Published online Apr 7, 2014. doi: 10.3748/wjg.v20.i13.3672
Revised: February 9, 2014
Accepted: March 8, 2014
Published online: April 7, 2014
Processing time: 120 Days and 16.8 Hours
AIM: To investigate the expression of ERG, CD34, CD31 (PECAM-1, platelet/endothelial cell adhesion molecule 1) and factor VIII-related antigen (FVIIIRAg) in the diagnosis of hepatic angiosarcoma patients.
METHODS: Patient samples were collected from January 1986 to December 2012 from the People’s Liberation Army General Hospital in Beijing, China. We obtained twenty-four samples of hepatic angiosarcoma (HAS) that were confirmed by two pathologist. The samples were the result of three autopsy cases, eight biopsy cases and 13 patients who underwent surgical tumor removal. The HAS cases accounted for 2.23% (24/1075) of all hepatic vascular tumors at the hospital during the same time period. Patient histories including age, gender, clinical manifestations, medical treatments, laboratory tests, radiological images, histological observations and outcomes for each case were analyzed in detail. All samples were evaluated histologically with hematoxylin and eosin staining. Using immunohistochemistry, the expression and localization of ERG was examined in all HAS specimens and compared to the known endothelial markers CD34, CD31 and FVIIIRAg. The endothelial markers were also evaluated in a panel of non-HAS tumors.
RESULTS: This cohort of 24 HAS cases is, to the best of our knowledge, currently the largest cohort in the world in the publicly available literature. Hepatic angiosarcoma tissue samples were obtained from 14 males and 10 females with a mean age of 50.6 years (range: 7-86 years). The patients presented with the following clinical manifestations: abdominal pain (16/24), back pain (3/24), heart palpitations (1/20), cough (1/24) or no clinical symptoms (3/24). Tumors were predominantly localized in the right hepatic lobe (15/24) or left hepatic lobe (6/24), or a diffuse growth on the right and left hepatic lobes (3/24). Eleven patients underwent surgical resection (45.8%), two patients received a liver transplant (8.3%), eight patients received interventional therapy (33.3%) and three patients received no treatment (lesions discovered at autopsy, 12.5%). Postoperative follow-up of patients revealed that 87.5% (21/24) of patients had died and three cases were not able to be tracked. In all cases, the mean survival time was 12.1 mo. While 100% of the HAS samples were positive for ERG expression, expression of the other markers was more variable. CD31 was expressed in 79.2% (19/24) of samples, CD34 was expressed in 87.5% (21/24) of samples and FVIIIRAg was expressed in 41.7% (10/24) of samples.
CONCLUSION: ERG is a more sensitive and specific diagnostic marker for hepatic angiosarcoma in comparison to CD31, CD34 and FVIIIRAg.
Core tip: Hepatic angiosarcoma (HAS) is a rare disease and formal therapeutic guidelines have not been established. A method to detect HAS early using molecular markers would improve patient survival. Here, we have assembled the largest cohort to date of 24 HAS cases to determine if ERG, CD31, CD34 or factor VIII-related antigen (FVIIIRAg) represent a diagnostic marker for the disease. Expression of CD31, CD34 and FVIIIRAg was variable across the 24 samples, while ERG was consistently expressed in all HAS samples. ERG showed increased sensitivity and specificity in comparison to the other markers examined and represents a novel diagnostic marker for HAS.
- Citation: Wang ZB, Yuan J, Chen W, Wei LX. Transcription factor ERG is a specific and sensitive diagnostic marker for hepatic angiosarcoma. World J Gastroenterol 2014; 20(13): 3672-3679
- URL: https://www.wjgnet.com/1007-9327/full/v20/i13/3672.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i13.3672
Angiosarcoma is a malignant neoplasm that affects endothelial cells and can occur in any location throughout the body[1,2]. The location of tumor origin often permits metastases to distant sites. Although hepatic angiosarcoma (HAS) is a very rare disease and accounts for only 2% of primary liver malignancies[3], it is still the third most common primary liver malignancy[4,5]. Case-control studies have shown that approximately one-third of HAS cases appear to be caused by environmental carcinogens via exposure to thorium dioxide, arsenical insecticide or polyvinyl chloride[6].
The diagnosis of HAS is difficult, especially if the patient has no history of carcinogen exposure. Long-term survival in HAS patients is poor due to its rapid progression, high recurrence rate and resistance to traditional chemo- and radiotherapies[7-9]. Due to a high recurrence rate and poor surgical outcomes, liver transplantation as a form of therapy for HAS is no longer performed[5]. No formal guidelines exist for the treatment of HAS. Currently, the best treatment option for HAS is partial surgical resection of the liver to remove the tumor. Thus, a means to detect HAS early using specific molecular markers will provide a critical time window during which surgical resection can be performed, which will ultimately improve patient survival.
CD31, CD34 and factor VIII-related antigen (FVIIIRAg) are expressed in endothelial cells and have been hypothesized to serve as diagnostic markers of angiosarcoma[10]. CD31, a transmembrane glycoprotein adhesion molecule, is expressed by platelets, megakaryocytes, and endothelial cells[11]. CD34 is a cell-surface marker expressed in both endothelial cells and hematopoietic stem cells[12]. FVIIIRAg is expressed in Weibel-Palade bodies of endothelial cells and in megakaryocytes[13]. Although these markers are touted as diagnostic for angiosarcoma, the precise role of each of these markers in HAS remains unclear.
ERG (avian v-ets erythroblastosis virus E26 oncogene homolog), a member of the ETS family of transcription factors, is expressed in endothelial cells[14]. ERG plays essential roles in the regulation of angiogenesis and apoptosis of endothelial cells[15]. Chromosomal translocations affecting ERG have been implicated in the development of several cancers[16,17]. Recent reports have demonstrated that ERG is a specific and sensitive biological marker in the diagnosis of angiosarcoma[18-20]. The diagnostic value of ERG in HAS is largely unknown and only one previous publication has documented ERG expression in a HAS patient[18]. To this end, we assembled the largest cohort to date of HAS tissues to evaluate the role of ERG expression in HAS. Moreover, we analyzed how ERG expression compares to CD31, CD34 and FVIIIRAg endothelial markers.
All patient specimens were collected from January 1986 to December 2012 at the Chinese People’s Liberation Army General Hospital in Beijing, China. We confirmed 24 cases of HAS by pathology after 13 patients underwent surgical tumor removal, eight patients were biopsied and three patients were autopsied. These cases accounted for 2.23% (24/1075) of hepatic vascular tumors during the same time period. Detailed clinical histories, including gender, age, clinical symptoms, tumor location, size, imaging data, and laboratory test results were collected from each patient’s record. Follow-up information on each case was obtained through telephone conversations and hospital records to evaluate patient prognosis in November 2013. As controls, we obtained other types of liver spindle cell tumors, including hepatic epithelioid hemangioendotheliomas (EHE; n = 3), undifferentiated sarcomas (n = 3), leiomyosarcoma (n = 1), sarcomatoid carcinomas (n = 3) and gastrointestinal stromal tumor liver metastases (n = 2).
All hematoxylin and eosin-stained slides were reviewed for each case. Histological changes were evaluated by experienced pathologists using a light microscope.
Tissues were fixed in 4% formalin and embedded in paraffin for immunohistochemistry using the EnVision kit (DAKO; Denmark). The antibodies used in this study are listed in Table 1.
| Protein name | Antibody clone | Source | Concentration | Location |
| Vimentin | V9 | Invitrogen | 1:200 | C |
| CD31 | Jc70A | Gene Tech | 1:100 | M |
| CD34 | QBEnd-10 | Dako | 1:50 | M |
| FVIIIRAg | F8/86 | Dako | 1:50 | C |
| ERG | Ep111 | Epitomics | 1:100 | N |
| CK | Polyclonal | Dako | 1:100 | C |
| GPC-3 | 1G12 | Cell marque | 1:150 | C;M |
| Hepatocyte | OCH1E5 | Zeta | 1:100 | C |
| CD117 | Polyclonal | Dako | 1:400 | M |
| Desmin | D33 | Dako | 1:100 | C |
| Ki-67 | MIB-1 | Dako | 1:200 | N |
Statistical analysis was performed using the SPSS 17.0 statistical software package (SPSS Inc., Chicago, IL, United States). Cumulative survival curves were generated using the Kaplan-Meier method. The hazard ratio and 95% confidence interval were also calculated.
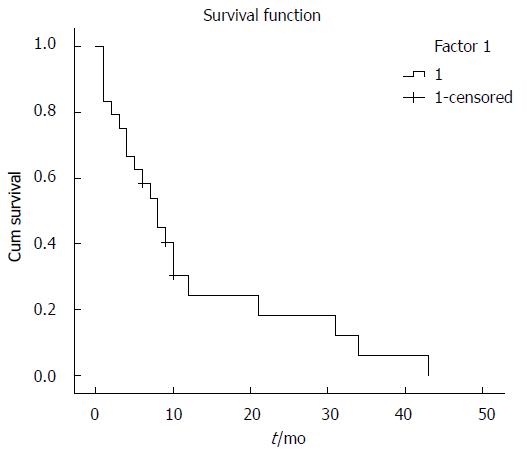
The HAS tissues were obtained from 14 males and 10 females, with a mean age of 50.6 (range: 7-86) years (Table 2). The patients presented with the following clinical manifestations: abdominal pain (16/24), back pain (3/24), heart palpitations (1/20), cough (1/24) or no clinical symptoms (3/24). Tumors were located in the right lobe (15/24), left hepatic lobe (6/24) or as a diffuse growth on the right and left hepatic lobes (3/24) (Table 2). Patients underwent surgical resection (11/24), liver transplantation (2/24), interventional therapy (8/24) or they received no treatment (lesions discovered at autopsy; 3/24). Postoperative follow-up of each case showed that 18 patients had died and three cases could not be accounted for. Kaplan-Meier survival curves demonstrated that the prognosis of HAS cases was poor (Figure 1, Table 3). Laboratory tests for alpha-fetoprotein, carcinoembryonic antigen, CA19-9, CA125 and other tumor markers were normal. Two patients were positive for the hepatitis B surface antigen and one patient had abnormal hemoglobin levels (90 g/L).
| No. | Gender | Age/yr | Clinical symptoms | Tumor location | Maximum tumor size/cm | Treatment methods | Follow-up |
| 11 | Male | 42 | Abdominal discomfort | Left lateral lobe of the liver | 5 × 3 × 3 | Adjuvant therapy | Spleen metastasis at treatment and died 10 mo later |
| 21 | Female | 30 | Abdominal discomfort | Left inner hepatic lobe | 6 × 4 × 3 | Adjuvant therapy | Unable to follow-up after 9 mo |
| 3 | Male | 60 | Upper abdominal pain | Right hepatic lobe | 16 × 15 × 13 | Surgery | Died after 12 mo |
| 41 | Female | 58 | Liver pain | Left lobe of liver | 9.5 × 9.4 × 6 | Adjuvant therapy | Unable to follow-up after 6 mo |
| 51 | Female | 52 | Back pain | Left and right hepatic lobes | 13 × 8 × 6 | Adjuvant therapy | Thoracic metastasis at treatment and died 8 mo later |
| 6 | Male | 7 | Abdominal pain, fever | Right hepatic lobe | 7 × 6 × 3 | Surgery | Died 1 mo later |
| 7 | Female | 52 | Upper abdominal pain | Left hepatic lobe | 12.5 × 12 × 4 | Surgery | Post-operative thoracic and lung metastases and died 31 mo later |
| 8 | Female | 62 | Abdominal discomfort | Left hepatic lobe | 7.5 × 6 × 3 | Surgery | Post-operative lung metastases and died 34 mo later |
| 91 | Male | 68 | Upper abdominal pain | Right hepatic lobe | 10 × 8 × 6 | Adjuvant therapy | Portal vein cancer thrombosis at diagnosis and died 9 mo later |
| 10 | Female | 29 | Hypo-proteinemia | Left and right hepatic lobes | 27 × 20 × 9 | Liver transplant | Spleen metastasis at diagnosis and died during surgery |
| 11 | Male | 49 | Asymptomatic | Right hepatic lobe | 3 × 3 × 2 | Surgery | Bone metastases and died 43 mo later |
| 12 | Female | 62 | Upper abdominal pain | Right hepatic lobe | 3 × 2 × 2 | Liver Transplant | Recurrence of tumor in the transplanted liver and died 4 mo later |
| 13 | Female | 32 | Asymptomatic | Right hepatic lobe | 6 × 5 × 3 | Surgery | Died 3 mo later |
| 14 | Female | 51 | Upper abdominal pain | Right hepatic lobe | 10 × 6 × 5 | Surgery | Died 8 mo later |
| 15 | Male | 53 | Upper abdominal pain | Left hepatic lobe | 27 × 17 × 6 | Surgery | Recurrence after 4 mo and died 7 mo later |
| 16 | Male | 49 | Upper abdominal pain | Right hepatic lobe | 11 × 7 × 5 | Surgery | Died 10 mo later |
| 171 | Male | 66 | Back pain | Right hepatic lobe | 6 × 5 × 4 | Adjuvant therapy | Thoracic metastasis at diagnosis and died 5 mo later |
| 18 | Male | 86 | Upper abdominal pain | Left and right hepatic lobes | 8 × 7 × 3 | Autopsy findings without treatment | Died due to tumor rupture |
| 19 | Male | 86 | Upper abdominal pain | Right hepatic lobe | 9 × 9 × 6 | Autopsy findings without treatment | Tumor metastasis and died due to heart failure |
| 20 | Male | 70 | Palpitations | Right hepatic lobe | 4.5 × 4 × 3 | Autopsy findings without treatment | Tumor metastasis and died due to heart failure |
| 211 | Female | 45 | Cough | Right hepatic lobe | 4.5 × 4 × 4 | Adjuvant therapy | Lung metastasis at diagnosis and died 6 mo later |
| 22 | Male | 21 | Right shoulder pain | Right hepatic lobe | 16.5 × 15 × 8 | Surgery | Tumor recurrence and died 8 mo later |
| 231 | Male | 21 | Upper abdominal pain | Right hepatic lobe | 3.5 × 3 × 3 | Adjuvant therapy | Unable to follow-up after 10 mo |
| 24 | Male | 63 | Upper abdominal pain | Right hepatic lobe | 3.5 × 3.5 × 3 | Surgery | Tumor recurrence after 18 mo and died 21 mo later |
| Factor 1 | Mean1 | |||
| Estimate | Std. error | 95%CI | ||
| Lower bound | Upper bound | |||
| 1 | 12.196 | 2.788 | 6.731 | 17.660 |
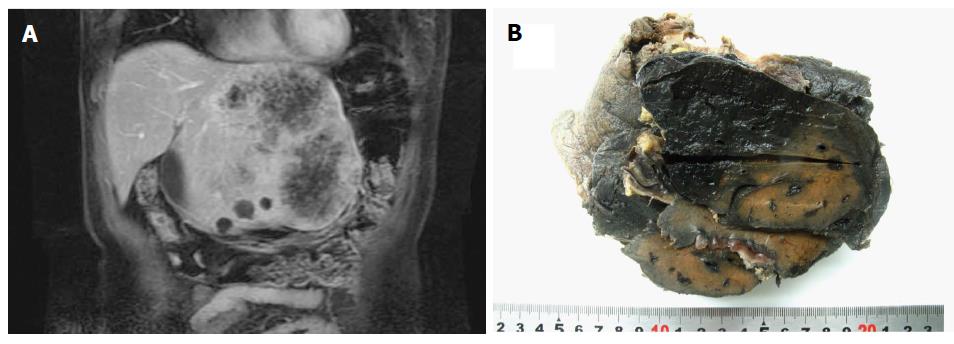
Radiological imaging, which included ultrasound, CT and magnetic resonance imaging, showed cystic or solid masses with varying textures and ill-defined borders (Figure 2A). Sixteen of the 24 samples were obtained via surgery or autopsy and of these, 10 were classified as single huge nodules (62.5%), two with multiple nodules (12.5%), three with single large nodules with peripheral small nodules (18.8%) and one with diffuse micronodules (6.3%). The mean tumor diameter was 9.58 cm (range: 3-27 cm). Tumor tissues were dark red in color and had a honeycomb appearance (Figure 2B).
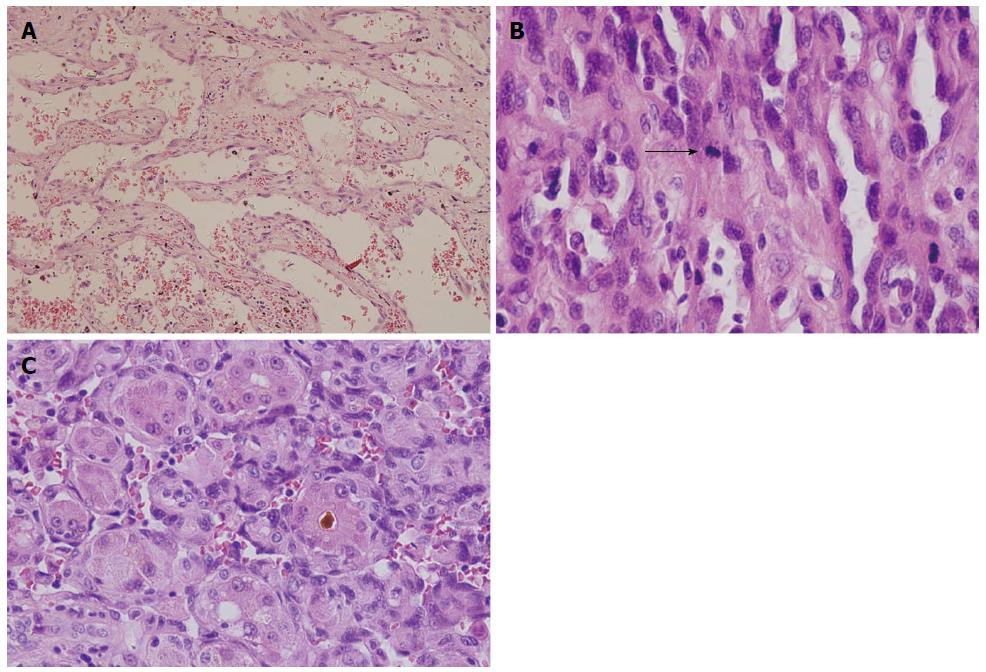
When tumor histology was examined, one layer or multiple layers of tumor cells lined a vascular lumen and gave rise to the lumen-like structure (Figure 3A and B). The tumor cells grew along the sinusoids, with the liver serving as a scaffold for tumor growth (Figure 3C). Three of the samples had overt compartments that were filled with red blood cells. The tumors predominantly contained atypical spindle cells and mitotic cells were frequently observed. Moreover, we observed significant necrosis and calcification within the tumors. Areas of epithelial differentiation were apparent in some tumors. In patients with recurrent tumors following liver transplantation, the tumors contained a large blood chamber that differed from the morphology observed in the primary lesion.
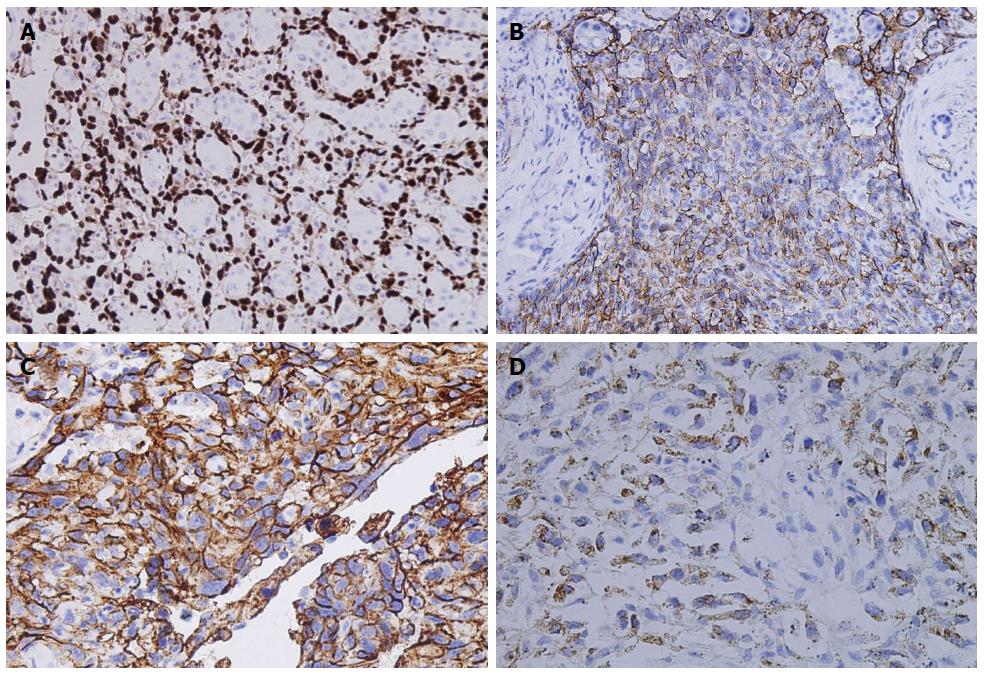
To evaluate ERG, CD31, CD34 and FVIIIRAg as diagnostic markers of HAS, we evaluated their expression by immunohistochemistry. ERG expression was observed in all HAS samples examined (24/24; Figure 4A). In contrast, the other three endothelial markers examined were not uniformly expressed across HAS samples. CD34, CD31, and FVIIIRAg were expressed in 21/24, 19/24 and 10/24 cases, respectively (Figure 4B-D). Moreover, only one case was positive for pan-cytokeratin (CK) expression (1/24). The cell proliferation index was assayed by Ki-67 staining. In all samples, the cell proliferation index was greater than 10% and up to 60%. Desmin, c-kit proto-oncogene (CD117) and Glypican-3 (GPC-3) expression was absent in all samples.
When control samples were evaluated for expression of the endothelial markers, all three cases of EHE tumors were positive for ERG, CD34 and CD31 expression, while only one was positive for FVIIIRAg (33.3%). The undifferentiated sarcoma samples (n = 3) lacked ERG, CD31 and FVIIIRAg expression and only one sample (33.3%) was positive for CD34 expression. The leiomyosarcoma sample was positive for CD34 expression, but lacked ERG, CD31 and FVIIIRAg expression. Similarly, only one sarcomatoid carcinoma (33.3%) showed CD34 expression while ERG, CD31 and FVIIIRAg were absent. Both gastrointestinal stromal tumor liver metastases were positive for CD34, but lacked ERG, CD31 and FVIIIRAg expression.
Primary sarcomas of the liver are rare. The most common hepatic sarcomas are HAS, embryonal sarcoma, leiomyosarcoma, EHE, fibrosarcoma and malignant fibrous histiocytoma[21]. HAS is primarily observed in the elderly and often presents with nonspecific symptoms such discomfort or distension of the abdomen, weight loss or fatigue, which makes the diagnosis difficult[3]. HAS is frequently caused by exposure to carcinogens such as thorium dioxide, arsenical insecticide or polyvinyl chloride[6,22-26]. However, in all the Chinese patients that comprised our cohort, carcinogen exposure was not an important factor in disease onset. Our data are consistent with reports on HAS development from Taiwan and South Korea[27,28]. To better address the role of carcinogens and HAS in China, an approach involving a survey of multiple medical centers across the country would be beneficial. Infection from hepatitis B virus (HBV) has also been implicated as a risk factor for HAS. In our study, the frequency of HBV infection was no different to that in the normal, healthy Chinese population, indicating that HBV infection did not play a role in HAS onset[29,30].
HAS often appears as a single mass when viewed histologically, however, multiple masses have also been observed and HAS may affect the whole liver. In the clinic, four primary patterns of disease have been observed: (1) multiple nodules; (2) a large dominant mass; (3) a dominant mass combined with multiple nodules; and (4) diffuse micronodular infiltration of the liver, although this is observed less frequently[31]. In the HAS patient cohort, a large dominant mass was most frequently observed (62.5%). The remaining patients had a dominant mass and multiple nodules (18.8%), multiple nodules (12.5%) or diffuse micronodular infiltration of the liver (6.3%). When HAS samples are viewed by microscopy, the tumor cells are often mitotic, have a spindle or polygonal morphology and may be in a single layer or multi-layered surrounding a vascular source, connected with thick hepatic cables. HAS tumor cells have the ability to form papillary structures and may show epithelial differentiation.
HAS patient survival is currently very poor, with a median survival of 6 mo without treatment. Even with treatment, only 3% of patients are reported to survive longer than 2 years[5]. Poor prognosis of HAS patients is primarily due to early metastases and an extended time between disease onset and correct diagnosis. Surgical resection is currently the standard treatment for HAS. Early diagnosis of HAS is required for surgical resection to be beneficial to the patient. Other treatments such as chemotherapy or radiotherapy have not shown a conclusive survival benefit in HAS patients[7-9]. Thus, the identification of specific and sensitive biomarkers to diagnose HAS would significantly improve patient outcome. Here, we have assembled the largest HAS cohort to date and provide evidence that ERG is a specific diagnostic marker for HAS.
ERG regulates angiogenesis and differentiation of embryonic stem cells into endothelial cells. Chromosomal translocations affecting ERG expression have been shown to play a role in the development of several cancers, including prostate cancer[16,17]. The diagnostic value of ERG in HAS, however, has not been evaluated. Here, we have shown that ERG expression was detected in all HAS cases examined. While three cases of EHE, one of our controls, also showed positive expression of ERG, the remaining controls-undifferentiated sarcoma, sarcomatoid carcinoma and gastrointestinal stromal tumor liver metastases-were negative for ERG expression. These results suggest that ERG expression is relatively specific to HAS tumors. In comparison to the other endothelial markers (i.e., CD31, CD34 and FVIIIRAg) examined, ERG was more sensitive and had increased specificity for HAS. Thus, ERG may represent a novel diagnostic marker for HAS. Because ERG expression shows a similar pattern in both benign and malignant vascular populations, histological examination is required to differentiate angiosarcomas from other endothelial neoplasms[18]. Tumor cell mitotic activities, atypical mitotic features and necrosis have been fundamentally recognized as probable factors determining propensity for HAS.
In conclusion, ERG is a new biomarker for the diagnosis of HAS. While CD31, CD34 and FVIIIRAg were variably expressed across our HAS cohort, ERG was expressed in all tumors examined. Because HAS is a relatively rare malignancy, our cohort (n = 24) is, to the best of our knowledge, the largest in the world to date. The data provided from this patient cohort will help to establish a standard for symptoms, diagnosis and treatment of HAS. Moreover, our data implicate ERG as a potent marker in the diagnosis of HAS tumors.
Hepatic angiosarcoma (HAS) is a rare disease characterized by poor prognosis due to its rapid progression, high recurrence rate and resistance to traditional chemo- and radiotherapies. Surgical resection, which requires a prompt diagnosis, is currently the best treatment option for HAS. Because the initial symptoms of HAS can be nonspecific, it is difficult to diagnose. Thus, a specific molecular marker for the early diagnosis of HAS would provide a critical time window for surgical resection and greatly improve patient survival. ERG (V-Ets Erythroblastosis Virus E26 Oncogene Homolog) is a marker of endothelial cells that has been implicated in other cancers, but its value as a diagnostic marker in HAS has not yet been evaluated.
ERG is a member of the ETS family of transcription factors and is expressed in endothelial cells. Angiogenesis, endothelial cell differentiation and endothelial homeostasis are regulated by ERG. Previous reports have shown that ERG is a specific and sensitive biological marker for the diagnosis of angiosarcoma. The diagnostic value of ERG expression in HAS has not been evaluated. In addition, because HAS is quite rare, it has been difficult to evaluate diagnostic criteria in a large patient cohort.
This study has assembled the largest HAS cohort to date (n = 24 cases) to evaluate the role of ERG expression in liver angiosarcoma. Moreover, this study compares ERG expression to other known endothelial markers such as CD31, CD34 and factor VIII-related antigen (FVIIIRAg) as a means of HAS diagnosis. While CD31, CD34 and FVIIIRAg had variable expression across the cohort samples, ERG expression was found in 100% of the cases examined. The data provided from this patient cohort will help to establish a standard for symptoms, diagnosis and treatment of HAS. This is the first study to identify ERG expression as a marker for HAS.
The findings in this study highlight ERG expression as a potent and novel marker for HAS tumors, which will aid in developing diagnostic tests for the disease.
HAS is a rare type of cancer that affects the endothelial cells of the liver and is difficult to diagnose until the disease has spread. ERG, CD31, CD34 and FVIIIRAg are genes expressed in endothelial cells, the cell population affected in HAS, and may therefore represent a way of identifying HAS tumors.
This is a good original research article that demonstrates that ERG is a specific and sensitive diagnostic biomarker for hepatic angiosarcoma. Moreover, ERG is a more sensitive and specific diagnostic biomarker for hepatic angiosarcoma in comparison to CD31, CD34 and FVIIIRAg. This study represents the largest cohort of HAS cases to date and is well designed to evaluate the value of ERG in HAS diagnosis. The results of this study have a high potential for clinical application.
P- Reviewer: Munoz M S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Ma S
| 1. | Fayette J, Martin E, Piperno-Neumann S, Le Cesne A, Robert C, Bonvalot S, Ranchère D, Pouillart P, Coindre JM, Blay JY. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol. 2007;18:2030-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 259] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 2. | Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 344] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Mani H, Van Thiel DH. Mesenchymal tumors of the liver. Clin Liver Dis. 2001;5:219-257, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Molina E, Hernandez A. Clinical manifestations of primary hepatic angiosarcoma. Dig Dis Sci. 2003;48:677-682. [PubMed] |
| 5. | Locker GY, Doroshow JH, Zwelling LA, Chabner BA. The clinical features of hepatic angiosarcoma: a report of four cases and a review of the English literature. Medicine (Baltimore). 1979;58:48-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 154] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Thomas LB, Popper H. Pathology of angiosarcoma of the liver among vinyl chloride-polyvinyl chloride workers. Ann N Y Acad Sci. 1975;246:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 33] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Almogy G, Lieberman S, Gips M, Pappo O, Edden Y, Jurim O, Simon Slasky B, Uzieli B, Eid A. Clinical outcomes of surgical resections for primary liver sarcoma in adults: results from a single centre. Eur J Surg Oncol. 2004;30:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Jennings RN, Miller MA, Ramos-Vara JA. Comparison of CD34, CD31, and factor VIII-related antigen immunohistochemical expression in feline vascular neoplasms and CD34 expression in feline nonvascular neoplasms. Vet Pathol. 2012;49:532-537. [PubMed] |
| 11. | Ilan N, Madri JA. PECAM-1: old friend, new partners. Curr Opin Cell Biol. 2003;15:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 177] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Simmons DL, Satterthwaite AB, Tenen DG, Seed B. Molecular cloning of a cDNA encoding CD34, a sialomucin of human hematopoietic stem cells. J Immunol. 1992;148:267-271. [PubMed] |
| 13. | Lemos QT, Andrade ZA. Angiogenesis and experimental hepatic fibrosis. Mem Inst Oswaldo Cruz. 2010;105:611-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Reddy ES, Rao VN, Papas TS. The erg gene: a human gene related to the ets oncogene. Proc Natl Acad Sci USA. 1987;84:6131-6135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 126] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Birdsey GM, Dryden NH, Amsellem V, Gebhardt F, Sahnan K, Haskard DO, Dejana E, Mason JC, Randi AM. Transcription factor Erg regulates angiogenesis and endothelial apoptosis through VE-cadherin. Blood. 2008;111:3498-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Hossain D, Bostwick DG. Significance of the TMPRSS2: ERG gene fusion in prostate cancer. BJU Int. 2013;111:834-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Navarro R, Laguna A, de Torres C, Cigudosa JC, Suñol M, Cruz O, Mora J. Primary Ewing sarcoma of the tentorium presenting with intracranial hemorrhage in a child. J Neurosurg. 2007;107:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Miettinen M, Wang ZF, Paetau A, Tan SH, Dobi A, Srivastava S, Sesterhenn I. ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am J Surg Pathol. 2011;35:432-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 253] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 19. | Agaimy A, Kirsche H, Semrau S, Iro H, Hartmann A. Cytokeratin-positive epithelioid angiosarcoma presenting in the tonsil: a diagnostic challenge. Hum Pathol. 2012;43:1142-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | McKay KM, Doyle LA, Lazar AJ, Hornick JL. Expression of ERG, an Ets family transcription factor, distinguishes cutaneous angiosarcoma from histological mimics. Histopathology. 2012;61:989-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Weitz J, Klimstra DS, Cymes K, Jarnagin WR, D’Angelica M, La Quaglia MP, Fong Y, Brennan MF, Blumgart LH, Dematteo RP. Management of primary liver sarcomas. Cancer. 2007;109:1391-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Ito Y, Kojiro M, Nakashima T, Mori T. Pathomorphologic characteristics of 102 cases of thorotrast-related hepatocellular carcinoma, cholangiocarcinoma, and hepatic angiosarcoma. Cancer. 1988;62:1153-1162. [PubMed] |
| 23. | Wagoner JK. Toxicity of vinyl chloride and poly(vinyl chloride): a critical review. Environ Health Perspect. 1983;52:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Makarov IA, Fedotova IV. [Mechanisms of the carcinogenic effects of vinyl chloride (literature review)]. Gig Tr Prof Zabol. 1983;42-45. [PubMed] |
| 25. | Zaeva GN. [Carcinogenic properties of vinyl chloride (a review of the literature)]. Gig Tr Prof Zabol. 1976;46-48. [PubMed] |
| 26. | Bartsch H, Montesano R. Mutagenic and carcinogenic effects of vinyl chloride. Mutat Res. 1975;32:93-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 72] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Huang NC, Wann SR, Chang HT, Lin SL, Wang JS, Guo HR. Arsenic, vinyl chloride, viral hepatitis, and hepatic angiosarcoma: a hospital-based study and review of literature in Taiwan. BMC Gastroenterol. 2011;11:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Kim HR, Rha SY, Cheon SH, Roh JK, Park YN, Yoo NC. Clinical features and treatment outcomes of advanced stage primary hepatic angiosarcoma. Ann Oncol. 2009;20:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 29. | Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y. Epidemiological serosurvey of hepatitis B in China--declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27:6550-6557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 712] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 30. | Zhou YM, Li B, Yin ZM, Xu F, Wang B, Xu W, Liu P, Yang JM. Results of hepatic resection for primary hepatic angiosarcoma in adults. Med Sci Monit. 2010;16:CR61-CR66. [PubMed] |
| 31. | Koyama T, Fletcher JG, Johnson CD, Kuo MS, Notohara K, Burgart LJ. Primary hepatic angiosarcoma: findings at CT and MR imaging. Radiology. 2002;222:667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |