L, Tanskanen A, Wahlbeck K, Tiihonen J. Incidence of cancer and statin usage--record linkage study. Int J Cancer. 2010;126:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 34. | Coogan PF, Smith J, Rosenberg L. Statin use and risk of colorectal cancer. J Natl Cancer Inst. 2007;99:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Vinogradova Y, Hippisley-Cox J, Coupland C, Logan RF. Risk of colorectal cancer in patients prescribed statins, nonsteroidal anti-inflammatory drugs, and cyclooxygenase-2 inhibitors: nested case-control study. Gastroenterology. 2007;133:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Kaye JA, Jick H. Statin use and cancer risk in the General Practice Research Database. Br J Cancer. 2004;90:635-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 37. | Vinogradova Y, Coupland C, Hippisley-Cox J. Exposure to statins and risk of common cancers: a series of nested case-control studies. BMC Cancer. 2011;11:409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 38. | Siddiqui AA, Nazario H, Mahgoub A, Pandove S, Cipher D, Spechler SJ. The long-term use of statins is associated with a decreased incidence of adenomatous colon polyps. Digestion. 2009;79:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Siddiqui AA, Nazario H, Mahgoub A, Patel M, Cipher D, Spechler SJ. For patients with colorectal cancer, the long-term use of statins is associated with better clinical outcomes. Dig Dis Sci. 2009;54:1307-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Drori S, Girnun GD, Tou L, Szwaya JD, Mueller E, Xia K, Shivdasani RA, Spiegelman BM. Hic-5 regulates an epithelial program mediated by PPARgamma. Genes Dev. 2005;19:362-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Gupta RA, Brockman JA, Sarraf P, Willson TM, DuBois RN. Target genes of peroxisome proliferator-activated receptor gamma in colorectal cancer cells. J Biol Chem. 2001;276:29681-29687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 42. | Sarraf P, Mueller E, Jones D, King FJ, DeAngelo DJ, Partridge JB, Holden SA, Chen LB, Singer S, Fletcher C. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med. 1998;4:1046-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 752] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 43. | Yoshizumi T, Ohta T, Ninomiya I, Terada I, Fushida S, Fujimura T, Nishimura G, Shimizu K, Yi S, Miwa K. Thiazolidinedione, a peroxisome proliferator-activated receptor-gamma ligand, inhibits growth and metastasis of HT-29 human colon cancer cells through differentiation-promoting effects. Int J Oncol. 2004;25:631-639. [PubMed] |
| 44. | Peters JM, Shah YM, Gonzalez FJ. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat Rev Cancer. 2012;12:181-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 379] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 45. | Colmers IN, Bowker SL, Johnson JA. Thiazolidinedione use and cancer incidence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab. 2012;38:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 46. | Chen SW, Tsan YT, Chen JD, Hsieh HI, Lee CH, Lin HH, Wang JD, Chen PC. Use of thiazolidinediones and the risk of colorectal cancer in patients with diabetes: a nationwide, population-based, case-control study. Diabetes Care. 2013;36:369-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor gamma agonists. Lancet Oncol. 2004;5:419-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 350] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 48. | Koeffler HP. Peroxisome proliferator-activated receptor gamma and cancers. Clin Cancer Res. 2003;9:1-9. [PubMed] |
| 49. | Ogino S, Shima K, Baba Y, Nosho K, Irahara N, Kure S, Chen L, Toyoda S, Kirkner GJ, Wang YL. Colorectal cancer expression of peroxisome proliferator-activated receptor gamma (PPARG, PPARgamma) is associated with good prognosis. Gastroenterology. 2009;136:1242-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 50. | McAlpine CA, Barak Y, Matise I, Cormier RT. Intestinal-specific PPARgamma deficiency enhances tumorigenesis in ApcMin/+ mice. Int J Cancer. 2006;119:2339-2346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Lefebvre AM, Chen I, Desreumaux P, Najib J, Fruchart JC, Geboes K, Briggs M, Heyman R, Auwerx J. Activation of the peroxisome proliferator-activated receptor gamma promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nat Med. 1998;4:1053-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 466] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 52. | Saez E, Tontonoz P, Nelson MC, Alvarez JG, Ming UT, Baird SM, Thomazy VA, Evans RM. Activators of the nuclear receptor PPARgamma enhance colon polyp formation. Nat Med. 1998;4:1058-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 460] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 53. | Pino MV, Kelley MF, Jayyosi Z. Promotion of colon tumors in C57BL/6J-APC(min)/+ mice by thiazolidinedione PPARgamma agonists and a structurally unrelated PPARgamma agonist. Toxicol Pathol. 2004;32:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Yang K, Fan KH, Lamprecht SA, Edelmann W, Kopelovich L, Kucherlapati R, Lipkin M. Peroxisome proliferator-activated receptor gamma agonist troglitazone induces colon tumors in normal C57BL/6J mice and enhances colonic carcinogenesis in Apc1638 N/+ Mlh1+/- double mutant mice. Int J Cancer. 2005;116:495-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Erdmann E, Charbonnel B, Wilcox R. Thiazolidinediones and cardiovascular risk - a question of balance. Curr Cardiol Rev. 2009;5:155-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, Reid IR. The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2007;92:1305-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 309] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 57. | Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2205] [Cited by in RCA: 2108] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 58. | Schwartz AV, Sellmeyer DE. Thiazolidinedione therapy gets complicated: is bone loss the price of improved insulin resistance. Diabetes Care. 2007;30:1670-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Piccinni C, Motola D, Marchesini G, Poluzzi E. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care. 2011;34:1369-1371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 60. | Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 848] [Article Influence: 53.0] [Reference Citation Analysis (1)] |
| 61. | Lee JH, Kim TI, Jeon SM, Hong SP, Cheon JH, Kim WH. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. Int J Cancer. 2012;131:752-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 62. | Garrett CR, Hassabo HM, Bhadkamkar NA, Wen S, Baladandayuthapani V, Kee BK, Eng C, Hassan MM. Survival advantage observed with the use of metformin in patients with type II diabetes and colorectal cancer. Br J Cancer. 2012;106:1374-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 63. | Zhang ZJ, Zheng ZJ, Kan H, Song Y, Cui W, Zhao G, Kip KE. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: a meta-analysis. Diabetes Care. 2011;34:2323-2328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 64. | Hsieh MC, Lee TC, Cheng SM, Tu ST, Yen MH, Tseng CH. The influence of type 2 diabetes and glucose-lowering therapies on cancer risk in the Taiwanese. Exp Diabetes Res. 2012;2012:413782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 65. | Lee JH, Jeon SM, Hong SP, Cheon JH, Kim TI, Kim WH. Metformin use is associated with a decreased incidence of colorectal adenomas in diabetic patients with previous colorectal cancer. Dig Liver Dis. 2012;44:1042-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 66. | Algire C, Amrein L, Zakikhani M, Panasci L, Pollak M. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr Relat Cancer. 2010;17:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 67. | Hosono K, Endo H, Takahashi H, Sugiyama M, Uchiyama T, Suzuki K, Nozaki Y, Yoneda K, Fujita K, Yoneda M. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Mol Carcinog. 2010;49:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 68. | Emami Riedmaier A, Fisel P, Nies AT, Schaeffeler E, Schwab M. Metformin and cancer: from the old medicine cabinet to pharmacological pitfalls and prospects. Trends Pharmacol Sci. 2013;34:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 69. | Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1166] [Cited by in RCA: 1229] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 70. | Hosono K, Endo H, Takahashi H, Sugiyama M, Sakai E, Uchiyama T, Suzuki K, Iida H, Sakamoto Y, Yoneda K. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev Res (Phila). 2010;3:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 71. | Higurashi T, Takahashi H, Endo H, Hosono K, Yamada E, Ohkubo H, Sakai E, Uchiyama T, Hata Y, Fujisawa N. Metformin efficacy and safety for colorectal polyps: a double-blind randomized controlled trial. BMC Cancer. 2012;12:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 72. | Asano TK, McLeod RS. Non steroidal anti-inflammatory drugs (NSAID) and Aspirin for preventing colorectal adenomas and carcinomas. Cochrane Database Syst Rev. 2004;CD004079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 73. | Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1534] [Cited by in RCA: 1456] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 74. | Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6773] [Cited by in RCA: 6647] [Article Influence: 511.3] [Reference Citation Analysis (0)] |
| 75. | van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1325] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 76. | Vaiopoulos AG, Kostakis ID, Koutsilieris M, Papavassiliou AG. Colorectal cancer stem cells. Stem Cells. 2012;30:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 77. | Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA. 2012;109:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 656] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 78. | Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4327] [Article Influence: 240.4] [Reference Citation Analysis (0)] |
| 79. | Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci USA. 2011;108:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 431] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 80. | Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 565] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 81. | Harada K, Higaki S, Amano A, Hashimoto K, Hashimoto S, Gondo T, Sakaida I. A reduced COX-2 expression and a reduced number of pericryptal myofibroblasts are associated with depressed adenoma of the colon. Oncol Rep. 2007;17:1353-1358. [PubMed] |
| 82. | Andoh A, Bamba S, Brittan M, Fujiyama Y, Wright NA. Role of intestinal subepithelial myofibroblasts in inflammation and regenerative response in the gut. Pharmacol Ther. 2007;114:94-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 83. | Kim JH, Oh SH, Kim EJ, Park SJ, Hong SP, Cheon JH, Kim TI, Kim WH. The role of myofibroblasts in upregulation of S100A8 and S100A9 and the differentiation of myeloid cells in the colorectal cancer microenvironment. Biochem Biophys Res Commun. 2012;423:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 84. | Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 962] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 85. | Adegboyega PA, Mifflin RC, DiMari JF, Saada JI, Powell DW. Immunohistochemical study of myofibroblasts in normal colonic mucosa, hyperplastic polyps, and adenomatous colorectal polyps. Arch Pathol Lab Med. 2002;126:829-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 86. | Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature. 2011;474:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 349] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 87. | Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603-607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 535] [Cited by in RCA: 517] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 88. | Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1797] [Cited by in RCA: 1667] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 89. | Ziskin JL, Dunlap D, Yaylaoglu M, Fodor IK, Forrest WF, Patel R, Ge N, Hutchins GG, Pine JK, Quirke P. In situ validation of an intestinal stem cell signature in colorectal cancer. Gut. 2013;62:1012-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 90. | O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2977] [Cited by in RCA: 3048] [Article Influence: 160.4] [Reference Citation Analysis (0)] |
| 91. | Merlos-Suárez A, Barriga FM, Jung P, Iglesias M, Céspedes MV, Rossell D, Sevillano M, Hernando-Momblona X, da Silva-Diz V, Muñoz P. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8:511-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 741] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 92. | Todaro M, Francipane MG, Medema JP, Stassi G. Colon cancer stem cells: promise of targeted therapy. Gastroenterology. 2010;138:2151-2162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 354] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 93. | Nakanishi Y, Seno H, Fukuoka A, Ueo T, Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet. 2013;45:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 330] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 94. | Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183-1188. [PubMed] |
| 95. | Xia D, Wang D, Kim SH, Katoh H, DuBois RN. Prostaglandin E2 promotes intestinal tumor growth via DNA methylation. Nat Med. 2012;18:224-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 96. | Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 656] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 97. | Williams CS, Luongo C, Radhika A, Zhang T, Lamps LW, Nanney LB, Beauchamp RD, DuBois RN. Elevated cyclooxygenase-2 levels in Min mouse adenomas. Gastroenterology. 1996;111:1134-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 208] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 98. | Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell. 1996;87:803-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1733] [Cited by in RCA: 1678] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 99. | Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 720] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 100. | Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells. Clin Cancer Res. 2010;16:3153-3162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 392] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 101. | Moon CM, Kwon JH, Kim JS, Oh SH, Jin Lee K, Park JJ, Pil Hong S, Cheon JH, Kim TI, Kim WH. Nonsteroidal anti-inflammatory drugs suppress cancer stem cells via inhibiting PTGS2 (cyclooxygenase 2) and NOTCH/HES1 and activating PPARG in colorectal cancer. Int J Cancer. 2014;134:519-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 102. | Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001;15:2057-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 558] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 103. | Zhang H, Ye Y, Bai Z, Wang S. The COX-2 selective inhibitor-independent COX-2 effect on colon carcinoma cells is associated with the Delta1/Notch1 pathway. Dig Dis Sci. 2008;53:2195-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Beher D, Clarke EE, Wrigley JD, Martin AC, Nadin A, Churcher I, Shearman MS. Selected non-steroidal anti-inflammatory drugs and their derivatives target gamma-secretase at a novel site. Evidence for an allosteric mechanism. J Biol Chem. 2004;279:43419-43426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 105. | Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997;272:3406-3410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 844] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 106. | Vaish V, Tanwar L, Sanyal SN. The role of NF-κB and PPARγ in experimentally induced colorectal cancer and chemoprevention by cyclooxygenase-2 inhibitors. Tumour Biol. 2010;31:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 107. | Katoh M, Katoh M. Notch signaling in gastrointestinal tract (review). Int J Oncol. 2007;30:247-251. [PubMed] |
| 108. | Sikandar SS, Pate KT, Anderson S, Dizon D, Edwards RA, Waterman ML, Lipkin SM. NOTCH signaling is required for formation and self-renewal of tumor-initiating cells and for repression of secretory cell differentiation in colon cancer. Cancer Res. 2010;70:1469-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 109. | Chearwae W, Bright JJ. PPARgamma agonists inhibit growth and expansion of CD133+ brain tumour stem cells. Br J Cancer. 2008;99:2044-2053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 110. | Qiu W, Wang X, Leibowitz B, Liu H, Barker N, Okada H, Oue N, Yasui W, Clevers H, Schoen RE. Chemoprevention by nonsteroidal anti-inflammatory drugs eliminates oncogenic intestinal stem cells via SMAC-dependent apoptosis. Proc Natl Acad Sci USA. 2010;107:20027-20032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 111. | Li Y, Wicha MS, Schwartz SJ, Sun D. Implications of cancer stem cell theory for cancer chemoprevention by natural dietary compounds. J Nutr Biochem. 2011;22:799-806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 112. | Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, Liu S, Dontu G, Wicha MS. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122:777-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 331] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 113. | Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, Yu Y, Clouthier SG, Schwartz SJ, Wicha MS. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16:2580-2590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 400] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 114. | Kodach LL, Jacobs RJ, Voorneveld PW, Wildenberg ME, Verspaget HW, van Wezel T, Morreau H, Hommes DW, Peppelenbosch MP, van den Brink GR. Statins augment the chemosensitivity of colorectal cancer cells inducing epigenetic reprogramming and reducing colorectal cancer cell ‘stemness’ via the bone morphogenetic protein pathway. Gut. 2011;60:1544-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 115. | Vermeulen L, De Sousa E Melo F, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1301] [Cited by in RCA: 1443] [Article Influence: 96.2] [Reference Citation Analysis (1)] |
| 116. | Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Göktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 831] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 117. | Hirsch HA, Iliopoulos D, Struhl K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc Natl Acad Sci USA. 2013;110:972-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 348] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 118. | Arai M, Uchiba M, Komura H, Mizuochi Y, Harada N, Okajima K. Metformin, an antidiabetic agent, suppresses the production of tumor necrosis factor and tissue factor by inhibiting early growth response factor-1 expression in human monocytes in vitro. J Pharmacol Exp Ther. 2010;334:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 119. | Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med (Berl). 2011;89:667-676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 532] [Cited by in RCA: 670] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 120. | Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1016] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 121. | Suh N, Reddy BS, DeCastro A, Paul S, Lee HJ, Smolarek AK, So JY, Simi B, Wang CX, Janakiram NB. Combination of atorvastatin with sulindac or naproxen profoundly inhibits colonic adenocarcinomas by suppressing the p65/β-catenin/cyclin D1 signaling pathway in rats. Cancer Prev Res (Phila). 2011;4:1895-1902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 122. | Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 580] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 123. | Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2205] [Cited by in RCA: 2490] [Article Influence: 191.5] [Reference Citation Analysis (0)] |
| 124. | Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5794] [Cited by in RCA: 6588] [Article Influence: 506.8] [Reference Citation Analysis (1)] |
| 125. | Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2185] [Cited by in RCA: 2295] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 126. | Morgensztern D, McLeod HL. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs. 2005;16:797-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 365] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 127. | Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov. 2012;2:778-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 404] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 128. | Ben Sahra I, Laurent K, Giuliano S, Larbret F, Ponzio G, Gounon P, Le Marchand-Brustel Y, Giorgetti-Peraldi S, Cormont M, Bertolotto C. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010;70:2465-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 429] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 129. | Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti JF, Le Marchand-Brustel Y, Bost F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576-3586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 678] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 130. | Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507-7511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 870] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 131. | Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011;71:3196-3201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 363] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 132. | Gou S, Cui P, Li X, Shi P, Liu T, Wang C. Low concentrations of metformin selectively inhibit CD133+ cell proliferation in pancreatic cancer and have anticancer action. PLoS One. 2013;8:e63969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 133. | Cai Z, Ke J, He X, Yuan R, Chen Y, Wu X, Wang L, Wang J, Lan P, Wu X. Significance of mTOR signaling and its inhibitor against cancer stem-like cells in colorectal cancer. Ann Surg Oncol. 2014;21:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 134. | Fang DD, Zhang CC, Gu Y, Jani JP, Cao J, Tsaparikos K, Yuan J, Thiel M, Jackson-Fisher A, Zong Q. Antitumor Efficacy of the Dual PI3K/mTOR Inhibitor PF-04691502 in a Human Xenograft Tumor Model Derived from Colorectal Cancer Stem Cells Harboring a PIK3CA Mutation. PLoS One. 2013;8:e67258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 135. | Huang J, Nguyen-McCarty M, Hexner EO, Danet-Desnoyers G, Klein PS. Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nat Med. 2012;18:1778-1785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 136. | Ma L, Niknejad N, Gorn-Hondermann I, Dayekh K, Dimitroulakos J. Lovastatin induces multiple stress pathways including LKB1/AMPK activation that regulate its cytotoxic effects in squamous cell carcinoma cells. PLoS One. 2012;7:e46055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 137. | Yasuda Y, Shimizu M, Shirakami Y, Sakai H, Kubota M, Hata K, Hirose Y, Tsurumi H, Tanaka T, Moriwaki H. Pitavastatin inhibits azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Cancer Sci. 2010;101:1701-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 138. | Guh JH, Chang WL, Yang J, Lee SL, Wei S, Wang D, Kulp SK, Chen CS. Development of novel adenosine monophosphate-activated protein kinase activators. J Med Chem. 2010;53:2552-2561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 139. | Din FV, Valanciute A, Houde VP, Zibrova D, Green KA, Sakamoto K, Alessi DR, Dunlop MG. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142:1504-15.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 334] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 140. | Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green KA, Mustard KJ. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 588] [Article Influence: 45.2] [Reference Citation Analysis (0)] |