Published online Apr 14, 2014. doi: 10.3748/wjg.v20.i14.3719
Revised: November 18, 2013
Accepted: January 6, 2014
Published online: April 14, 2014
Processing time: 198 Days and 10.3 Hours
Colorectal cancer hepatic metastases represent the final stage of a multi-step biological process. This process starts with a series of mutations in colonic epithelial cells, continues with their detachment from the large intestine, dissemination through the blood and/or lymphatic circulation, attachment to the hepatic sinusoids and interactions with the sinusoidal cells, such as sinusoidal endothelial cells, Kupffer cells, stellate cells and pit cells. The metastatic sequence terminates with colorectal cancer cell invasion, adaptation and colonisation of the hepatic parenchyma. All these events, termed the colorectal cancer invasion-metastasis cascade, include multiple molecular pathways, intercellular interactions and expression of a plethora of chemokines and growth factors, and adhesion molecules, such as the selectins, the integrins or the cadherins, as well as enzymes including matrix metalloproteinases. This review aims to present recent advances that provide insights into these cell-biological events and emphasizes those that may be amenable to therapeutic targeting.
Core tip: The multi-step process of colorectal hepatic metastases includes numerous molecular pathways and cellular interactions with potential clinical interest. Mutations at the initial site of colorectal carcinogenesis, such as p53 and APC, neoplastic cell interrelationship with the stromal macrophages, neoangiogenesis through growth factors, such as the vascular endothelial growth factor and platelet-derived growth factor, the role of hepatic sinusoidal cells, such as Kupffer cells, the expression of adhesion molecules, including the selectins and the integrins, are all crucial stages/events within the metastatic process. The exploration and analysis of recent research data may contribute to a better understanding of their clinical significance and may lead to new therapeutic strategies.
- Citation: Paschos KA, Majeed AW, Bird NC. Natural history of hepatic metastases from colorectal cancer - pathobiological pathways with clinical significance. World J Gastroenterol 2014; 20(14): 3719-3737
- URL: https://www.wjgnet.com/1007-9327/full/v20/i14/3719.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i14.3719
Carcinogenesis [carcino (καρκίνος) = cancer, and genesis (γέννεσις) = birth] and metastasis [meta (μετά) = after, next and stasis (στάση) = arrest] are both words of Greek origin, expressing the onset and the advanced progression (spread to another location) of cancer, respectively. Cancer development is a complicated biological process that includes numerous poorly understood aspects and attracts the greatest interest of biomedical sciences. It is a succession of interrelated, well-defined stages, usually termed the invasion-metastasis cascade[1,2].
The primary stage in the natural history of carcinogenesis starts with the transformation of normal cells into malignant derivatives at their initial site. Most interestingly, this transformation is also multistep and includes several genetic and phenotypic cellular alterations. Thus, there are two processes that progress in parallel, the metastatic cascade and the cancer cell transformation[3,4].
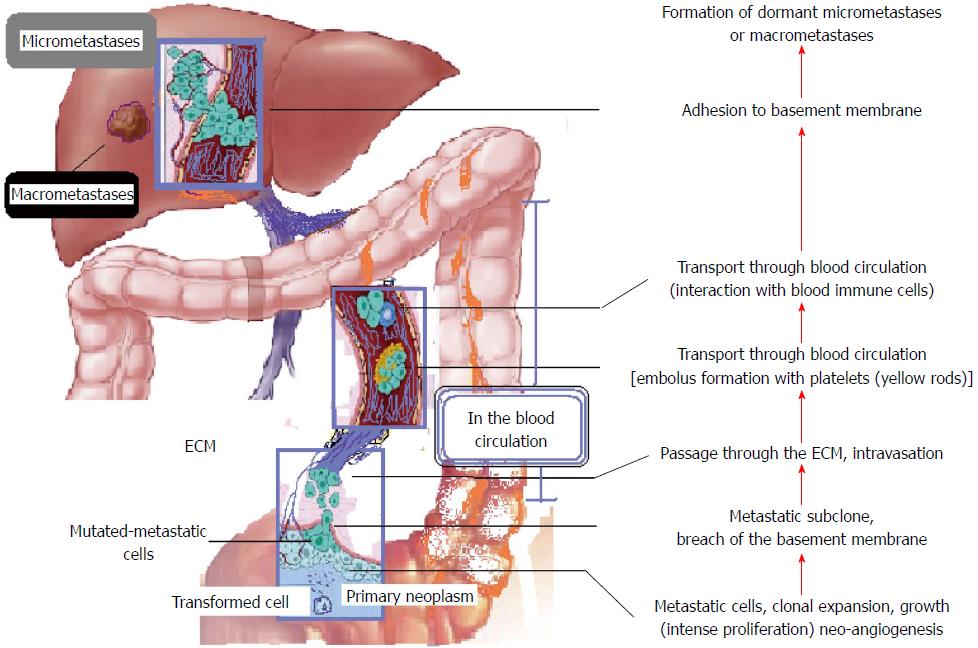
Primary mutations that signal the initiation of carcinogenesis lead to a progressive cellular proliferation and tumour formation. Neovascularisation (neoangiogenesis) and invasion of normal tissue follow, until several malignant cells are detached from the primary tumour (disaggregation), migrate and intravasate; this is the onset of metastasis. The next steps may be evasion of the immune system, cell survival in the hostile environment of the systemic circulation, arrest of the circulating tumour cells and adhesion to the endothelium lining the capillary bed of the target organ, and extravasation. The final stages of the metastatic cascade are evasion of host defences, establishment of a new blood network to supply the development of a new secondary tumour, and colonisation (Figure 1)[3,5,6].
All these successive stages demand multiple properties from malignant cells, and failure or inadequacy in any of them cancels the entire metastatic cascade. These properties are acquired through multiple mutations. It is uncertain whether cancer cells already possess most of their new potential when they begin the metastatic sequence or if this is gradually acquired. However, the colonising ability is strongly believed to appear later in the tumourigenicity[4,7]. Importantly, various laboratories have confirmed different cell subpopulations in the primary tumour site. Concurrently, the metastatic tumour created in a distant location by cancer cells is considered a different entity from the primary one, because metastatic cells show phenotypical and genetic differences from their ancestors[8-10].
While micrometastases are achieved by several cancer cells, macrometastases rarely occur. It is believed that micrometastases are the final metastatic stage for the vast majority of malignant cells, which never succeed in surviving or adapting in the inhospitable environment of the foreign tissue. In accordance with that belief, cancer patients may present myriad of micrometastases in multiple organs and tissues, although without any clinical evidence[11,12].
This general pattern of carcinogenesis and metastasis applies to most cancer types with certain modifications and adaptations according to implicated cells, tissues and organs[1]. Colorectal hepatic metastasis attracts a particular scientific interest due to the high frequency of colorectal cancer (CRC) (3rd most common cancer in the developed countries and 2nd leading cause of cancer-related mortality) and the unique properties, functions and role of the liver in the human body[13,14]. This review will describe the metastatic journey of CRC cells from the large intestine to the liver and will investigate the normal-malignant cell interactions, the role of the tissue microenvironment and the genetic and molecular pathways that mediate this carcinogenic-metastatic process.
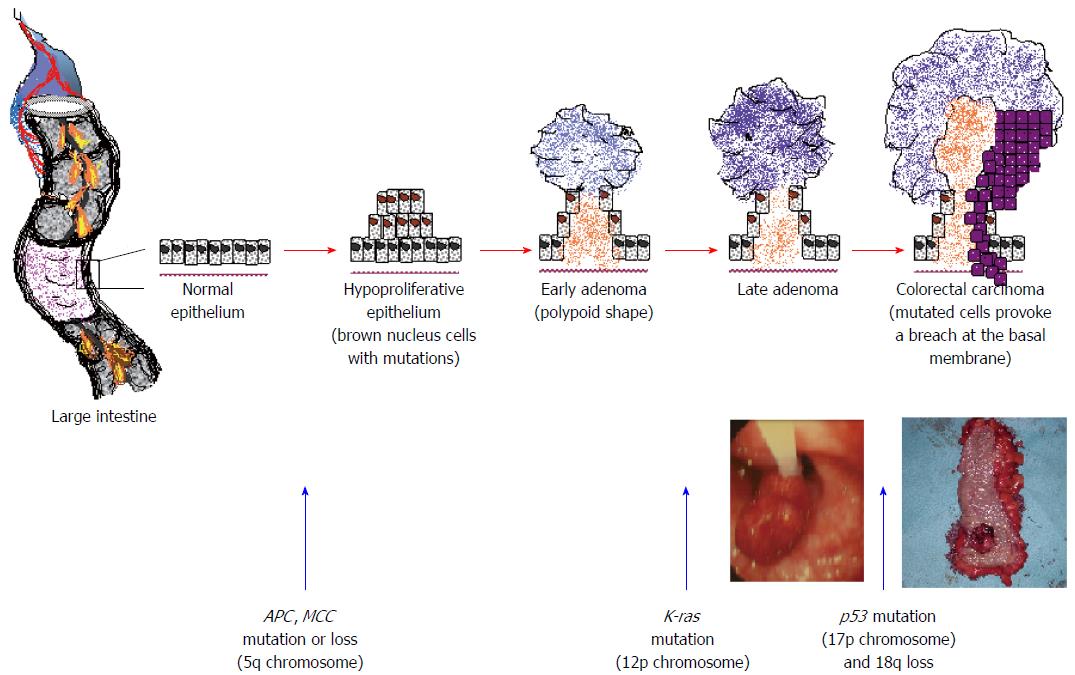
Mutations in CRC include alterations in tumour suppressor genes such as p53 and APC, DNA repair genes such as hMLH1 and hMSH2, and/or oncogenes such as K-ras and c-Myc. In this way, normal colorectal epithelial cells gain, among other abilities, the capacity of uncontrolled growth and evasion of apoptosis (programmed cellular death). Rapid cell proliferation usually generates the formation of a polypoid structure in the epithelium that may evolve to adenoma, which is a precancerous lesion. An adenoma may progress to CRC, as the mutations accumulate and CRC cells acquire all the necessary properties; single cancer cells or small clusters of them may finally detach from the initial intestinal tumour and migrate (Figure 2)[15,16].
There are at least two well-described genetic pathways that may generate CRC. The most common, which mediates up to 60% of carcinomas, is the chromosomal instability pathway. This is the result of mutations of the p53, APC and protooncogene K-ras, allelic loss of 18q and aneuploidy. The role of the APC gene is crucial in tumourigenesis, as 100% of patients with the familial adenomatous polyposis (FAP) syndrome who carry this mutation develop CRC if they receive no treatment. Another genetic pathway is the microsatellite instability (MIN) one, also termed replication error pathway, which is responsible for approximately 20% of carcinomas. These neoplasms have aberrant DNA mismatch, a near-diploid karyotype, sparse p53, K-ras and SMAD4 alterations, but also frequent BAX, BRAF and TGF-BIIR mutations. Hereditary nonpolyposis colon cancer (HNPCC) is attributed to the MIN pathway, accounts for 5%-6% of CRC, and 80% of these patients develop cancer in their lifetime. In HNPCC, MIN is a consequence of mutations in DNA mismatch repair genes (hMSH2, hPMS1, hPMS2 and hMLH1). Notably, other genetic pathways also exist, such as the cytosine phosphodiester guanosine island methylator phenotype pathway. While in the absence of methylation genes are normally expressed, in the presence of promoter methylation, genes are transcriptionally downregulated. When tumour suppressor genes are involved, carcinogenic mutations occur. A whole subclass of CRC tumours include high proportions of hypermethylated genes[17-19].
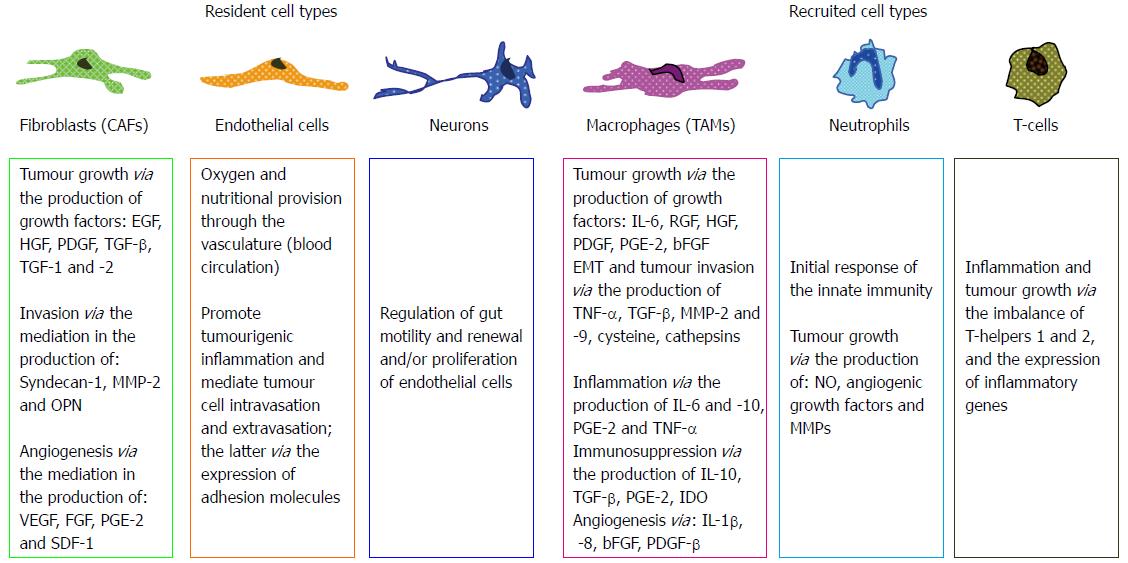
The promotion of the initial stages of colorectal carcinogenesis depends on the communication and interrelationship of neoplastic cells with the stroma. The term stroma, also referred to as reactive stroma in carcinogenesis, includes the endothelial cells, fibroblasts, immune cells (such as macrophages, lymphocytes and neutrophils), as well as non-cellular elements, such as the extracellular matrix (ECM). It appears that numerous special characteristics of the reactive tumour stroma, concerning cellular, architectural or chemical elements, support a reciprocal tumourigenic communication with neoplastic cells via the basement membrane[20]. These characteristics may be a high number of fibroblasts, altered molecular expression on the cellular surface and the cytoplasm of endothelial cells, macrophage recruitment, increased capillary density, ECM rich in fibrin and collagen-1. Furthermore, the production and secretion of a plethora of chemical compounds, including cytokines and growth factors in the colorectal stroma, mediate the promotion of carcinogenesis (Figure 3)[21-23].
Fibroblasts within a tumour appear to harbour mutations that transform them into myofibroblasts that are termed cancer-associated fibroblasts (CAFs). Apart from normal fibroblasts, CAFs may also originate from endothelial cells, epithelial cells, preadipocytes and bone marrow-derived progenitors[24,25]. Interestingly, CAF mutations may refer to a variety of genes encoding multiple growth factors, cytokines, enzymes and ECM-related proteins. Various studies have shown that CAFs have the potential to produce transforming growth factor beta (TGF-β) in an autocrine or paracrine way, triggering CRC cell detachment from their initial site[26,27]. Moreover, a recent study from Zhu et al[28] has demonstrated that TGF-1 may induce plasminogen activator 1 (PAI-1) transcription in CAFs. PAI-1 mediates the fibrinolytic activity in the vasculature, is widely expressed throughout tumours and is associated with malignant invasion and neoangiogenesis[29,30]. Taking together these experimental data, CAFs appear to play an important role in various aspects of carcinogenesis and metastasis, including migration, matrix degradation, invasion and angiogenesis[26,31].
The development of a tumour causes an inflammatory reaction where immune cells may be implicated. Macrophages are potentially the most important tumour-associated immune cells. They may constitute a considerable amount of the initial tumour mass and they correlate with tumour poor prognosis. Although macrophages act as tissue scavengers in general, eliminating any potential harmful element (invading cells or chemicals), cancer cells may use macrophage products in their favour, masking their surface antigens and thus avoiding the tumouricidal action of immune cells. In the invasion-metastasis cascade, macrophages play a significant role in the promotion of inflammation, stroma and ECM remodeling, angiogenesis, neoplastic cell invasion, intravasation and seeding at foreign sites[32-34].
Neoangiogenesis at the initial site of CRC is crucial for tumour development since oxygen diffusion alone from the normal capillary network is unable to supply a tumour larger than 1-2 mm. Macrophages regulate the critical process of neovascularisation through vascular endothelial growth factor (VEGF) production[35]. VEGF acts directly on endothelial cells promoting their proliferation, migration, invasion and high vascular permeability[36,37]. Another paradigm of the macrophage supporting role for malignant colorectal cells is through the macrophagic removal of apoptotic CRC cells that express sulfoglycolipids SM4. While such a process initially appears to be tumouricidal, the increased secretion of interleukins and TGF-β1 may contribute to tumour development and angiogenesis activation[38].
Lymphocytes constitute another immune cell category implicated in tumourigenesis with a favourable prognosis. In advanced CRC, the presence of T lymphocytes favours a better clinical outcome for patients suffering from the disease[39-41]. A recent study by de Miranda et al[42] showed that high tumour infiltration by activated CD8+ T cells in patients with Lynch CRC correlated with early staging of the primary tumour and absence of lymphatic metastases. While immune cells mainly support the defending system of normal tissue against neoplastic cells, the latter use genetic and molecular pathways that promote the evasion of immunosurveillance. CRC cells may express the Fas ligand on their surface and bind Fas-expressing immune cells, thus triggering apoptotic mechanisms for the latter[43,44]. An alternative mechanism of escaping immunosurveillance for tumour cells is the high expression of carbohydrates on their cellular membrane. This alters the CRC cell surface antigen profile, impeding their recognition and destruction by immune cells[45]. Furthermore, cells of normal colorectal tissue may also support neoplastic cells in evading immunosurveillance. Tumour-associated macrophages are able to produce arginine metabolites, which cause T-lymphocyte death. Similarly, in the lymph nodes of advanced neoplasms, dendritic cells play an immunosuppressive role rather than an immunoreactive one. Thus, instead of activating T-lymphocytes, they induce tumour tolerance[46,47].
ECM supports the cells mechanically and assists their physicochemical functions through the provision of pathways for migration and the maintenance of growth factors. Adhesion molecules play a critical role in the ECM-cell connection, with the integrins being particularly important. The ECM consists of 5 classes of molecules (collagens, fibronectins, glycans, hyaluronans, laminins and proteoglycans). Every class includes multiple isoforms, which are cell-dependent. ECM composition and structure vary with developmental stage, tissue and disease[48,49].
The basement membrane (BM) or basal lamina is a specific ECM-type meshwork produced by epithelial and stromal cells, and it plays a crucial role in carcinogenesis and metastasis[50,51]. BM chemical structure includes Type IV and VII collagen, laminins and heparan sulphate proteoglycans. Type IV collagen in particular is of great importance and provides the basic scaffold that supports other BM components, such as laminin networks and perlecan oligomers[52-54].
Any alteration in ECM structural profile or degradation of any of its molecules may cause cellular and tissue dysfunction and therefore disease. CRC induces numerous ECM changes, the BM included. Type VII collagen is altered or disappears during the development of multiple cancers, such as melanoma, breast and prostate. Moreover, laminin-5 is usually absent from the ECM structure in advanced CRC. Interestingly, new synthesis or accumulation of BM molecules (e.g., laminin-1 or 5) may also be imposed by tumours, and this phenomenon suggests the important role of BM in tumour invasion[53,54]. Similarly, modifications in ECM adhesion molecules also occur, while neoplastic cells express the appropriate adhesive receptors on their cellular membrane. Integrin α6β4, a molecule that normally supports cellular adhesion to the BM, is such a paradigm as, following the tumour-imposed ECM modifications, it changes its role and induces actin-mediated cell motility. In this way, integrin new distribution appears to correlate with considerable CRC aggressiveness[55]. Most importantly, neoplastic cells may also interfere in ECM metabolism through proteinase expression, of the matrix metalloproteinases (MMPs) in particular. These multi-functional degrading enzymes play a critical role in carcinogenesis but also in other stages of the invasion-metastasis cascade. CRC cells may induce the expression of MMP-2 and -9 by stromal cells, either directly or via a paracrine mechanism, thus modifying ECM and promoting tumour development[56-58].
Epithelial-mesenchymal transition (EMT) is a reversible morphogenetic biological process that involves the transition from stationary polarized epithelial cells to motile, multipolar or spindle-shaped mesenchymal cells. Epithelial cells are characterised by an atypical-basal polarity, the formation of tight junctions and the expression of intercellular adhesion molecules, such as E-cadherin. On the other hand, mesenchymal cells appear to be unable to build mature intercellular contacts, invade through the ECM and express molecules including fibronectin, vimentin, N-cadherin, Twist and Snail. While EMT occurs in embryogenesis and normal early development, accumulating evidence indicates that it also plays a crucial role in tumour development and metastasis; neoplastic cells usurp EMT to obtain properties that promote their detachment from their initial site and favour their migration to distant tissues[59,60].
The process of EMT is induced, promoted and regulated by multiple effectors that also regulate EMT during embryogenesis, including the cytokines interleukin 8 (IL-8), growth factors [epidermal growth factor (EGF), platelet-derived growth factor (PDGF), TGFβ] and ECM components. Interestingly, it has been shown that CRC cells are able to produce such molecules, including IL-1α, IL-1β and tumour necrosis factor alpha (TNF-α)[60-62]. Specifically, TGFβ plays a critical role through autocrine and/or paracrine mechanisms. This growth factor may be triggered by TNF-α, which is produced by tumour-infiltrating macrophages. The binding of TGFβ with TGFβR1 and/or TGFβR2 receptors triggers the phosphorylation of Smad2 and Smad3 dimers, which dissociate from the receptors to interact with Smad4; subsequently these dimers enter the nucleus to regulate EMT[63]. Additionally, the TGFβ-Smad pathway induces the high motility group A2 gene (HMGA2), which mediates EMT; HMGA2 is a nuclear factor that links TGFβ with the EMT-inducing transcription factors Twist and Snail1 and 2. TGFβ/Smad activities may also be associated with PDGF and PDGF receptor signalling, such as the phosphorylation of p68 RNA helicase to trigger nuclear translocation of β-catenin through a wingless Int (Wnt)-independent pathway[64-66].
In CRC, tumour cell EMT predominantly concerns cell adhesion mediated by E-cadherin. CRC cells present either mutations of the E-cadherin gene, proteolytic degradation of E-cadherin, insulin growth factor 1-promoted internalization of E-cadherin or disarrangement of E-cadherin and β-catenin connections[67,68]. Notably, intact cadherin-catenin complexes support the normal function of intercellular adhesion and guarantee stable “adherens junctions” and an unimpaired Wnt signalling pathway (the latter being a complex protein network controlling signal transduction)[69]. Neoplastic cells along with the E-cadherin deregulation present an accumulation of Src kinase and phospho-myosin associated with EMT[70,71], as well as high expression of guanine nucleotide exchange factor Tiam 1 that promotes metastatic potential through cellular adhesion reduction and resistance to anoikis[72]. Furthermore, CRC cells present a variable level of Ras homolog gene family, member C (RhoC), a protein that functions as a switch in signal transduction, promoting reorganisation of actin and regulating cell shape. It is noteworthy that increased expression of RhoC is associated with poor prognosis, as well as with an aberrant localization and expression of E-cadherin. The disruption of E-cadherin-mediated cell adhesion contributes to CRC cell detachment and motility. Moreover, its replacement with N-cadherin, a phenomenon named cadherin switch, further promotes cancer cell translocation and invasion of the surrounding stroma. In this way, EMT favours carcinogenesis and cellular transformation towards a metastatic phenotype[73].
Angiogenesis is defined as the formation of new vessels from preexisting ones[74]. Hypoxia or low oxygen tension is the primary triggering factor for the onset of this process. Early in carcinogenesis, tumour growth requires angiogenesis for the provision of oxygen and nutritional factors, while later new vessels provide CRC cells with a way to the systemic circulation (intravasation) and metastasis to distant sites. In normal conditions, the angiogenic process is accurately regulated by pro- and anti-angiogenic agents. In CRC, hypoxia induces the activation of hypoxia inducible factor-1 and subsequently the expression of angiogenic factors including VEGF, basic fibroblast growth factor and PDGF by CRC cells. Notably, prostaglandin E2 directly triggers CRC cells to express VEGF, appearing as a promising therapeutic target[75,76].
Moreover, stromal cells, such as cancer-associated fibroblasts (CAFs) and tumor-associated macrophages, mast cells, neutrophils and others, also produce proangiogenic factors inducing angiogenesis. The aforementioned factors interact with their respective receptors at the endothelial cell surface initiating signalling cascades involving p38, eNOS, as well as PI3 and ERK MAP kinases; the latter induce vasodilation, endothelial cell proliferation/migration and vessel assembly. Concurrently, angiogenesis suppressor proteins are downregulated, such as thrombospondin. The resulting vascular networks of tumours are chaotic and poorly functional due to abnormal endothelial cell structure, proliferation and apoptosis; additionally, abnormal pericytes appear to be loosely attached and do not fully cover the vessels. Consequently, neoplastic vasculature is leaky, haemorrhagic, with excessive branching, which results in oxygen depletion and extracellular acidosis[77,78].
It has been demonstrated that the microvascular density has a considerable prognostic value for tumours and may predict survival in patients with CRC[79]. To study this, a colour Doppler vascularity index (DVI) (ratio of coloured pixels/total pixels per tumour section) has been introduced, in an effort to predict distant metastasis and survival in CRC patients. It was concluded that patients who had a DVI > 15% at the primary site had worse overall survival compared to patients with DVI < 15%[80]. A similar parameter is the Doppler hepatic perfusion index (DHPI) (ratio of hepatic arterial flow/total liver flow), which appeared to be increased even in patients with occult micrometastases and is predictive of metastatic liver tumour recurrence[81]. The association between angiogenesis at the primary CRC site and in the hepatic metastases is further supported by imaging techniques, such as dynamic contrast-enhanced magnetic resonance imaging and contrast-enhanced computed tomography[82].
The lymphatic network is a major metastatic route and lymphatic metastases constitute an important prognostic indicator in solid tumours and CRC[83]. Excessive lymphangiogenesis is associated with metastasis in CRC. Lymphatic vessel density (LVD) has been introduced as a quantitative parameter for lymphangiogenesis. In vitro models demonstrated that VEGF-A and VEGF-C are the major regulators of this process and their levels correlate with LVD. In addition, PDGF-BB, FGF-2, hepatocyte growth factor (HGF), angiopoietin by binding to its receptor Tie2 and sphingosine-1-phosphate (S1P) cause lymphangiogenic effects. Neoplastic cells with high levels of sphingosine kinase 1 release S1P in the ECM, which in turn may lead to paracrine-induced angio- and lymphangiogenesis[84-86]. In vivo models suggested new pathways for lymphangiogenesis in CRC. The high levels of VEGF-C and VEGF-D appear to direct tumour lymphangiogenesis via the VEGF-C and -D pathway and their receptor VEGFR-3 (present on lymphatic endothelial cells), while VEGF-A may play a regulatory role during this process[85,87]. The activation of VEGFR-3 along with the β1 integrin subunit triggers multiple signalling pathways in the lymphatic endothelial cells, including Pyk2, NF-κB, ERK and JNK MAP kinases, which mediate proliferation and survival[88]. A recent study by Du et al[89] on human CRC samples indicated that metastasis associated protein 1 (MTA-1), which is expressed in various epithelial cancers and plays a critical role in metastasis, correlated with VEGF-C and mediated its expression. Hence, it was suggested that MTA-1 promotes lymphangiogenesis and therefore CRC metastasis.
Lymphangiogenesis may be initiated with the formation of lymphatic vessels by circulating endothelial progenitors (CEPs); the latter constitute a subpopulation of circulating endothelial cells derived from the bone marrow. It has been shown that CEPs are increased in the circulation in multiple pathologies, cancer included[27,90,91]. However, alternative pathways for the initiation and progress of lymphangiogenesis may also exist. The differentiation of other cells into lymphatic endothelial cells, such as macrophages and stromal cells, may be such pathways[92,93].
Interestingly, apart from invasion to lymph nodes, CRC LVD is also associated with liver metastasis. Although this association is rather unclear, a possible explanation may be that CRC cells express chemokine receptor CXCR5; its ligand BCA-1/CXCL13 is mutually expressed on lymphatic endothelial cells and the liver[94]. CRC cells may be directed through the lymphatic network to both lymph nodes and the liver. Moreover, lymphatic metastases are traced at the liver lymphatic drainage network, including portal, mediastinal and coeliac lymph nodes. These liver-associated lymphatic metastases may be generated from previous CRC liver metastases. All these findings show clearly the importance of angio- and lymphangiogenesis for carcinogenesis and metastasis and explain why they attract great research interest and constitute promising targets for anticancer therapy[27,95].
Malignant cell detachment from their primary tumour is the necessary step for their intravasation into existing or newly formed lymphatic or blood vessels. Certain molecular changes concerning the expression of degrading enzymes (such as the MMPs) and adhesion molecules (such as selectins, integrins and others) appear to contribute to CRC cell intravasation. Notably, the tortuous leaky neoplastic blood network, with its loose junctions among endothelial cells and the defective pericyte coverage, may substantially promote tumour cells entering into the lumina of vessels[78].
Sonoshita et al[96] studied murine and human tissue and showed that the transcriptional modulator enhancer of split (Aes) prevented CRC cells from intravasation through the impairment of trans-endothelial invasion that followed Notch-dependent mechanisms. Concurrently, urokinase plasminogen activator (uPA) expression is increased in patients with FAP during the transformation of normal to dysplastic epithelia[97,98]. The uPA system consists of uPA, the uPA receptor (uPAR), the tissue-type plasminogen activator (tPA), the plasminogen (Plg) and plasminogen activator inhibitors 1 and 2 (PAI-1 and PAI-2). This biological system is involved in multiple molecular pathways, including adhesion, chemotaxis, cytokine release, protease expression, neutrophil proliferation and activation for oxidant production. It modulates inflammation, growth, invasion, angiogenesis and metastasis of multiple tumour types[99,100]. When uPA connects with uPAR, it activates Plg along with other proteases such as MMP-2 and -9. These events promote ECM fragmentation and CRC cell detachment and intravasation.
Interestingly, when normal cells lose their contact with the ECM and their neighbouring cells, they undergo apoptosis that is triggered by broken integrin bonds. The ability of CRC cells to survive in the presence of fragmented ECM constitutes a crucial property of metastatic cells. This apoptosis resistance might be attributed to the overexpression of focal adhesion kinase by CRC cells, which contributes to conferring survival by activating certain molecular pathways, including ERK and AKT. Hence, tumour cells can by-pass integrin signalling and survive without contact with the ECM[101,102].
Following their detachment, access to the blood and lymphatic vessels is the next step for metastatic CRC cells. The microvessel diameter and their leaky structure are important factors that promote passive CRC cell entry into the circulation. Although lymphangiogenesis is less studied than angiogenesis, it appears that LECs, stimulated by VEGF-C, secrete mitogenic and/or chemotactic factors, which may attract tumour cells to adhere to, pass through and intravasate into the newly-built lymph vessels[90,103].
CRC cells are transported through the hepatic-portal circulatory system to reach the liver. The inferior and superior mesenteric veins and the portal vein, along with their neighbouring lymphatic vessels, constitute the dominant metastatic routes for hepatic metastases. In cancer patients, a large number of circulating tumour cells may be detected, although the intravasated cellular populations do not correlate with the density and the extension of metastases. Accumulating experimental evidence has suggested that entrance into the systemic circulation is lethal for the vast majority of tumour cells; only 0.01% of metastatic cancer cells may trigger the formation of metastasis when injected into the systemic circulation[27]. In vitro studies using videomicroscopy to monitor intravasated cancer cells have indicated that most of the cells undergo apoptosis during their passage through the vascular wall or soon after their entrance into the circulation. It is generally accepted that two major mechanisms contribute to tumour cell death following their intravasation: mechanical stress and the immune system[5,104].
Haematogenous dissemination exposes tumour cells to the strong mechanical forces generated by blood flow. In particular, when they circulate within a narrow capillary network or the microvasculature of contractile organs, such as the heart and the skeletal muscles, they are forced to transform their shape from spherical to cylindrical, which is lethal to the majority of tumour cells[5]. However, as tumour cells get progressively more invasive and metastatic through a series of mutations, they display larger cell deformations and shape alterations. In vivo studies have demonstrated that metastatic cells are quite deformable and both the cell nucleus and cytoplasm may undergo strong compression and shape deformation in small capillaries. Specifically, the length of the cell nucleus may increase 1.6-fold and the cytoplasmic major axis up to 3.9-fold in comparison with the same cells in larger microvessels[105-107]. Also, tumour cells reaching capillary bifurcation points can stretch and extend their cytoplasms in both directions[105,106]. Mechanical forces, such as endothelial contraction, shear or pulsatile stresses, cause the production of oxygen radicals including NO and ROS, which generate apoptosis of CRC cells.
Metastatic tumour cells have developed multiple molecular pathways in order to survive the mechanical forces in the circulation. CRC cells interact with platelets and/or themselves to form large emboli that protect them from shear stress[108]. Cancer cells may activate adhesion pathways involving integrins, leading to their attachment to endothelial cells and thus evasion of anoikis. Programmed cell death may also implicate metabolic pathways including pentose phosphate and control of glucose uptake[109]. Moreover, the tyrosine kinase TrkB was demonstrated to suppress anoikis and appeared to be crucial for metastatic progression in CRC cells[110].
The second major mechanism responsible for metastatic cell elimination in the circulation is immunosurveillance. Numerous cytokines released by cancer cells, endothelial cells or immune cells, such as IL-2, -12 and -18, may activate various subsets of immune cells including T-lymphocytes and natural killer (NK) cells. The latter eliminate cancer cells through Trail and/or NKG2/perforin pathways. Similar to mechanical forces adaptations, metastatic cells have developed mechanisms to evade immunosurveillance. It has been shown that cancer patients present high levels of acute phase glycoproteins in their blood, correlated with the disease. These proteins may protect malignant cells against anoikis and immune cell attacks[111]. Furthermore, tumour cells interact with platelets forming large emboli, a process mediated by the expression of tissue factor and L- and P-selectins. In this way, they shield themselves against both immune detection and shear forces[112]. Undoubtedly, immune system and cancer cell interactions attract great research interest and the manipulation of immunesurveillance is a major strategy in anticancer treatment.
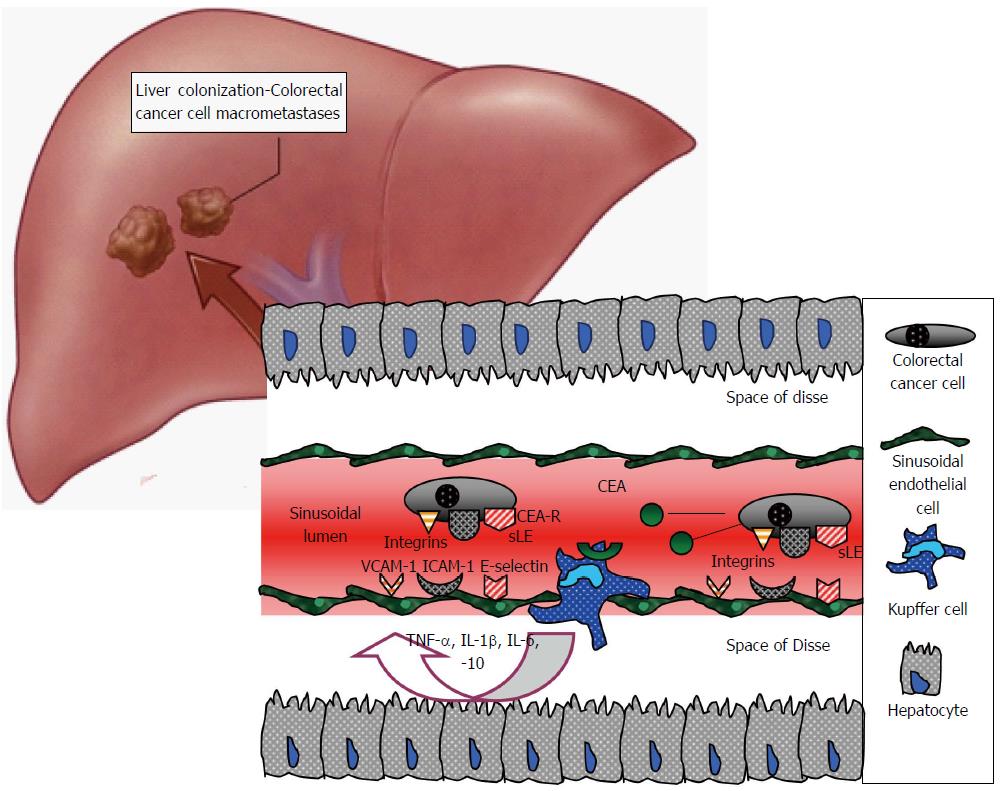
The sinusoids constitute the specific hepatic capillary network where four different cell populations reside within the sinusoidal lumen or in proximity to the sinusoidal wall: sinusoidal endothelial cells (SECs), Kupffer cells (KCs), hepatic stellate cells (HSCs) and pit cells. Each of these cell types plays a crucial role in hepatic homeostasis and CRC metastasis[113].
The progression of CRC hepatic metastasis is divided into four interrelated phases: (1) microvascular phase of liver-infiltrating malignant cells; (2) interlobular micrometastasis phase; (3) angiogenic micrometastasis phase; and (4) established hepatic metastasis phase. The first phase mainly occurs within the sinusoids, whereas the following ones describe further metastatic steps that affect the inner hepatic parenchyma. Although sinusoidal cells contribute to all four, they predominantly mediate the first phase[114].
During the microvascular phase, resident cells generate multiple tumouricidal and tumourigenic effects, which may either promote the invading cell elimination or liver colonisation. A plethora of molecular pathways are involved, including NO and reactive oxygen release by SECs, expression of adhesion molecules, such as selectins, integrins and others by the same cells, phagocytosis, cytokine and growth factor release by KCs and HSCs, release of cytotoxic agents from pit cells. The aforementioned examples demonstrate the complexity and the importance of the intercellular reactions that occur within the sinusoids in colorectal liver metastasis[114-116].
SECs were first described by Wisse at the beginning of 1970s. Their cytoplasm includes characteristic canals, arranged in sieve plates named fenestrae. Fenestration constitutes a unique marker and distinguishes SECs from all other endothelial and liver cells (Figure 4)[117,118]. They form a major scavenger cell system and accomplish receptor-mediated endocytosis and pinocytosis through three major types of endocytic receptors: the liver sinusoidal cell mannose receptor, the liver sinusoidal cell scavenger receptor and the liver sinusoidal cell receptor IIb2, which is an Fc-γ receptor[114]. The scavenging properties of SECs are critical in CRC metastasis. In vivo murine experiments indicated that autotaxin, a phosphodiesterase that promotes metastasis and angiogenesis, was rapidly removed from the blood circulation and degraded by SECs[119]. This study further supported previous data which demonstrated that hepatectomy or chemically-induced injury resulted in an increase of serum autotoxin[120].
Although SECs appear to play a tumouricidal role, they may also aid tumour cells to arrest and metastasise within the liver. Under cytokine activation, SECs may express adhesion molecules, such as E-selectin, which mediate cancer cell attachment to the endothelium, thus facilitating their extravasation into the hepatic parenchyma[121,122].
Experimental studies have attempted to investigate and exploit SEC properties in anticancer therapy. Gene therapy uses certain vectors, such as adenoviruses, to reach target cells and is considered a new and promising treatment of acquired diseases, including liver neoplasms and metastases. SECs, in conjunction with KCs, may endocytose and destroy these vectors, cancelling any therapy. On the other hand, as hepatocytes constitute the primary target cells for anti-cancer therapy, gene vectors should reach the space of Disse, in order to interrelate with hepatocytes. The space of Disse may be accessible only through SEC fenestration, and the diameter of the latter ranges according to species and liver condition; ageing or liver diseases substantially alter fenestrae, impairing the vector access to the space of Disse[123].
These constitute the biggest (more than 80%) tissue macrophage population in the body of vertebrates and approximately 15% of all liver cells[124,125]. KCs mainly act as scavengers around the sinusoids, but also as antigen-presenting cells and liver regeneration mediators. When activated, KCs produce a wide variety of molecules, such as growth control mediators, inflammatory agents, proteolytic and hydrolytic enzymes, oxygen and nitrogen species and lipid metabolites. All these products modulate acute and chronic liver responses to pathogens, chemicals, drugs, injury, as well as cancer and metastasis (Table 1)[126-128].
| Group | Molecules |
| Cytokines and chemokines | Tumour necrosis factor alpha |
| Transforming growth factor beta | |
| Interleukins (IL-1α, -1β, -6, -8, -10, -12 and -18) | |
| Interferon gamma | |
| Platelet-activating factor | |
| Monocyte chemotactic protein-1 | |
| Macrophage inflammatory protein (MIP-1) | |
| Hydrolytic and | Urokinase-type plasminogen activator |
| proteolytic enzymes | Metalloproteinases (MMP-1, -7 and -13) |
| Lipid metabolites | Prostaglandin E2 |
| (prostanoids) | Thromboxane |
| Oxygen species (superoxide) and hydrogen peroxide | |
| Nitrogen species (nitric oxide) | |
KCs play a crucial role in the “host tumoural surveillance system”. As they constantly reside around the sinusoids, they discriminate and remove neoplastic cells that reach the liver. Interestingly, CRC cells become vulnerable to macrophage tumouricidal activity during endothelial adhesion and extravasation[129,130]. Specifically, KCs may recruit inflammatory cells, they may arrest CRC cells and inhibit their growth acting in a cytostatic way, and they may bind and eliminate them in a cytotoxic way[130]; the latter occurs by several mechanisms: phagocytic release of TNF, secretion of proteases and production of oxygen metabolites[131-133].
Rat experiments demonstrated that in the early stages of CRC liver metastasis, KCs exerted tumouricidal activity in conjunction with NK cells. One hour after CRC cells had reached the liver, more than 70% were already in contact with KCs; the KC population was considerably increased from the first day and phagocytosed more than 90% of malignant cells. However, NK depletion left 35% of the cancer free from KC interactions. The responsible mechanism may be that activated NK cells secrete pro-inflammatory cytokines, such as granulocyte macrophage colony stimulating factor (GM-CSF) and interferon gamma (IFN-γ), which in turn activate KCs or sensitise tumour cells to cytotoxic effects. Alternatively, NKs may induce CRC cell apoptosis, causing exposure of phosphatidylserine and enhancing phagocytosis by KCs[115]. The opposite interaction was also reported: activated KCs may produce IL-12 and/or IL-18, which enhance IFN-γ release by pit cells that exhibit high tumouricidal activity, thus inhibiting CRC haematogenous metastasis in murine livers[134,135]. It appears that in the metastatic process, KCs and pit cells act in close cooperation against the invading cancer cells. Both produce cytokines and interact stimulating one another, eliminating cancer cells directly or mediating cancer cell death by their counterparts.
High mobility group box 1 (HMGB1) is a protein produced by normal and cancer cells which triggers apoptosis in macrophages and monocytes. Experimental data from Dukes’ C and D surgical specimens showed that HGMB1 levels in the portal blood were higher for Dukes’ D and correlated with the levels in the primary tumours. When HMGB1 concentration was raised, KC numbers were substantially decreased. Moreover, in vitro murine experiments performed in the same study showed that the administration of the protein decreased KC populations and promoted liver metastases[136]. These findings support the tumouricidal role of KCs in the metastatic process.
The interaction between KCs and invading tumour cells is not always in favour of liver homeostasis. Binding to KCs facilitates CRC cell arrest in the liver; if the killing process is not accomplished promptly or is partially completed, then the binding process substantially contributes to liver colonisation. Experimental data in rats advocated that KCs exert a limited capacity for tumour surveillance; when malignant cells reach the liver in very high numbers or present great antigenic diversity, KCs are eventually saturated and metastasis progresses[130].
Additionally, KCs produce growth factors, such as HGF, which facilitate tumour cell proliferation[137]. Furthermore, the ability of KCs to secrete MMPs, especially MMP-9 and MMP-14, as well as their inhibitors, may enhance angiogenesis and tumour invasion, via alterations of the ECM[138]. Notably, MMP-9-deficient mice presented considerably fewer liver metastatic lesions when CRC cells were injected in their spleen. MMP-9 was primarily derived from KCs, independently of its expression by tumour cells[139]. Moreover, in vitro experiments have indicated that highly malignant cells reduce, in their favour, the phagocytic capacity of KCs and promote colonisation[140]. The preceding experimental evidence suggests that KCs prevent tumour outgrowth and liver metastasis when the burden and the rate of invading cells are relatively low. However, KCs may contribute to liver colonisation if their tumouricidal ability is saturated due to excessive numbers of invading cells or when metastases are already established.
KCs present an 80 kDa carcinoembryonic antigen (CEA) receptor, which mediates binding and subsequent degradation of CEA[141,142]. When CEA is complexed, KCs are activated and secrete through β-2 adrenergic pathways large amounts of cytokines, including TNF-α, IL-1β, IL-6 and IL-10[141,143]. These pro- and anti-inflammatory agents generate alterations in the sinusoidal endothelium and SECs express adhesion molecules of the selectin family, which promote the arrest and extravasation of tumour cells[144]. Murine studies indicated successful adhesion of CRC cells to KCs and SECs, without the immediate mediation of CEA, excluding CEA’s function as an adhesion molecule in hepatic colonisation[145]. The last observation was further investigated and it was revealed that CEA supports CRC cell survival via the induction of IL-10 and subsequent decrease of NO concentration. IL-10 is probably released by stimulated KCs and NO levels decrease due to inhibition of inducible nitric oxide synthetase[146].
Accumulating data have demonstrated the important role of immunoglobulin superfamily adhesion molecules in colorectal liver metastases, referring to KCs. Murine experiments demonstrated that CRC cells trigger KCs to produce pro-inflammatory cytokines, which in turn stimulate SECs to express high levels of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1)[147] or VCAM-1 and platelet endothelial cell adhesion molecule 1[148]. These molecules mediate cancer cell adhesion, follow E-selectin expression and promote subsequent extravasation. Similarly, in vitro experiments using CEA-activated murine KCs indicated that cytokines were released, which activated endothelial cells to express ICAM-1 and VCAM-1[122,149].
Consequently, several lines of evidence support the hypothesis that when CRC cells reach the hepatic sinusoids, they secrete CEA that may activate KCs to produce cytokines; the latter stimulate SECs to express cell adhesion molecules that bind colorectal metastasising cells. This mechanism obviously contributes to malignant cell arrest, proliferation and invasion (Figure 5).
The cytostatic and cytotoxic actions of KCs were taken into consideration in liver metastasis research and adjuvant strategies were created in order to maintain and increase their potential antitumour role. As transient peri- and post-operative immunosuppression has been observed in patients undergoing major surgical interventions, further enhancement of KC activities is particularly useful during and after hepatectomy[150]. GM-CSF was tested for this purpose and experimental data from human liver wedge biopsies showed that it could stimulate KCs to exert increased cytotoxicity against a SW948 colon carcinoma cell line[151]. In syngenic rat models, the administration of this factor increased KC numbers and significantly enhanced their cytotoxicity ex vivo, cancelling the development of small metastatic foci[152]. IFN-γ was also tested targeting KC activity; in syngenic murine models, the preoperative administration of IFN-γ caused high anti-metastatic function of KCs and NKs in early liver metastasis[153]. These promising results remain to be further analysed and repeated in bigger studies using human hepatic tissue.
HSCs are located in the space of Disse, comprising about 15% of the nonparenchymal hepatic cells. They have a unique morphology, due to long cytoplasmic processes that form a spindle-shaped cellular body (Figure 6)[154]. KCs produce chemokines that induce mono- and polymorphonuclear leukocyte infiltration, activate neutrophils and control lymphocyte populations exerting immuno-regulatory activity[155,156]. Furthermore, HSCs act as antigen-presenting cells that may activate T lymphocytes[157,158] and release pro-inflammatory cytokines when exposed to bacterial endotoxins through toll-like receptors[158,159].
HSCs secrete and respond to a wide variety of cytokines. They modify the activity of various growth factors, express adhesion molecules, such as ICAM-1 and VCAM-1, and regulate the detoxification of ethanol and xenobiotics[156,160]. Activated HSCs display contractility, chemotaxis, fibrogenesis, matrix degradation activity, proliferation, and retinoid loss. They mediate inflammation, cell survival and apoptosis, liver regeneration and monitoring of cellular pH[161-163].
HSCs are also key cells in tumour growth and CRC metastatic processes. Experimental studies in rats revealed that conditioned media from cultures of hepatocellular carcinoma hepatocytes could activate HSCs[164]. Moreover, in vitro experiments with metastatic melanoma cells concluded that tumour cells triggered HSC activation; the latter promoted angiogenesis through VEGF expression[165]. Injection of colon carcinoma cells in nude mice favoured the formation of hepatic metastatic foci through HGF and TGF-β1 produced by HSCs. Similarly, tumour cells secreted PDGF-AB and enhanced HSC proliferation and locomotion[166].
Co-cultures of SECs and HSCs caused spontaneous cellular differentiation, with HSCs forming the core and SECs the surface of the cell population. Concurrently, activated HSCs cultured with SECs expressed functional smooth muscle cell phenotype and formed capillary-like structures in angiogenesis assays. Taking into consideration that tumours activate HSCs, their contribution to neoangiogenesis through interactions with SECs was implicated in these studies[114,167].
The name pit cell is related to their cytoplasmic granules. The cells contain granules with lysosomal enzymes, perforin and various phosphatases, but their structural characteristic is the presence of cytoplasmic rod vesicles. Their shape varies, due to the presence of pseudopodia[113,168,169].
Pit cells substantially contribute to hepatic immunity and exert strong antitumour action. In collaboration with KCs, they represent the first line of liver defence against cancer cells. Experimental studies in rodents demonstrated that pit cells are highly cytotoxic against multiple malignant cell lines, including murine fibrosarcoma L929, colon carcinoma CC531 and colorectal carcinoma DHD-K12 rat cells[169].
Pit cells exert their cytotoxicity through binding to target cells, a process named conjugation. Various adhesion molecules on pit cells mediate this process, such as CD2, a member of the immunoglobulin superfamily, CD28 and lymphocyte function-associated antigen 1, while CD54 and CD58 may be present on target cells[170,171]. In addition, interactions between β2 integrins and ICAMs are crucial, supporting these cell-cell conjugations[169,170,172].
Following conjugation, various receptors may be stimulated, triggering or inhibiting pit cell cytotoxicity. Three superfamilies of NK cell receptors are presented primarily on human pit cells, while others, named co-receptors, still remain under investigation: the killer immunoglobulin receptor that recognizes major histocompatibility complex (MHC) class I molecules, the c-type lectin, recognising non classical MHC class I or class I-like molecules, and the natural cytotoxicity receptor superfamily, which remains to be further studied[173].
Pit cell tumouricidal actions are exerted mainly through three mechanisms[168,174], as follows:
Perforin-granzyme pathway: Through calcium-dependent molecular reactions pit cells adhere to tumour cells and release perforin and proteases into the intercellular space. Subsequently, perforin induces pores in the tumour cytoplasmic membrane and then proteases may generate DNA segmentation.
Apoptosis pathway: Pit cells express both the FasL and the tumour necrosis factor-related apoptosis-inducing ligand. When pit cells interact with tumour cells, these ligands bind to respective receptors and cancer cells undergo apoptosis.
Cytokine pathway: Pit cells secrete cytokines, such as IFN-γ, and thus activate lymphocytes and macrophages against invading cancer cells.
The adherence of metastasising CRC cells to SECs is a primary step of great importance toward liver invasion and colonisation[140]. Malignant cells bind to SECs initially through selectins. However, these bonds do not appear to be strong enough to guarantee stable cell adhesion. Integrins are necessary to stabilise tumour cell-SEC adhesion. If integrins do not conform, then the bonds are broken and cancer cells are released into the blood or undergo mechanical damage[175]. The development of strong intercellular bonds allows CRC cells to resist the attractive forces of plasma flow and circulating blood cells, when they adhere to the hepatic sinusoids[176]. Multiple signalling molecules, including focal adhesion kinase, paxillin, and cytoskeletal proteins, are probably required for tumour cell adhesion and stabilisation under the hydrodynamic conditions of blood flow[175].
Shimizu et al[177], studying CRC liver metastases in murine models, reported that shortly after endothelial adhesion occurred within the sinusoids, metastasising cells extended cytoplasmic projections towards the space of Disse, through the pores of SECs. Forty-eight hours after their injection in mice, CRC cells may reach the hepatocytes and enter their cytoplasm, and 72 h later, they developed metastatic foci.
From the moment of extravasation, cytotoxic T cells, monocytes and macrophages which occupy extra-sinusoidal hepatic tissue are activated against the metastatic cells, though not always successfully[178]. Ultimately, few CRC cells cause micrometastases in the hepatic parenchyma. They remain in a dormant state, the duration of which is unknown. It is probable that these micrometastases will be reactivated after an unspecified time period and will create macrometastases. The last stage of the invasion-metastasis cascade is then accomplished[179,180].
It has been proposed that carcinoma cells address the problem of an incompatible microenvironment at the distant metastatic site through the establishment of a “premetastatic niche”[181]. In that state, CRC cells may release soluble CD44 that mediates resistance to apoptosis[182]. The crosstalk among cancer cells, immune cells, endothelial and stromal cells causes the production of various chemokines and growth factors, such as the EGF and TGF-α, which promote metastatic cell growth in the liver. Interestingly, in order to survive and develop a secondary neoplasm in the liver, CRC cells must undergo the reverse transition from EMT, which is termed mesenchymal-epithelial transition (MET). Consequently, CRC cells express epithelial cell markers such as E-cadherin[183,184]. Additionally, Belluco et al[185] showed that CRC cell kinase profile at the hepatic metastatic sites differs considerably from the initial intestinal site. These data indicate that CRC cells adjust their signalling network at the hepatic microenvironment in order to survive and generate metastatic foci. The activation of MET is probably triggered by the liver ECM. Hepatic CAFs appear to promote this process through overexpression of COX2 and TGF-β2.
The transition of metastatic microcolonies to macroscopic metastases may occur after weeks or months or they may persist as microcolonies in a state of long-term dormancy. However, similarly to the initial CRC tumours, this transition also needs additional vascular supply through neoangiogenesis. VEGF-A is the major growth factor that regulates neovascularisation and VEGFR1 is the main receptor.
The invasion-metastatic process from the initial site at the large intestine to the liver is a long process, where multiple molecular pathways and numerous cell types are involved. Recent research has elucidated various aspects of this process, such as the role of EMT and stromal microenvironment cellular crosstalk, the importance of adhesion molecules (Table 2), the significance of proteases, as well as the role of VEGF members in angio- and lymphangiogenesis.
| Adhesion molecule family | Adhesion molecule | Expression |
| Cadherins | E-cadherin | Colon epithelial cells |
| P-cadherin | Colon epithelial cells | |
| Lectins | sLEx | Colon epithelial cells |
| sLEa (CA 19-9) | ||
| Galectin-3 | ||
| Selectins | E-Selectin | ECs |
| L-Selectin | Leukocytes | |
| P-Selectin | ECs and platelets | |
| Integrins | LFA-1 (αLβ2) | Colon epithelial cells, ECs, fibroblasts, leukocytes and platelets |
| VLA-4 (α4β1) | ||
| Immunoglobulin superfamily CAMs | ICAMs | ECs, fibroblasts and leukocytes |
| PECAM-1 | ECs, platelets and leukocytes | |
| VCAM-1 | ECs and epithelial cells | |
| MadCAM | Colon epithelial cells | |
| CEA | Colon epithelial cells | |
| Proteoglycan receptors | CD44 | Colon cells and ECs |
Furthermore, technological advances have revolutionised the study of metastasis, since imaging techniques have provided real-time visualisation of metastatic cells in the ECM and the circulation[186]. Also, new genetic, molecular and biochemical techniques may permit the investigation of tumour cell heterogeneity at the initial and their metastatic sites and may explain its functional significance[187].
The progress in basic research concerning CRC hepatic metastasis over the last decade has been accompanied by the rapid translation of experimental data to oncological treatment. An anti-VEGF monoclonal antibody, named bevacizumab, has been introduced in CRC therapy and has contributed to prolonged patient survival[188]. Concurrently, clinical trials are in progress for antiCEA antibodies[189], MMP inhibitors[190], antibodies against integrins[191] or molecules that increase immunosurveillance[192,193]. Although comprehension of CRC hepatic metastasis has substantially evolved, the invasion-metastasis cascade remains partially understood and future basic and clinical research still has multiple issues to clarify.
P- Reviewers: Arshad A, He H, Langner C S- Editor: Gou SX L- Editor: Logan S E- Editor: Ma S
| 1. | Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2388] [Cited by in RCA: 2954] [Article Influence: 211.0] [Reference Citation Analysis (0)] |
| 2. | Ghobrial IM. Myeloma as a model for the process of metastasis: implications for therapy. Blood. 2012;120:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 3. | Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 2009;28:113-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 774] [Cited by in RCA: 682] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 4. | Weinberg R. The biology of cancer. 1st ed. Taylor & Francis Group: Garland Science 2007; . |
| 5. | Rejniak KA. Investigating dynamical deformations of tumor cells in circulation: predictions from a theoretical model. Front Oncol. 2012;2:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Chong HC, Tan CK, Huang RL, Tan NS. Matricellular proteins: a sticky affair with cancers. J Oncol. 2012;2012:351089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Miranda E, Destro A, Malesci A, Balladore E, Bianchi P, Baryshnikova E, Franchi G, Morenghi E, Laghi L, Gennari L. Genetic and epigenetic changes in primary metastatic and nonmetastatic colorectal cancer. Br J Cancer. 2006;95:1101-1107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Nakao K, Shibusawa M, Ishihara A, Yoshizawa H, Tsunoda A, Kusano M, Kurose A, Makita T, Sasaki K. Genetic changes in colorectal carcinoma tumors with liver metastases analyzed by comparative genomic hybridization and DNA ploidy. Cancer. 2001;91:721-726. [PubMed] |
| 10. | Aragane H, Sakakura C, Nakanishi M, Yasuoka R, Fujita Y, Taniguchi H, Hagiwara A, Yamaguchi T, Abe T, Inazawa J. Chromosomal aberrations in colorectal cancers and liver metastases analyzed by comparative genomic hybridization. Int J Cancer. 2001;94:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Athanassiadou P, Grapsa D. Recent advances in the detection of bone marrow micrometastases: A promising area for research or just another false hope A review of the literature. Cancer Metastasis Rev. 2006;25:507-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Pelkey TJ, Frierson HF, Bruns DE. Molecular and immunological detection of circulating tumor cells and micrometastases from solid tumors. Clin Chem. 1996;42:1369-1381. [PubMed] |
| 13. | Garborg K, Holme Ø, Løberg M, Kalager M, Adami HO, Bretthauer M. Current status of screening for colorectal cancer. Ann Oncol. 2013;24:1963-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Slesser AA, Georgiou P, Brown G, Mudan S, Goldin R, Tekkis P. The tumour biology of synchronous and metachronous colorectal liver metastases: a systematic review. Clin Exp Metastasis. 2013;30:457-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 15. | Ballinger AB, Anggiansah C. Colorectal cancer. BMJ. 2007;335:715-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Jänne PA, Mayer RJ. Chemoprevention of colorectal cancer. N Engl J Med. 2000;342:1960-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 312] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 17. | Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, Kang GH, Widschwendter M, Weener D, Buchanan D. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1423] [Cited by in RCA: 1494] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 18. | Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology. 2010;138:2059-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 604] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 19. | Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5:19-27. [PubMed] |
| 20. | Droujinine IA, Eckert MA, Zhao W. To grab the stroma by the horns: from biology to cancer therapy with mesenchymal stem cells. Oncotarget. 2013;4:651-664. [PubMed] |
| 21. | Yoo J, Perez CE, Nie W, Sinnett-Smith J, Rozengurt E. TNF-alpha and LPA promote synergistic expression of COX-2 in human colonic myofibroblasts: role of LPA-mediated transactivation of upregulated EGFR. BMC Gastroenterol. 2013;13:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Peddareddigari VG, Wang D, Dubois RN. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron. 2010;3:149-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (2)] |
| 23. | Quante M, Varga J, Wang TC, Greten FR. The gastrointestinal tumor microenvironment. Gastroenterology. 2013;145:63-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 24. | Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR, Wright NA. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492-8495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 418] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 25. | Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331-4339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 724] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 26. | Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol. 2010;21:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 277] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 27. | Gout S, Huot J. Role of cancer microenvironment in metastasis: focus on colon cancer. Cancer Microenviron. 2008;1:69-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Zhu Y, Yin WL, Ba YF, Tian L, Gu ZQ, Zhang MS, Zhong CN. Transforming growth factor-1 promotes the transcriptional activation of plasminogen activator inhibitor type 1 in carcinoma-associated fibroblasts. Mol Med Rep. 2012;6:1001-1005. [PubMed] |
| 29. | Dass K, Ahmad A, Azmi AS, Sarkar SH, Sarkar FH. Evolving role of uPA/uPAR system in human cancers. Cancer Treat Rev. 2008;34:122-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 316] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 30. | Dimova EY, Kietzmann T. Metabolic, hormonal and environmental regulation of plasminogen activator inhibitor-1 (PAI-1) expression: lessons from the liver. Thromb Haemost. 2008;100:992-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Berdiel-Acer M, Bohem ME, López-Doriga A, Vidal A, Salazar R, Martínez-Iniesta M, Santos C, Sanjuan X, Villanueva A, Molleví DG. Hepatic carcinoma-associated fibroblasts promote an adaptative response in colorectal cancer cells that inhibit proliferation and apoptosis: nonresistant cells die by nonapoptotic cell death. Neoplasia. 2011;13:931-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218:1402-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 451] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 33. | Kruse J, von Bernstorff W, Evert K, Albers N, Hadlich S, Hagemann S, Günther C, van Rooijen N, Heidecke CD, Partecke LI. Macrophages promote tumour growth and liver metastasis in an orthotopic syngeneic mouse model of colon cancer. Int J Colorectal Dis. 2013;28:1337-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 677] [Cited by in RCA: 803] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 35. | Rudmik LR, Magliocco AM. Molecular mechanisms of hepatic metastasis in colorectal cancer. J Surg Oncol. 2005;92:347-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Pavlidis ET, Pavlidis TE. Role of bevacizumab in colorectal cancer growth and its adverse effects: a review. World J Gastroenterol. 2013;19:5051-5060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (4)] |
| 37. | Martinelli E, Troiani T, Morgillo F, Orditura M, De Vita F, Belli G, Ciardiello F. Emerging VEGF-receptor inhibitors for colorectal cancer. Expert Opin Emerg Drugs. 2013;18:25-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Popovic ZV, Sandhoff R, Sijmonsma TP, Kaden S, Jennemann R, Kiss E, Tone E, Autschbach F, Platt N, Malle E. Sulfated glycosphingolipid as mediator of phagocytosis: SM4s enhances apoptotic cell clearance and modulates macrophage activity. J Immunol. 2007;179:6770-6782. [PubMed] |
| 39. | Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654-2666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1553] [Cited by in RCA: 1627] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 40. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4318] [Cited by in RCA: 4902] [Article Influence: 258.0] [Reference Citation Analysis (0)] |
| 41. | Correale P, Rotundo MS, Botta C, Del Vecchio MT, Tassone P, Tagliaferri P. Tumor infiltration by chemokine receptor 7 (CCR7)(+) T-lymphocytes is a favorable prognostic factor in metastatic colorectal cancer. Oncoimmunology. 2012;1:531-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | de Miranda NF, Goudkade D, Jordanova ES, Tops CM, Hes FJ, Vasen HF, van Wezel T, Morreau H. Infiltration of Lynch colorectal cancers by activated immune cells associates with early staging of the primary tumor and absence of lymph node metastases. Clin Cancer Res. 2012;18:1237-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Liu K. Role of apoptosis resistance in immune evasion and metastasis of colorectal cancer. World J Gastrointest Oncol. 2010;2:399-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | O’Connell J, O’Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075-1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 641] [Cited by in RCA: 645] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 45. | Le QT, Shi G, Cao H, Nelson DW, Wang Y, Chen EY, Zhao S, Kong C, Richardson D, O’Byrne KJ. Galectin-1: a link between tumor hypoxia and tumor immune privilege. J Clin Oncol. 2005;23:8932-8941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 46. | Lu B, Finn OJ. T-cell death and cancer immune tolerance. Cell Death Differ. 2008;15:70-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 47. | Shurin MR, Shurin GV, Lokshin A, Yurkovetsky ZR, Gutkin DW, Chatta G, Zhong H, Han B, Ferris RL. Intratumoral cytokines/chemokines/growth factors and tumor infiltrating dendritic cells: friends or enemies. Cancer Metastasis Rev. 2006;25:333-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Guven Maiorov E, Keskin O, Gursoy A, Nussinov R. The structural network of inflammation and cancer: merits and challenges. Semin Cancer Biol. 2013;23:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 49. | Beaty BT, Sharma VP, Bravo-Cordero JJ, Simpson MA, Eddy RJ, Koleske AJ, Condeelis J. β1 integrin regulates Arg to promote invadopodial maturation and matrix degradation. Mol Biol Cell. 2013;24:1661-1675, S1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 50. | Pöschl E, Schlötzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 577] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 51. | Tanner K. Regulation of the basement membrane by epithelia generated forces. Phys Biol. 2012;9:065003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Peranzoni E, Rivas-Caicedo A, Bougherara H, Salmon H, Donnadieu E. Positive and negative influence of the matrix architecture on antitumor immune surveillance. Cell Mol Life Sci. 2013;70:4431-4448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 53. | Zhang YY, Chen B, Ding YQ. Metastasis-associated factors facilitating the progression of colorectal cancer. Asian Pac J Cancer Prev. 2012;13:2437-2444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Zapatka M, Zboralski D, Radacz Y, Böckmann M, Arnold C, Schöneck A, Hoppe S, Tannapfel A, Schmiegel W, Simon-Assmann P. Basement membrane component laminin-5 is a target of the tumor suppressor Smad4. Oncogene. 2007;26:1417-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | Mizutani K, Kawano S, Minami A, Waseda M, Ikeda W, Takai Y. Interaction of nectin-like molecule 2 with integrin alpha6beta4 and inhibition of disassembly of integrin alpha6beta4 from hemidesmosomes. J Biol Chem. 2011;286:36667-36676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Müller-Edenborn B, Roth-Zʼgraggen B, Bartnicka K, Borgeat A, Hoos A, Borsig L, Beck-Schimmer B. Volatile anesthetics reduce invasion of colorectal cancer cells through down-regulation of matrix metalloproteinase-9. Anesthesiology. 2012;117:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 57. | Kryczka J, Stasiak M, Dziki L, Mik M, Dziki A, Cierniewski C. Matrix metalloproteinase-2 cleavage of the β1 integrin ectodomain facilitates colon cancer cell motility. J Biol Chem. 2012;287:36556-36566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 58. | Stenzinger A, Wittschieber D, von Winterfeld M, Goeppert B, Kamphues C, Weichert W, Dietel M, Rabien A, Klauschen F. High extracellular matrix metalloproteinase inducer/CD147 expression is strongly and independently associated with poor prognosis in colorectal cancer. Hum Pathol. 2012;43:1471-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 59. | Todosi AM, Gavrilescu MM, Aniţei GM, Filip B, Scripcariu V. Colon cancer at the molecular level--usefulness of epithelial-mesenchymal transition analysis. Rev Med Chir Soc Med Nat Iasi. 2012;116:1106-1111. [PubMed] |
| 60. | Zhu QC, Gao RY, Wu W, Qin HL. Epithelial-mesenchymal transition and its role in the pathogenesis of colorectal cancer. Asian Pac J Cancer Prev. 2013;14:2689-2698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 61. | Narita T, Kawakami-Kimura N, Kasai Y, Hosono J, Nakashio T, Matsuura N, Sato M, Kannagi R. Induction of E-selectin expression on vascular endothelium by digestive system cancer cells. J Gastroenterol. 1996;31:299-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 62. | Matsuo Y, Sawai H, Ma J, Xu D, Ochi N, Yasuda A, Takahashi H, Funahashi H, Takeyama H. IL-1alpha secreted by colon cancer cells enhances angiogenesis: the relationship between IL-1alpha release and tumor cells’ potential for liver metastasis. J Surg Oncol. 2009;99:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Borthwick LA, Gardner A, De Soyza A, Mann DA, Fisher AJ. Transforming Growth Factor-β1 (TGF-β1) Driven Epithelial to Mesenchymal Transition (EMT) is Accentuated by Tumour Necrosis Factor α (TNFα) via Crosstalk Between the SMAD and NF-κB Pathways. Cancer Microenviron. 2012;5:45-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 64. | Fuxe J, Vincent T, Garcia de Herreros A. Transcriptional crosstalk between TGF-β and stem cell pathways in tumor cell invasion: role of EMT promoting Smad complexes. Cell Cycle. 2010;9:2363-2374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 65. | Katsuno Y, Lamouille S, Derynck R. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 657] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 66. | Thuault S, Tan EJ, Peinado H, Cano A, Heldin CH, Moustakas A. HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition. J Biol Chem. 2008;283:33437-33446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 295] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 67. | Xiong H, Hong J, Du W, Lin YW, Ren LL, Wang YC, Su WY, Wang JL, Cui Y, Wang ZH. Roles of STAT3 and ZEB1 proteins in E-cadherin down-regulation and human colorectal cancer epithelial-mesenchymal transition. J Biol Chem. 2012;287:5819-5832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 260] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 68. | Stoops SL, Pearson AS, Weaver C, Waterson AG, Days E, Farmer C, Brady S, Weaver CD, Beauchamp RD, Lindsley CW. Identification and optimization of small molecules that restore E-cadherin expression and reduce invasion in colorectal carcinoma cells. ACS Chem Biol. 2011;6:452-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | Qi J, Zhu YQ. Targeting the most upstream site of Wnt signaling pathway provides a strategic advantage for therapy in colorectal cancer. Curr Drug Targets. 2008;9:548-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 70. | Avizienyte E, Fincham VJ, Brunton VG, Frame MC. Src SH3/2 domain-mediated peripheral accumulation of Src and phospho-myosin is linked to deregulation of E-cadherin and the epithelial-mesenchymal transition. Mol Biol Cell. 2004;15:2794-2803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 71. | Avizienyte E, Brunton VG, Fincham VJ, Frame MC. The SRC-induced mesenchymal state in late-stage colon cancer cells. Cells Tissues Organs. 2005;179:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Minard ME, Ellis LM, Gallick GE. Tiam1 regulates cell adhesion, migration and apoptosis in colon tumor cells. Clin Exp Metastasis. 2006;23:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Bellovin DI, Simpson KJ, Danilov T, Maynard E, Rimm DL, Oettgen P, Mercurio AM. Reciprocal regulation of RhoA and RhoC characterizes the EMT and identifies RhoC as a prognostic marker of colon carcinoma. Oncogene. 2006;25:6959-6967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 74. | Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4077] [Cited by in RCA: 3939] [Article Influence: 140.7] [Reference Citation Analysis (0)] |
| 75. | Fukuda R, Kelly B, Semenza GL. Vascular endothelial growth factor gene expression in colon cancer cells exposed to prostaglandin E2 is mediated by hypoxia-inducible factor 1. Cancer Res. 2003;63:2330-2334. [PubMed] |
| 76. | Finetti F, Donnini S, Giachetti A, Morbidelli L, Ziche M. Prostaglandin E(2) primes the angiogenic switch via a synergic interaction with the fibroblast growth factor-2 pathway. Circ Res. 2009;105:657-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 77. | Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med. 2012;2:a006627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 376] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 78. | Owen JL, Mohamadzadeh M. Macrophages and chemokines as mediators of angiogenesis. Front Physiol. 2013;4:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 79. | Choi HJ, Hyun MS, Jung GJ, Kim SS, Hong SH. Tumor angiogenesis as a prognostic predictor in colorectal carcinoma with special reference to mode of metastasis and recurrence. Oncology. 1998;55:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 119] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 80. | Chen CN, Cheng YM, Liang JT, Lee PH, Hsieh FJ, Yuan RH, Wang SM, Chang MF, Chang KJ. Color Doppler vascularity index can predict distant metastasis and survival in colon cancer patients. Cancer Res. 2000;60:2892-2897. [PubMed] |
| 81. | Leen E, Goldberg JA, Angerson WJ, McArdle CS. Potential role of doppler perfusion index in selection of patients with colorectal cancer for adjuvant chemotherapy. Lancet. 2000;355:34-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 82. | Goh V, Padhani AR, Rasheed S. Functional imaging of colorectal cancer angiogenesis. Lancet Oncol. 2007;8:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 83. | Royston D, Jackson DG. Mechanisms of lymphatic metastasis in human colorectal adenocarcinoma. J Pathol. 2009;217:608-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 84. | Yoon CM, Hong BS, Moon HG, Lim S, Suh PG, Kim YK, Chae CB, Gho YS. Sphingosine-1-phosphate promotes lymphangiogenesis by stimulating S1P1/Gi/PLC/Ca2+ signaling pathways. Blood. 2008;112:1129-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 85. | Nagahashi M, Ramachandran S, Rashid OM, Takabe K. Lymphangiogenesis: a new player in cancer progression. World J Gastroenterol. 2010;16:4003-4012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 86. | Anelli V, Gault CR, Snider AJ, Obeid LM. Role of sphingosine kinase-1 in paracrine/transcellular angiogenesis and lymphangiogenesis in vitro. FASEB J. 2010;24:2727-2738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 87. | Saad RS, Kordunsky L, Liu YL, Denning KL, Kandil HA, Silverman JF. Lymphatic microvessel density as prognostic marker in colorectal cancer. Mod Pathol. 2006;19:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 88. | Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 923] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 89. | Du B, Yang ZY, Zhong XY, Fang M, Yan YR, Qi GL, Pan YL, Zhou XL. Metastasis-associated protein 1 induces VEGF-C and facilitates lymphangiogenesis in colorectal cancer. World J Gastroenterol. 2011;17:1219-1226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 90. | Langheinrich MC, Schellerer V, Perrakis A, Lohmüller C, Schildberg C, Naschberger E, Stürzl M, Hohenberger W, Croner RS. Molecular mechanisms of lymphatic metastasis in solid tumors of the gastrointestinal tract. Int J Clin Exp Pathol. 2012;5:614-623. [PubMed] |
| 91. | Sundlisaeter E, Dicko A, Sakariassen PØ, Sondenaa K, Enger PØ, Bjerkvig R. Lymphangiogenesis in colorectal cancer--prognostic and therapeutic aspects. Int J Cancer. 2007;121:1401-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 92. | He Y, Rajantie I, Ilmonen M, Makinen T, Karkkainen MJ, Haiko P, Salven P, Alitalo K. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 2004;64:3737-3740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 93. | Kerjaschki D. The crucial role of macrophages in lymphangiogenesis. J Clin Invest. 2005;115:2316-2319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 178] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 94. | Meijer J, Zeelenberg IS, Sipos B, Roos E. The CXCR5 chemokine receptor is expressed by carcinoma cells and promotes growth of colon carcinoma in the liver. Cancer Res. 2006;66:9576-9582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 95. | Gomes FG, Nedel F, Alves AM, Nör JE, Tarquinio SB. Tumor angiogenesis and lymphangiogenesis: tumor/endothelial crosstalk and cellular/microenvironmental signaling mechanisms. Life Sci. 2013;92:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 96. | Sonoshita M, Aoki M, Fuwa H, Aoki K, Hosogi H, Sakai Y, Hashida H, Takabayashi A, Sasaki M, Robine S. Suppression of colon cancer metastasis by Aes through inhibition of Notch signaling. Cancer Cell. 2011;19:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 97. | Sier CF, Vloedgraven HJ, Griffioen G, Ganesh S, Nagengast FM, Lamers CB, Verspaget HW. Plasminogen activators and inhibitor type 1 in neoplastic colonic tissue from patients with familial adenomatous polyposis. Br J Cancer. 1995;71:393-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 98. | Ploplis VA, Tipton H, Menchen H, Castellino FJ. A urokinase-type plasminogen activator deficiency diminishes the frequency of intestinal adenomas in ApcMin/+ mice. J Pathol. 2007;213:266-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 99. | Hildenbrand R, Gandhari M, Stroebel P, Marx A, Allgayer H, Arens N. The urokinase-system--role of cell proliferation and apoptosis. Histol Histopathol. 2008;23:227-236. [PubMed] |
| 100. | Kwaan HC, McMahon B. The role of plasminogen-plasmin system in cancer. Cancer Treat Res. 2009;148:43-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 101. | Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, Liu ET, Cance WG. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55:2752-2755. [PubMed] |
| 102. | Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1307] [Cited by in RCA: 1362] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 103. | Karkkainen MJ, Mäkinen T, Alitalo K. Lymphatic endothelium: a new frontier of metastasis research. Nat Cell Biol. 2002;4:E2-E5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 136] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 104. | Shin MK, Kim SK, Jung H. Integration of intra- and extravasation in one cell-based microfluidic chip for the study of cancer metastasis. Lab Chip. 2011;11:3880-3887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 105. | Stoletov K, Montel V, Lester RD, Gonias SL, Klemke R. High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc Natl Acad Sci USA. 2007;104:17406-17411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 247] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 106. | Yamauchi K, Yang M, Jiang P, Yamamoto N, Xu M, Amoh Y, Tsuji K, Bouvet M, Tsuchiya H, Tomita K. Real-time in vivo dual-color imaging of intracapillary cancer cell and nucleus deformation and migration. Cancer Res. 2005;65:4246-4252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 107. | Chow KH, Factor RE, Ullman KS. The nuclear envelope environment and its cancer connections. Nat Rev Cancer. 2012;12:196-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 108. | Placke T, Kopp HG, Salih HR. The wolf in sheep’s clothing: Platelet-derived “pseudo self” impairs cancer cell “missing self” recognition by NK cells. Oncoimmunology. 2012;1:557-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 109. | Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, Gao S, Puigserver P, Brugge JS. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 890] [Cited by in RCA: 833] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 110. | Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 447] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 111. | Samak R, Israël L. [Extraction and identification of circulating immune complexes from the serum of cancer patient by affinity chromatography followed by high pressure steric exclusion chromatography. Demonstration of their effect on the mitogenesis of normal lymphocytes]. Ann Med Interne (Paris). 1982;133:362-366. [PubMed] |
| 112. | Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2949] [Cited by in RCA: 2764] [Article Influence: 172.8] [Reference Citation Analysis (0)] |
| 113. | Vekemans K, Braet F. Structural and functional aspects of the liver and liver sinusoidal cells in relation to colon carcinoma metastasis. World J Gastroenterol. 2005;11:5095-5102. [PubMed] |
| 114. | Smedsrød B, Le Couteur D, Ikejima K, Jaeschke H, Kawada N, Naito M, Knolle P, Nagy L, Senoo H, Vidal-Vanaclocha F. Hepatic sinusoidal cells in health and disease: update from the 14th International Symposium. Liver Int. 2009;29:490-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 115. | Timmers M, Vekemans K, Vermijlen D, Asosingh K, Kuppen P, Bouwens L, Wisse E, Braet F. Interactions between rat colon carcinoma cells and Kupffer cells during the onset of hepatic metastasis. Int J Cancer. 2004;112:793-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 116. | Enns A, Gassmann P, Schlüter K, Korb T, Spiegel HU, Senninger N, Haier J. Integrins can directly mediate metastatic tumor cell adhesion within the liver sinusoids. J Gastrointest Surg. 2004;8:1049-159; discussion 1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 117. | Wisse E, De Zanger RB, Charels K, Van Der Smissen P, McCuskey RS. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology. 1985;5:683-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 490] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 118. | Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol. 2002;1:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 539] [Cited by in RCA: 523] [Article Influence: 22.7] [Reference Citation Analysis (2)] |
| 119. | Jansen S, Andries M, Vekemans K, Vanbilloen H, Verbruggen A, Bollen M. Rapid clearance of the circulating metastatic factor autotaxin by the scavenger receptors of liver sinusoidal endothelial cells. Cancer Lett. 2009;284:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 120. | Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, Tomiya T, Tejima K, Nishikawa T, Arai M, Yanase M. Plasma lysophosphatidic acid level and serum autotaxin activity are increased in liver injury in rats in relation to its severity. Life Sci. 2007;81:1009-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 121. | Laferriere J, Houle F, Taher MM, Valerie K, Huot J. Transendothelial migration of colon carcinoma cells requires expression of E-selectin by endothelial cells and activation of stress-activated protein kinase-2 (SAPK2/p38) in the tumor cells. J Biol Chem. 2001;276:33762-33772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 122. | Gangopadhyay A, Lazure DA, Thomas P. Adhesion of colorectal carcinoma cells to the endothelium is mediated by cytokines from CEA stimulated Kupffer cells. Clin Exp Metastasis. 1998;16:703-712. [PubMed] |
| 123. | Jacobs F, Wisse E, De Geest B. The role of liver sinusoidal cells in hepatocyte-directed gene transfer. Am J Pathol. 2010;176:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 124. | Bouwens L, De Bleser P, Vanderkerken K, Geerts B, Wisse E. Liver cell heterogeneity: functions of non-parenchymal cells. Enzyme. 1992;46:155-168. [PubMed] |
| 125. | Laskin DL. Nonparenchymal cells and hepatotoxicity. Semin Liver Dis. 1990;10:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 101] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 126. | Laskin DL, Weinberger B, Laskin JD. Functional heterogeneity in liver and lung macrophages. J Leukoc Biol. 2001;70:163-170. [PubMed] |
| 127. | Roberts RA, Ganey PE, Ju C, Kamendulis LM, Rusyn I, Klaunig JE. Role of the Kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicol Sci. 2007;96:2-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 128. | Tu Z, Bozorgzadeh A, Pierce RH, Kurtis J, Crispe IN, Orloff MS. TLR-dependent cross talk between human Kupffer cells and NK cells. J Exp Med. 2008;205:233-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 129. | Gardner CR, Wasserman AJ, Laskin DL. Differential sensitivity of tumor targets to liver macrophage-mediated cytotoxicity. Cancer Res. 1987;47:6686-6691. [PubMed] |
| 130. | Bayón LG, Izquierdo MA, Sirovich I, van Rooijen N, Beelen RH, Meijer S. Role of Kupffer cells in arresting circulating tumor cells and controlling metastatic growth in the liver. Hepatology. 1996;23:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 131. | Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1898] [Cited by in RCA: 1875] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 132. | Decker T, Lohmann-Matthes ML, Karck U, Peters T, Decker K. Comparative study of cytotoxicity, tumor necrosis factor, and prostaglandin release after stimulation of rat Kupffer cells, murine Kupffer cells, and murine inflammatory liver macrophages. J Leukoc Biol. 1989;45:139-146. [PubMed] |
| 133. | Gardner CR, Wasserman AJ, Laskin DL. Liver macrophage-mediated cytotoxicity toward mastocytoma cells involves phagocytosis of tumor targets. Hepatology. 1991;14:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 134. | Seki S, Habu Y, Kawamura T, Takeda K, Dobashi H, Ohkawa T, Hiraide H. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol Rev. 2000;174:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 267] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 135. | Su W, Kitagawa T, Ito T, Oyama T, Lee CM, Kim YK, Matsuda H. Antitumor effect to IL-12 administration into the portal vein on murine liver metastasis. J Hepatobiliary Pancreat Surg. 2002;9:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 136. | Luo Y, Ohmori H, Fujii K, Moriwaka Y, Sasahira T, Kurihara M, Tatsumoto N, Sasaki T, Yamashita Y, Kuniyasu H. HMGB1 attenuates anti-metastatic defence of the liver in colorectal cancer. Eur J Cancer. 2010;46:791-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 137. | Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 616] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 138. | Knittel T, Mehde M, Kobold D, Saile B, Dinter C, Ramadori G. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-alpha and TGF-beta1. J Hepatol. 1999;30:48-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 238] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 139. | Gorden DL, Fingleton B, Crawford HC, Jansen DE, Lepage M, Matrisian LM. Resident stromal cell-derived MMP-9 promotes the growth of colorectal metastases in the liver microenvironment. Int J Cancer. 2007;121:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 140. | Bird NC, Mangnall D, Majeed AW. Biology of colorectal liver metastases: A review. J Surg Oncol. 2006;94:68-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 141. | Thomas P, Hayashi H, Zimmer R, Forse RA. Regulation of cytokine production in carcinoembryonic antigen stimulated Kupffer cells by beta-2 adrenergic receptors: implications for hepatic metastasis. Cancer Lett. 2004;209:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 142. | Gangopadhyay A, Lazure DA, Kelly TM, Thomas P. Purification and analysis of an 80-kDa carcinoembryonic antigen-binding protein from Kupffer cells. Arch Biochem Biophys. 1996;328:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 143. | Gangopadhyay A, Lazure DA, Thomas P. Carcinoembryonic antigen induces signal transduction in Kupffer cells. Cancer Lett. 1997;118:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 144. | Aarons CB, Bajenova O, Andrews C, Heydrick S, Bushell KN, Reed KL, Thomas P, Becker JM, Stucchi AF. Carcinoembryonic antigen-stimulated THP-1 macrophages activate endothelial cells and increase cell-cell adhesion of colorectal cancer cells. Clin Exp Metastasis. 2007;24:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 145. | Jessup JM, Ishii S, Mitzoi T, Edmiston KH, Shoji Y. Carcinoembryonic antigen facilitates experimental metastasis through a mechanism that does not involve adhesion to liver cells. Clin Exp Metastasis. 1999;17:481-488. [PubMed] |
| 146. | Jessup JM, Samara R, Battle P, Laguinge LM. Carcinoembryonic antigen promotes tumor cell survival in liver through an IL-10-dependent pathway. Clin Exp Metastasis. 2004;21:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 147. | Khatib AM, Auguste P, Fallavollita L, Wang N, Samani A, Kontogiannea M, Meterissian S, Brodt P. Characterization of the host proinflammatory response to tumor cells during the initial stages of liver metastasis. Am J Pathol. 2005;167:749-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 148. | Auguste P, Fallavollita L, Wang N, Burnier J, Bikfalvi A, Brodt P. The host inflammatory response promotes liver metastasis by increasing tumor cell arrest and extravasation. Am J Pathol. 2007;170:1781-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 149. | Minami S, Furui J, Kanematsu T. Role of carcinoembryonic antigen in the progression of colon cancer cells that express carbohydrate antigen. Cancer Res. 2001;61:2732-2735. [PubMed] |
| 150. | van der Bij GJ, Oosterling SJ, Meijer S, Beelen RH, van Egmond M. Therapeutic potential of Kupffer cells in prevention of liver metastases outgrowth. Immunobiology. 2005;210:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 151. | Schuurman B, Heuff G, Beelen RH, Meyer S. Enhanced killing capacity of human Kupffer cells after activation with human granulocyte/macrophage-colony-stimulating factor and interferon gamma. Cancer Immunol Immunother. 1994;39:179-184. [PubMed] |
| 152. | Schuurman B, Koenen HJ, Beelen RH, Meyer S. Granulocyte macrophage colony stimulating factor pretreatment induces an increase of rat Kupffer cells with enhanced cytotoxicity in vitro and prevention of tumor outgrowth in vivo. Anticancer Drugs. 1997;8:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 153. | Rushfeldt C, Sveinbjørnsson B, Seljelid R, Smedsrød B. Early events of hepatic metastasis formation in mice: role of Kupffer and NK-cells in natural and interferon-gamma-stimulated defense. J Surg Res. 1999;82:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 154. | Sawitza I, Kordes C, Reister S, Häussinger D. The niche of stellate cells within rat liver. Hepatology. 2009;50:1617-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 155. | Maher JJ. Interactions between hepatic stellate cells and the immune system. Semin Liver Dis. 2001;21:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 143] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 156. | Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2244] [Cited by in RCA: 2197] [Article Influence: 129.2] [Reference Citation Analysis (0)] |
| 157. | Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, Liblau RS, Gressner AM, Kaufmann SH. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 304] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 158. | Winau F, Quack C, Darmoise A, Kaufmann SH. Starring stellate cells in liver immunology. Curr Opin Immunol. 2008;20:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 159. | Brun P, Castagliuolo I, Pinzani M, Palù G, Martines D. Exposure to bacterial cell wall products triggers an inflammatory phenotype in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G571-G578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 160. | March S, Graupera M, Rosa Sarrias M, Lozano F, Pizcueta P, Bosch J, Engel P. Identification and functional characterization of the hepatic stellate cell CD38 cell surface molecule. Am J Pathol. 2007;170:176-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 161. | Di Sario A, Baroni GS, Bendia E, D’Ambrosio L, Ridolfi F, Marileo JR, Jezequel AM, Benedetti A. Characterization of ion transport mechanisms regulating intracellular pH in hepatic stellate cells. Am J Physiol. 1997;273:G39-G48. [PubMed] |
| 162. | Aleffi S, Petrai I, Bertolani C, Parola M, Colombatto S, Novo E, Vizzutti F, Anania FA, Milani S, Rombouts K. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005;42:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 256] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 163. | Qamar A, Sheikh SZ, Masud A, Jhandier MN, Inayat IB, Hakim W, Mehal WZ. In vitro and in vivo protection of stellate cells from apoptosis by leptin. Dig Dis Sci. 2006;51:1697-1705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 164. | Faouzi S, Lepreux S, Bedin C, Dubuisson L, Balabaud C, Bioulac-Sage P, Desmoulière A, Rosenbaum J. Activation of cultured rat hepatic stellate cells by tumoral hepatocytes. Lab Invest. 1999;79:485-493. [PubMed] |
| 165. | Olaso E, Salado C, Egilegor E, Gutierrez V, Santisteban A, Sancho-Bru P, Friedman SL, Vidal-Vanaclocha F. Proangiogenic role of tumor-activated hepatic stellate cells in experimental melanoma metastasis. Hepatology. 2003;37:674-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 166. | Shimizu S, Yamada N, Sawada T, Ikeda K, Kawada N, Seki S, Kaneda K, Hirakawa K. In vivo and in vitro interactions between human colon carcinoma cells and hepatic stellate cells. Jpn J Cancer Res. 2000;91:1285-1295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 167. | Wirz W, Antoine M, Tag CG, Gressner AM, Korff T, Hellerbrand C, Kiefer P. Hepatic stellate cells display a functional vascular smooth muscle cell phenotype in a three-dimensional co-culture model with endothelial cells. Differentiation. 2008;76:784-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 168. | Nakatani K, Kaneda K, Seki S, Nakajima Y. Pit cells as liver-associated natural killer cells: morphology and function. Med Electron Microsc. 2004;37:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 169. | Luo DZ, Vermijlen D, Ahishali B, Triantis V, Plakoutsi G, Braet F, Vanderkerken K, Wisse E. On the cell biology of pit cells, the liver-specific NK cells. World J Gastroenterol. 2000;6:1-11. [PubMed] |
| 170. | Timonen T, Helander TS. Natural killer cell-target cell interactions. Curr Opin Cell Biol. 1997;9:667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 171. | Berke G. The binding and lysis of target cells by cytotoxic lymphocytes: molecular and cellular aspects. Annu Rev Immunol. 1994;12:735-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 172. | Luo D, Vanderkerken K, Bouwens L, Kuppen PJ, Baekeland M, Seynaeve C, Wisse E. The role of adhesion molecules in the recruitment of hepatic natural killer cells (pit cells) in rat liver. Hepatology. 1996;24:1475-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 173. | Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2006;20:123-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 357] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 174. | Vermijlen D, Luo D, Robaye B, Seynaeve C, Baekeland M, Wisse E. Pit cells (Hepatic natural killer cells) of the rat induce apoptosis in colon carcinoma cells by the perforin/granzyme pathway. Hepatology. 1999;29:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 175. | Haier J, Nicolson GL. Tumor cell adhesion under hydrodynamic conditions of fluid flow. APMIS. 2001;109:241-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 176. | Haier J, Nicolson GL. The role of tumor cell adhesion as an important factor in formation of distant colorectal metastasis. Dis Colon Rectum. 2001;44:876-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 177. | Shimizu S, Yamada N, Sawada T, Ikeda K, Nakatani K, Seki S, Kaneda K, Hirakawa K. Ultrastructure of early phase hepatic metastasis of human colon carcinoma cells with special reference to desmosomal junctions with hepatocytes. Pathol Int. 2000;50:953-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 178. | Braet F, Nagatsuma K, Saito M, Soon L, Wisse E, Matsuura T. The hepatic sinusoidal endothelial lining and colorectal liver metastases. World J Gastroenterol. 2007;13:821-825. [PubMed] |
| 179. | Hosch SB, Steffani KD, Scheunemann P, Izbicki JR. Micrometastases from HBP malignancies and metastatic cancer. J Hepatobiliary Pancreat Surg. 2002;9:583-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 180. | Ikawa K, Terashima Y, Sasaki K, Tashiro S. Genetic detection of liver micrometastases that are undetectable histologically. J Surg Res. 2002;106:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 181. | Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 953] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 182. | Du L, Rao G, Wang H, Li B, Tian W, Cui J, He L, Laffin B, Tian X, Hao C. CD44-positive cancer stem cells expressing cellular prion protein contribute to metastatic capacity in colorectal cancer. Cancer Res. 2013;73:2682-2694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 183. | Chen X, Wang Y, Xia H, Wang Q, Jiang X, Lin Z, Ma Y, Yang Y, Hu M. Loss of E-cadherin promotes the growth, invasion and drug resistance of colorectal cancer cells and is associated with liver metastasis. Mol Biol Rep. 2012;39:6707-6714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 184. | Han X, Fang X, Lou X, Hua D, Ding W, Foltz G, Hood L, Yuan Y, Lin B. Silencing SOX2 induced mesenchymal-epithelial transition and its expression predicts liver and lymph node metastasis of CRC patients. PLoS One. 2012;7:e41335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 185. | Belluco C, Mammano E, Petricoin E, Prevedello L, Calvert V, Liotta L, Nitti D, Lise M. Kinase substrate protein microarray analysis of human colon cancer and hepatic metastasis. Clin Chim Acta. 2005;357:180-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 186. | Sahai E. Illuminating the metastatic process. Nat Rev Cancer. 2007;7:737-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 413] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 187. | Kuiper T, van den Broek FJ, van Eeden S, Fockens P, Dekker E. Feasibility and accuracy of confocal endomicroscopy in comparison with narrow-band imaging and chromoendoscopy for the differentiation of colorectal lesions. Am J Gastroenterol. 2012;107:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 188. | Dai F, Shu L, Bian Y, Wang Z, Yang Z, Chu W, Gao S. Safety of bevacizumab in treating metastatic colorectal cancer: a systematic review and meta-analysis of all randomized clinical trials. Clin Drug Investig. 2013;33:779-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 189. | Chong G, Bhatnagar A, Cunningham D, Cosgriff TM, Harper PG, Steward W, Bridgewater J, Moore M, Cassidy J, Coleman R. Phase III trial of 5-fluorouracil and leucovorin plus either 3H1 anti-idiotype monoclonal antibody or placebo in patients with advanced colorectal cancer. Ann Oncol. 2006;17:437-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 190. | Ganea E, Trifan M, Laslo AC, Putina G, Cristescu C. Matrix metalloproteinases: useful and deleterious. Biochem Soc Trans. 2007;35:689-691. [PubMed] |
| 191. | Le Tourneau C, Faivre S, Raymond E. The role of integrins in colorectal cancer. Oncology (Williston Park). 2007;21:21-24. [PubMed] |
| 192. | Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 700] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 193. | Fujii SI, Shimizu K. Immunotherapy with artificial adjuvant vector cells: Harnessing both arms of the immune response. Oncoimmunology. 2013;2:e23432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 194. | Smith CW. 3. Adhesion molecules and receptors. J Allergy Clin Immunol. 2008;121:S375-S39; quiz S414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |