Published online Apr 7, 2014. doi: 10.3748/wjg.v20.i13.3485
Revised: January 7, 2014
Accepted: January 20, 2014
Published online: April 7, 2014
Processing time: 175 Days and 10.9 Hours
Endoscopy plays a key role in the diagnosis and treatment of patients with inflammatory bowel disease (IBD). Colonoscopy has been traditionally used in the diagnosis of IBD and helps in determination of an important end point in patient management, “mucosal healing”. However, the involvement of an advanced endoscopist has expanded with innovations in therapeutic and newer imaging techniques. Endoscopists are increasingly being involved in the management of anastomotic and small bowel strictures in these patients. The advent of balloon enteroscopy has helped us access areas not deemed possible in the past for dilations. An advanced endoscopist also plays an integral part in managing ileal pouch-anal anastomosis complications including management of pouch strictures and sinuses. The use of rectal endoscopic ultrasound has been expanded for imaging of perianal fistulae in patients with Crohn’s disease and appears much more sensitive than magnetic resonance imaging and exam under anesthesia. Advanced endoscopists also play an integral part in detection of dysplasia by employing advanced imaging techniques. In fact the paradigm for neoplasia surveillance in IBD is rapidly evolving with advancements in endoscopic imaging technology with pancolonic chromoendoscopy becoming the main imaging modality for neoplasia surveillance in IBD patients in most institutions. Advanced endoscopists are also called upon to diagnose primary sclerosing cholangitis (PSC) and also offer options for endoscopic management of strictures through endoscopic retrograde cholangiopancreatography (ERCP). In addition, PSC patients are at increased risk of developing cholangiocarcinoma with a 20% lifetime risk. Brush cytology obtained during ERCP and use of fluorescence in situ hybridization which assesses the presence of chromosomal aneuploidy (abnormality in chromosome number) are established initial diagnostic techniques in the investigation of patients with biliary strictures. Thus advanced endoscopists play an integral part in the management of IBD patients and our article aims to summarize the current evidence which supports this role and calls for developing and training a new breed of interventionalists who specialize in the management of IBD patients and complications specific to those patients.
Core tip: Endoscopy plays a key role in the diagnosis and treatment of patients with inflammatory bowel disease. The involvement of an advanced endoscopist has expanded with innovations in designs of endoscopes and newer imaging techniques. Our article aims to summarize the current evidence which supports the role of an advanced endoscopist in the management of colonic and ileal pouch strictures, biliary strictures in patients with primary sclerosing cholangitis, endoscopic diagnosis of colonic fistulae and surveillance of colon neoplasia and cholangiocarcinoma.
- Citation: Modha K, Navaneethan U. Advanced therapeutic endoscopist and inflammatory bowel disease: Dawn of a new role. World J Gastroenterol 2014; 20(13): 3485-3494
- URL: https://www.wjgnet.com/1007-9327/full/v20/i13/3485.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i13.3485
Crohn’s disease (CD) and ulcerative colitis (UC) are a group of inflammatory bowel diseases (IBD) that have environmental, immunological and bacterial etiologies. The role of an endoscopist has been well defined in the initial diagnosis of these disorders, assessment of disease severity and differentiation between the two disease processes. Previous studies have reported that in 80% of CD patients, at last one surgical resection will be required within 10 years of CD diagnosis[1,2]. Although surgical treatment is effective for CD strictures, there is invariably a high risk of recurrence of CD which may result in repeat surgery in up to 34% of patients[1,3]. Repeated surgery can result in complications related to short bowel syndrome, requirement for total parenteral nutrition and its attendant complications. The advent of endoscopy in the management of complicated CD strictures has changed the approach to the management of anastomotic and small bowel strictures in these patients. The advent of balloon enteroscopy has helped us access areas not deemed possible in the past for dilations. An advanced endoscopist also plays an integral part in managing ileal pouch-anal anastomosis (IPAA) complications including management of pouch strictures and sinuses. Colorectal cancer (CRC) is a serious potential complication of IBD. Advanced endoscopists play an important role in detection of dysplasia by employing advanced imaging techniques to identify early and subtle neoplastic lesions[4].
In addition, primary sclerosing cholangitis (PSC) a chronic, cholestatic disorder is seen in 2.4%-7.5% of patients with UC[5] and about 3.4% of patients with CD[6]. Advanced endoscopists are called upon to diagnose PSC and also offer options for endoscopic management of strictures through endoscopic retrograde cholangiopancreatography (ERCP). In addition, PSC patients are at increased risk of developing cholangiocarcinoma (CCA) with a 20% lifetime risk[7,8]. Brush cytology obtained during ERCP and use of fluorescence in situ hybridization (FISH) which assesses the presence of abnormality in chromosome number are established initial diagnostic techniques in the investigation of patients with biliary strictures[9]. The aim of our review is to highlight the current evidence which supports the role of an advanced endoscopist in the management of IBD and complications specific to them.
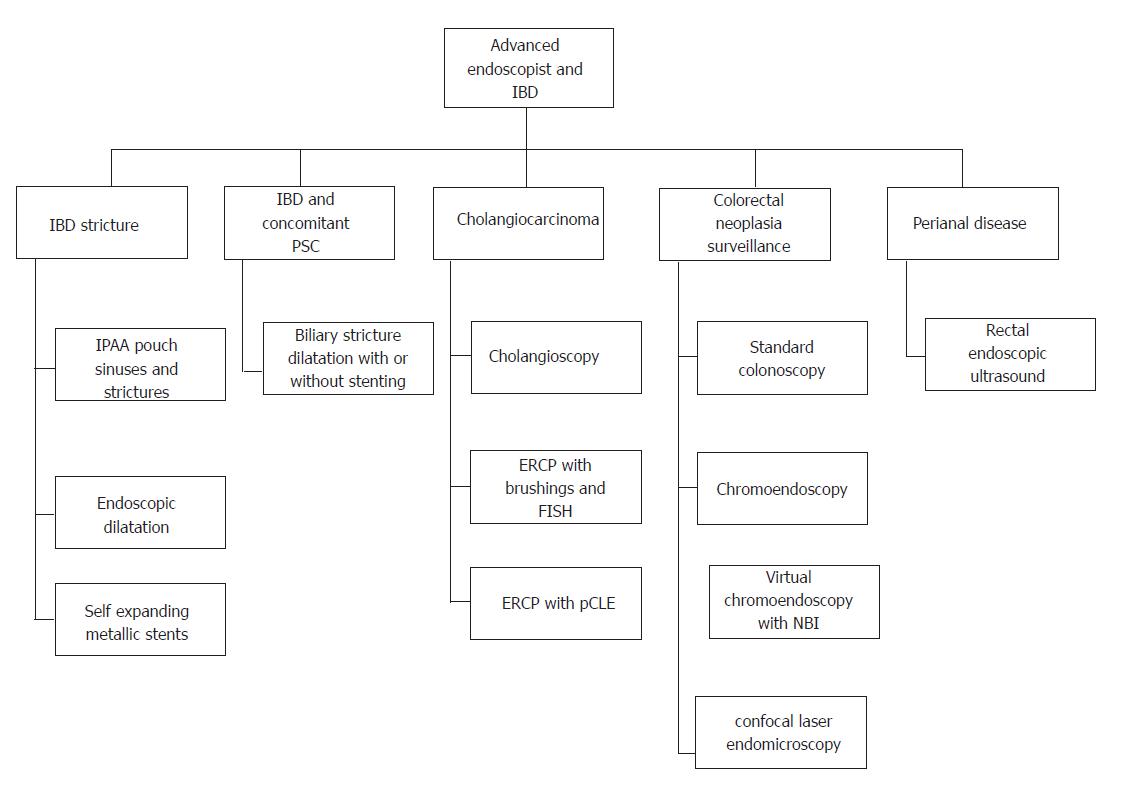
For the purpose of this article, we will discuss the role of an advanced endoscopist under various sections. Figure 1 illustrates the various roles of an advanced endoscopist in managing inflammatory bowel disease patient.
The Vienna Classification describes three distinct groups of CD: inflammatory, stricturing and penetrating. It also demonstrated an association between location and disease behavior. Stricturing disease is predominant in the terminal ileum and ileocolonic locations[10]. Subsequent studies using this classification have confirmed that most patients over the course of time will develop a penetrating or stricturing complication with stricture formation being the second most common and that this change is governed by location of disease[11,12]. Data from the TREAT (the Crohn’s therapy, resource, evaluation, and assessment tool) registry suggested factors associated with stricture formation. These were CD severity at the time of event onset (HR = 2.35, 95%CI: 1.35-4.09); CD duration (HR = 1.02, 95%CI: 1.00-1.04); ileal disease (HR = 1.56, 95%CI: 1.04-2.36); and new corticosteroid use (HR = 2.85, 95%CI: 1.23-6.57)[13].
Strictures in CD can occur de novo, at sites of bowel anastomosis or in the ileal pouch. Strictures are believed to be either inflammatory or fibrotic[13]. Inflammatory strictures have the option of being treated by medical therapy. Even then, when these patients are followed, 64% of these patients require surgery. CD patients with ileocolonic disease do worse with medical therapy (P = 0.026) and may require surgery sooner than patients with ileal disease (P = 0.023)[14]. The fibrotic strictures are largely treated surgically with either intestinal resection or strictureplasty[15]. Although the latter has the advantage of preserving bowel length, it has still been associated with a significant operative recurrence rate of 34% during a median follow up period of 7.5 years[16]. These results seem comparable to those from other review studies[17,18], one of them a meta-analysis[17], in which surgical recurrence rates have been cited as 24% (median surgical rate at 46 mo) and 23%. Younger patients tend to run an aggressive course with a shorter duration to reoperation[16]. Moreover, an 82% rate of second operation has been reported with upto 33% patients requiring more than 2 surgeries leading to risk of developing short bowel syndrome[19]. This means that avoidance of repeated surgeries is an important factor in considering alternative therapies. Endoscopic balloon dilation has been one such treatment alternative. Additionally, endoscopic balloon dilatation could be considered an adjunct to surgery given that it has been shown to add at least 50% efficacy to the initial surgery by prolonging the surgery free period. This was deduced after comparing interval of 6 years from surgery to first endoscopic dilation with the post dilatation surgery free period of 3 years[20]. It also has the advantages of reduced invasiveness and bowel preservation.
There have been several studies aimed at reporting the clinical efficacy, technical feasibility and short and long term results of endoscopic balloon dilatation. Almost all studies have used resolution of symptoms and/or surgery free period as outcomes. A systemic review summarized the results of important studies[20]. Most studies included had less than 60 patients. Most of them included postsurgical strictures. The meta-analysis clearly highlighted the drawbacks with making clear conclusions from the literature because of varying caliber of endoscopic balloons used for dilation from 18 to 25 mm, different approaches that were used, number of dilatations in the same endoscopic sessions and heterogeneity concerning the duration of each single dilatation[20]. Overall, technical success was achieved in 86% patients. The complication rates were < 5% in all studies barring two which reported a higher complication rate[21,22]. The reasons for this have been unclear, aggressive dilatation in one of them[21] may have been a contributing factor. The only factor that significantly affected dilatation efficacy and surgery-free follow up was length of stricture. Naïve vs postsurgical, steroid injection, active vs inactive CD were all deemed to be non significant[20]. Importantly, endoscopic dilatation was successful in avoiding surgery at the end of the follow-up in 112 of the total 347 (67%) patients that were included in this review[20]. If the patients who had failed for technical reasons were excluded, the success rate measured by avoidance of surgery was up to 78%[20].
Subsequent to the meta-analysis, more studies have been published on the efficacy of endoscopic dilation and have shown similar short (51%-89%) and long (52%-89%) term success[23-28]. Most strictures included in these studies have been anastomotic with the exception of the Mueller et al[26] study, in which 69% had de novo strictures. The study by Gustavsson et al[27] is the largest study to date, including a total of 178 patients, and the one with the longest follow-up period (median 12 years). Most patients had either ileal or ileocolonic disease, and approximately 40% had stricturing disease at presentation. Most of the patients (80%) had anastomotic strictures. There was no difference in efficacy based on the etiology of their strictures whether anastomotic or de novo. Bowel perforation occurred in 1.4% of patients and use of 25 mm balloon was associated with a 9.3% complication rate, as compared to 3.5% for the other sizes (P < 0.01).
Another prospective single center study from Germany of 55 patients, with 74 symptomatic strictures reported their efficacy[26]. Majority of patients in this study (69%) had de novo strictures. There was a 95% initial success rate, and 76% patients never required repeat treatment over the period of follow-up. 24% patients did eventually receive surgery over the follow-up period. Stricture length was the main factor that predicted the need for surgery. Interestingly, stricture location at ileocecal valve and stricture associated with fistula were significant predictors of a negative outcome[28]. Smoking has been reported to be a significant patient variable that negatively affects surgery free period[29,30] and doubles the risk of recurrent stricture formation requiring a new dilatation after the first one (P = 0.022)[31].
Given the high rate of stricture recurrence after dilation, intralesional injection of medications after dilation has been studied. A pilot study comparing intralesional steroid injection after balloon dilatation vs placebo did not find a reduction in time to redilatation[32]. This is in contrast to a pediatric study of 29 patients that reported a significant trend of patients who did not receive intralesional steroids towards redilation and surgery[33]. Effect of intralesional injection of infliximab has been studied in small number of patients but consensus is lacking. Thienpont et al[34] reported no significant effect of active disease at the time of dilatation or systemic medical therapy afterwards on redilatation or surgery. Thus, there is no clear evidence at this time to support the role of intralesional injection of medications following dilation.
We routinely perform endoscopic dilation with a 16-, 17-, and 18-mm through the scope (TTS) balloon (Boston Scientific, Inc, Boston, MA) or a 18-, 19-, and 20-mm TTS balloon with guidewire assistance. If possible, retrograde dilation with passage of the endoscope beyond the stricture and introduction of the balloon and pulling the endoscope backward and dilating the stricture is preferred.
Metal stents have been reported as an effective alternative to surgery for the palliation of patients with colorectal neoplastic obstruction. Data regarding their use in treating benign naïve or postsurgical strictures in CD is limited and controversial. Recent studies that have used self expanding metallic[35,36] and biodegradable[37-39] stents have reported a high incidence of migration[37,39]. On the other hand clinical success has ranged from 45% to 80%. The majority of stents in these studies were placed in postsurgical strictures.
Overall, efficacy of endoscopic dilatation in the treatment of small and large bowel strictures is promising with an acceptable rate of complications. Length of stricture and location of stricture are important considerations. We have not employed stenting in the management of IBD related strictures. We prefer the use of needle-knife for strictures which are refractory to balloon dilation for management.
The role of an advanced endoscopist in managing IPAA complications mainly caters to strictures and sinuses. IPAA strictures can occur at the pouch inlet, outlet, afferent limb or pouch body. An 11-year-experience with 1005 patients after restorative proctocolectomy and IPAA reported stricture formation in 14% of patients of which the majority (97.9%) were successfully treated with digital or bougie dilatation and only 2.1% required surgery[40]. Another large retrospective study with 1884 patient reported a similar incidence (11.2%) of stricture formation but a much higher (12%) rate of surgery to salvage pouch function. Dilatation of non fibrotic strictures was more successful than fibrotic strictures (P = 0.0001)[41]. A more recent study in which a cumulative of 646 strictures were dilated reported that 87.3% patients over a median follow up of 9.6 years were able to retain their pouches. It concurred that endoscopic dilatation of pouch strictures is efficacious and safe with a low rate of complications when attempted by an experienced endoscopist[42].
Pouch sinus is typically a late presentation of an initial anastomotic leak. The most common location of a pouch sinus is the pouch-anal anastomotic site at the presacral space. Presenting symptoms include perianal pain, pelvic pressure/discomfort and/or evidence of pelvic sepsis or pouchitis, CD of the pouch, or refractory cuffitis; others may be asymptomatic. Sinus opening and sinus tract can be detected by a combined application of pouchoscopy, contrast pouchogram, examination under anesthesia and pelvic magnetic resonance imaging (MRI). Treatment usually includes incision and drainage of the chronically infected superficial sinuses[43]. Fibrin glue injection of the sinus may be attempted[44]. Patients with a long sinus track who fail to heal are suitable for redo pouch.
An alternative strategy that has only recently been introduced and studied at our institution is endoscopic needle knife therapy for pouch sinuses. A total of 65 patients with pouch sinuses were treated with needle-knife therapy of which 84.6% achieved a complete or partial response. Duration from colectomy to needle knife treatment and complex nature of sinuses were inversely proportional to healing of sinuses[45].
Approximately, 25% of all patients with CD develop a perianal fistula, with fistulae more frequent in patients with involvement of the rectum[46,47]. Identification of fistulae is difficult with digital rectal examination alone or even with exam under anesthesia (EUA) because perianal disease is associated with induration and scarring. Endoscopic ultrasonography (EUS) has been used in this setting to help in the evaluation of CD fistulae.
Rectal EUS is performed by introducing a radial probe into the distal rectum and anal canal while the patient is in the left lateral position[48]. On EUS, the fistulae in the setting of CD appear as hypoechoic structures, but the presence of air or gas in the fistula tract may make the tract internally hyperechoic[48]. An abscess in the setting of CD fistulae can also be visualized as an anechoic or hypoechoic mass in the perianal region[48]. Some investigators have used hydrogen peroxide injection into the cutaneous fistula site to enhance visualization as it creates bubbles that appear hyperechoic and thus make the fistula easier to identify.
The accuracy of rectal EUS in the evaluation of perianal disease has been demonstrated in 3 prospective, blinded studies[49-51]. One of these studies compared EUS to computerized tomography (CT) in 25 patients with suspected perianal CD[49]. Confirmation of fistulae was done at the time of surgery. EUS was found to be more accurate than CT in the evaluation of perianal fistulae (82% vs 24%)[49]. In another study, rectal EUS was compared with pelvic MRI and EUA in 22 patients with CD and perianal fistulae. Rectal EUS was found to be the most sensitive modality for imaging (82%) when compared with pelvic MRI (50%)[50]. Rectal EUS in this study was performed with only a 7-MHz linear scanning probe. Another study comparing EUS to MRI found both to be equally accurate in the assessment of CD perianal fistulae (91% vs 87%)[51].
Studies have also used EUS to monitor fistula healing and/or guide treatment. A small, randomized, prospective study showed a benefit to the use of EUS monitoring for fistula healing[52]. Thus, it seems that a team of colorectal surgeons and advanced endoscopists seem to be the future in the evaluation and management of perianal CD.
PSC is a chronic, cholestatic liver disease characterized by inflammation and fibrosis of both intrahepatic and extrahepatic bile duct leading to the formation of bile duct strictures. An advanced endoscopist is integral in diagnosing PSC. Although magnetic resonance cholangiopancreatography (MRCP) is the initial preferred non-invasive technique to diagnose PSC, often ERCP is required to confirm diagnosis and rule out a dominant stricture. An advanced endoscopist is often called upon to manage patients with IBD and PSC who have concomitant dominant biliary stricture in the setting of PSC.
A “dominant stricture” has been defined as a stenosis with a diameter of 1.5 mm in the common bile duct or of 1 mm in the hepatic duct[53,54]. It is a frequent finding and occurs in 36%-57% of patients during follow up[55]. It should always raise the suspicion of the presence of a CCA. The stricturing process may cause extrahepatic biliary obstruction leading to development of symptoms. Patient with jaundice, pruritis, right upper quadrant pain and abnormal biochemical studies have been deemed appropriate candidates for therapy. The goal of therapy is to relieve biliary obstruction.
The optimal non surgical management of these dominant strictures is still debatable. Endoscopic balloon dilatation both with and without stenting has been studied. Long term stent therapy (3 mo) has been shown to have a high rate (close to 50%) of complications of cholangitis/jaundice attributed to stent occlusion[56]. However, short term stenting (11 d) was associated with a lower rate (7%) of these complications, at the same time producing significant effects in symptom reduction and biochemical resolution of cholestasis. Additionally, 81% remained asymptomatic over a 19 mo follow up period and none of the patients had recurrence of clinical/biochemical cholestasis[57]. Another study reported similar positive results after short term stent therapy with significant effects on resolution of symptoms and biochemical cholestasis with 80% of patients remaining re-intervention free at the end of 1 year[58]. A randomized trial comparing balloon dilatation and stenting has not been performed. However, a retrospective study that compared at endoscopic dilatation with dilatation + stenting reported an increased number of complications and incidence of cholangitis in the group that received both without significant difference in improvement of cholestasis[59]. It has been suggested that the group that received both interventions may have been sicker as stenting was done only when dilatation alone did not improve biliary drainage[60]. Moreover, half of the stents were placed percutaneously and the authors reported a significantly higher rate of complications with percutaneous placement of stents as compared to the endoscopic approach. One study aimed at assessing a survival benefit of endoscopic treatment of strictures reported a 5 years survival that was significantly (P = 0.027) higher over that predicted by the Mayo risk score[61]. Indirect evidence from other studies has supported this finding[62,63].
Overall, endoscopic therapy of strictures has been has been proven to be a safe and efficacious mode of treating primary sclerosing cholangitis associated strictures that helps in amelioration of symptoms and cholestasis with a low rate of complications. If stenting is considered, short term therapy is deemed best.
Patients with PSC are at risk of developing CCA. The risk after 10 years and 20 years is 9% and 19% respectively[64]. Patients with deterioration in functional status, worsening liver functions and/or weight loss should be evaluated for CCA. The distinction between a benign dominant stricture and CCA, however, has remained a challenge.
A cut off value of CA19-9 (cancer antigen 19-9) of > 130 U/mL in symptomatic patients has a sensitivity and specificity of 79% and 98% respectively[65]. Its value as a screening tool in asymptomatic patients remains to be defined. CCA often presents in its advanced stage when identification of a mass lesion makes a diagnosis of CCA very likely. In early stages, however, diagnosis is difficult. A study that followed 230 patients over 6 years reported sensitivity of ultrasound, CT, MRI as 57%, 75% and 63% respectively when imaging alone was considered. The positive predictive value of ERCP, MRCP and MRCP + MRI was 23%, 21% and 23 % respectively[66].
Bile duct brushings are the most commonly used method for tissue sampling during ERCP are now routinely obtained at the time of ERCP[7-9]. The vast majority of extrahepatic CCA are periductal, cancers, and do not demonstrate mass lesions on imaging studies. Brush cytology obtained during ERCP is the usual diagnostic technique in the investigation of patients with biliary strictures. However the sensitivity of brushings is low for distinguishing benign strictures and CCA with a 43% sensitivity and a diagnostic accuracy of 50%-60%[7-9].
At the time of ERCP brushings of stricture for cytology, another set of brushings for FISH are obtained in our institution. FISH probes are used to target the centromeric regions of chromosomes 3, 7 and 17 and the 9p21 band (p16). Some studies have considered positive FISH based on polysomy only, while some have considered trisomy or tetrasomy as positive too. In a recent meta-analysis from our group, we pooled all the available evidence in order to better define the utility of FISH for detection of CCA. Our unpublished observations show that any FISH positivity has a pooled sensitivity and specificity of 68% (95%CI: 61%-74%) and 70% (95%CI: 66%-73%) respectively. Thus both brushings for cytology and FISH obtained at the time of ERCP contribute significantly in diagnosing CCA in PSC patients[9].
Recent studies have demonstrated promising results of cholangioscopy in the diagnosis of CCA. Tischendorf et al[67] prospectively studied 53 patients out of which 12 patients were found to have CCA based on tissue sampling. Patients underwent cholangioscopy in addition to ERCP, cholangioscopy was found to have higher sensitivity and significantly higher specificity, positive predictive value and negative predictive value than endoscopic retrograde cholangiography. A single center prospective study involving 36 patients and using peroral cholangioscopy and biopsy reported an overall accuracy of 89% in differentiating benign from malignant stenoses[68].
ERCP with probe-based confocal laser endomicroscopy is an emerging technology that enables high resolution assessment of gastrointestinal mucosal histology thereby allowing examination of “optical biopsies” and exponentially expanding the scope of imaging capabilities. The examination is done in vivo and images are displayed in real time. A single center small case series of 15 patients and 21 dominant stenoses reported 100% sensitivity and 100% negative predictive value in excluding neoplasia[69]. It concluded that if verified in large prospective studies, this technology could be used to risk stratify strictures in patients with PSC. Also, chromoendoscopy using methylene blue was studied for the first time in staining tissue of the bile duct[70]. It helped indentify normal, dysplastic and inflamed mucosa of the biliary tract as was subsequently proven by follow up or histology. A homogenous staining was suggestive normal tissue, absence of it on circumscribed lesions or diffuse staining predicted neoplastic or inflamed tissue[70].
We routinely send 2 sets of brushes with PSC-related dominant strictures; one for routine cytology and the other for FISH analysis. Thus an advanced endoscopist forms an integral part of the team managing IBD patients with associated PSC and its related complications and surveillance of these patients for CCA.
Patients with IBD are at increased risk of CRC. Incidence of cancer has been more extensively studied in relation to UC than Crohn’s. The risk in UC is increased with the duration and extent of disease. A meta-analysis reported the risk is 2% at 10 years, 8% at 20 years, and 18% at 30 years of disease[71]. Patients with extensive colitis are at increased risk as compared to those with left sided colitis. Compared to age-matched controls, the risk begins to increase about 8-10 years after onset of symptoms. Patients with PSC are also more predisposed to developing CRC. Earlier studies on the risk of CRC with CD have been inconclusive. It is now believed that risk of developing CRC in UC and CD is nearly identical[72].
There have not been randomized controlled trials proving the effectiveness of surveillance colonoscopy. There have been three case series that have studied this[73-75]. A Cochrane analysis of these studies concluded that there was no clear evidence that surveillance improves survival. There is evidence that cancer tends to be detected earlier in patients who undergo surveillance and they are likely to have a better prognosis although lead time bias may affect this apparent benefit. It stated that there may be indirect evidence that surveillance is effective at reducing death associated with IBD and that it may be acceptably cost-effective[76].
Interval colonoscopies with random biopsies of abnormally appearing mucosa and targeted biopsies of suspicious lesions have been recommended. Newer methods aimed at detecting abnormal/dysplastic mucosa have been studied to help make this more effective. An advanced endoscopist with experience in complex mucosal imaging would play an important role in the surveillance of IBD patients for CRC. Newer endoscopic imaging modalities including high-definition endoscopy, chromoendoscopy, virtual chromoendoscopy and confocal laser endomicroscopy have the potential to significantly improve the detection and characterization of flat and subtle dysplasia, thereby setting the stage for a system of targeted neoplasia detection without random biopsies in IBD patients.
Chromoendoscopy is a dye-spraying technique that highlights the borders and surface architecture of neoplastic lesions, thereby unmasking and delineating subtle lesions and aiding in the differentiation of neoplastic and non-neoplastic tissue[76]. Chromoendoscopy has been compared to standard-definition endoscopy for detection of neoplasia in both IBD and non-IBD patients and shown to be superior[77]. However, careful cleansing and inspection of the entire colonic mucosal surface needs to be done to detect neoplasia[76]. Importantly, the use of chromoendoscopy does not increase the mean procedure time and has been shown to be no better than white-light endoscopy with random and targeted biopsies[78,79]. Chromoendoscopy described above using methylene blue has been studied in UC patients. The study included 102 patients, each of who had biopsies by different 3 techniques: standard colonoscopy with random biopsies, targeted biopsies and dye targeted biopsies in which methylene blue was segmentally applied throughout colon and abnormal mucosa that was made visible by dye spray was then biopsied. It concluded that dye targeted biopsies detected more dysplasia than random biopsies (P = 0.001) and more than targeted non dye biopsies. (P = 0.057)[78]. The disadvantages of this technique include the cost, time consuming nature and the fact the dye does not always coat the mucosa evenly and does not allow for a detailed analysis of the subepithelial network.
Virtual chromoendoscopy technologies have also been employed including narrowband imaging (NBI; Olympus, Tokyo, Japan), i-scan (Pentax, Tokyo, Japan), Fuji Intelligent Chromo Endoscopy (FICE; Fujinon, Tokyo, Japan) with use of selective light filters or post-image processing techniques to improve visualization of vascular structures[80]. A recent randomized parallel group study with 112 patients randomized to get colonoscopy using either NBI or white lights reported no significant difference in detection of dysplasia[81]. Another study involving 60 patients concluded that NBI was a less time consuming and equally efficacious method compared to chromoendoscopy to detect intraepithelial neoplasia in long standing IBD patients but had a high lesion and patient miss rate and cannot be recommended as a standard technique[82].
Confocal laser endomicroscopy is another advanced imaging technique that permits direct histologic assessment of mucosa at the cellular and subcellular levels in vivo[83]. The potential application of technology in IBD patients would be in combination with white-light endoscopy and chromoendoscopy to more accurately predict lesion histology and thus aid in decision making regarding lesion resectability in the setting of chronic colitis[84].
Endoscopic mucosal resection (EMR) is being used for management of specific raised lesions in chronic UC patients getting surveillance colonoscopy for dysplasia. In a study, EMR was used to remove 79 flat Paris 0-II lesions with a recurrence rate of 2.4% at 3 mo with no additional lesions detected in a 4-year follow-up period[85]. Thus management of dysplasia is emerging as a new intervention to develop in the area of IBD.
Even though considerable research has been done in the area of interventional IBD, future research is needed for better imaging techniques in the diagnosis of CCA and role of cholangioscopy in the management of PSC strictures. In addition, randomized controlled trials aimed at studying endoscopic balloon dilations and stenting in treating bowel strictures in the setting of IBD and comparing its efficacy with surgery are required.
The above review underlines the fundamental role of an advanced endoscopist in a team of IBD specialists, colorectal surgeons and hepatologists that manages patients with IBD. Further research and studies will better define the scope of the advanced endoscopist in the coming years.
P- Reviewers: Rerknimitr R, Vigsnaes LK S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg. 2000;231:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 475] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 2. | Farmer RG, Whelan G, Fazio VW. Long-term follow-up of patients with Crohn’s disease. Relationship between the clinical pattern and prognosis. Gastroenterology. 1985;88:1818-1825. [PubMed] |
| 3. | Michelassi F, Balestracci T, Chappell R, Block GE. Primary and recurrent Crohn’s disease. Experience with 1379 patients. Ann Surg. 1991;214:230-238; discussion 238-240. [PubMed] |
| 4. | Murthy SK, Kiesslich R. Evolving endoscopic strategies for detection and treatment of neoplastic lesions in inflammatory bowel disease. Gastrointest Endosc. 2013;77:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Fausa O, Schrumpf E, Elgjo K. Relationship of inflammatory bowel disease and primary sclerosing cholangitis. Semin Liver Dis. 1991;11:31-39. [PubMed] |
| 6. | Rasmussen HH, Fallingborg JF, Mortensen PB, Vyberg M, Tage-Jensen U, Rasmussen SN. Hepatobiliary dysfunction and primary sclerosing cholangitis in patients with Crohn’s disease. Scand J Gastroenterol. 1997;32:604-610. [PubMed] |
| 7. | Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A single center study. Am J Gastroenterol. 2007;102:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 271] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 8. | Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 833] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 9. | Trikudanathan G, Navaneethan U, Njei B, Vargo JJ, Parsi MA. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2013;Epub ahead of print. [PubMed] |
| 10. | Gasche C, Scholmerich J, Brynskov J, D’Haens G, Hanauer SB, Irvine EJ, Jewell DP, Rachmilewitz D, Sachar DB, Sandborn WJ. A simple classification of Crohn’s disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 124] [Reference Citation Analysis (0)] |
| 11. | Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 981] [Cited by in RCA: 979] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 12. | Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 705] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 13. | Lichtenstein GR, Olson A, Travers S, Diamond RH, Chen DM, Pritchard ML, Feagan BG, Cohen RD, Salzberg BA, Hanauer SB. Factors associated with the development of intestinal strictures or obstructions in patients with Crohn’s disease. Am J Gastroenterol. 2006;101:1030-1038. [PubMed] |
| 14. | Samimi R, Flasar MH, Kavic S, Tracy K, Cross RK. Outcome of medical treatment of stricturing and penetrating Crohn’s disease: a retrospective study. Inflamm Bowel Dis. 2010;16:1187-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Vrabie R, Irwin GL, Friedel D. Endoscopic management of inflammatory bowel disease strictures. World J Gastrointest Endosc. 2012;4:500-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Dietz DW, Laureti S, Strong SA, Hull TL, Church J, Remzi FH, Lavery IC, Fazio VW. Safety and longterm efficacy of strictureplasty in 314 patients with obstructing small bowel Crohn’s disease. J Am Coll Surg. 2001;192:330-337; discussion 337-338. [PubMed] |
| 17. | Yamamoto T, Fazio VW, Tekkis PP. Safety and efficacy of strictureplasty for Crohn’s disease: a systematic review and meta-analysis. Dis Colon Rectum. 2007;50:1968-1986. [PubMed] |
| 18. | Wibmer AG, Kroesen AJ, Gröne J, Buhr HJ, Ritz JP. Comparison of strictureplasty and endoscopic balloon dilatation for stricturing Crohn’s disease--review of the literature. Int J Colorectal Dis. 2010;25:1149-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Krupnick AS, Morris JB. The long-term results of resection and multiple resections in Crohn’s disease. Semin Gastrointest Dis. 2000;11:41-51. [PubMed] |
| 20. | Hassan C, Zullo A, De Francesco V, Ierardi E, Giustini M, Pitidis A, Taggi F, Winn S, Morini S. Systematic review: Endoscopic dilatation in Crohn’s disease. Aliment Pharmacol Ther. 2007;26:1457-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 197] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Couckuyt H, Gevers AM, Coremans G, Hiele M, Rutgeerts P. Efficacy and safety of hydrostatic balloon dilatation of ileocolonic Crohn’s strictures: a prospective longterm analysis. Gut. 1995;36:577-580. [PubMed] |
| 22. | Singh VV, Draganov P, Valentine J. Efficacy and safety of endoscopic balloon dilation of symptomatic upper and lower gastrointestinal Crohn’s disease strictures. J Clin Gastroenterol. 2005;39:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Stienecker K, Gleichmann D, Neumayer U, Glaser HJ, Tonus C. Long-term results of endoscopic balloon dilatation of lower gastrointestinal tract strictures in Crohn’s disease: a prospective study. World J Gastroenterol. 2009;15:2623-2627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Scimeca D, Mocciaro F, Cottone M, Montalbano LM, D’Amico G, Olivo M, Orlando R, Orlando A. Efficacy and safety of endoscopic balloon dilation of symptomatic intestinal Crohn’s disease strictures. Dig Liver Dis. 2011;43:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Foster EN, Quiros JA, Prindiville TP. Long-term follow-up of the endoscopic treatment of strictures in pediatric and adult patients with inflammatory bowel disease. J Clin Gastroenterol. 2008;42:880-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Mueller T, Rieder B, Bechtner G, Pfeiffer A. The response of Crohn’s strictures to endoscopic balloon dilation. Aliment Pharmacol Ther. 2010;31:634-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Gustavsson A, Magnuson A, Blomberg B, Andersson M, Halfvarson J, Tysk C. Endoscopic dilation is an efficacious and safe treatment of intestinal strictures in Crohn’s disease. Aliment Pharmacol Ther. 2012;36:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | de’Angelis N, Carra MC, Borrelli O, Bizzarri B, Vincenzi F, Fornaroli F, De Caro G, de’Angelis GL. Short- and long-term efficacy of endoscopic balloon dilation in Crohn’s disease strictures. World J Gastroenterol. 2013;19:2660-2667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Sabaté JM, Villarejo J, Bouhnik Y, Allez M, Gornet JM, Vahedi K, Modigliani R, Lémann M. Hydrostatic balloon dilatation of Crohn’s strictures. Aliment Pharmacol Ther. 2003;18:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Hoffmann JC, Heller F, Faiss S, von Lampe B, Kroesen AJ, Wahnschaffe U, Schulzke JD, Zeitz M, Bojarski C. Through the endoscope balloon dilation of ileocolonic strictures: prognostic factors, complications, and effectiveness. Int J Colorectal Dis. 2008;23:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Gustavsson A, Magnuson A, Blomberg B, Andersson M, Halfvarson J, Tysk C. Smoking is a risk factor for recurrence of intestinal stricture after endoscopic dilation in Crohn’s disease. Aliment Pharmacol Ther. 2013;37:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | East JE, Brooker JC, Rutter MD, Saunders BP. A pilot study of intrastricture steroid versus placebo injection after balloon dilatation of Crohn’s strictures. Clin Gastroenterol Hepatol. 2007;5:1065-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Di Nardo G, Oliva S, Passariello M, Pallotta N, Civitelli F, Frediani S, Gualdi G, Gandullia P, Mallardo S, Cucchiara S. Intralesional steroid injection after endoscopic balloon dilation in pediatric Crohn’s disease with stricture: a prospective, randomized, double-blind, controlled trial. Gastrointest Endosc. 2010;72:1201-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 34. | Thienpont C, D’Hoore A, Vermeire S, Demedts I, Bisschops R, Coremans G, Rutgeerts P, Van Assche G. Long-term outcome of endoscopic dilatation in patients with Crohn’s disease is not affected by disease activity or medical therapy. Gut. 2010;59:320-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 35. | Attar A, Maunoury V, Vahedi K, Vernier-Massouille G, Vida S, Bulois P, Colombel JF, Bouhnik Y. Safety and efficacy of extractible self-expandable metal stents in the treatment of Crohn’s disease intestinal strictures: a prospective pilot study. Inflamm Bowel Dis. 2012;18:1849-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Loras C, Pérez-Roldan F, Gornals JB, Barrio J, Igea F, González-Huix F, González-Carro P, Pérez-Miranda M, Espinós JC, Fernández-Bañares F. Endoscopic treatment with self-expanding metal stents for Crohn’s disease strictures. Aliment Pharmacol Ther. 2012;36:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Rejchrt S, Kopacova M, Brozik J, Bures J. Biodegradable stents for the treatment of benign stenoses of the small and large intestines. Endoscopy. 2011;43:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 38. | Pérez Roldán F, González Carro P, Villafáñez García MC, Aoufi Rabih S, Legaz Huidobro ML, Sánchez-Manjavacas Múñoz N, Roncero García-Escribano O, Ynfante Ferrús M, Bernardos Martín E, Ruiz Carrillo F. Usefulness of biodegradable polydioxanone stents in the treatment of postsurgical colorectal strictures and fistulas. Endoscopy. 2012;44:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Repici A, Pagano N, Rando G, Carlino A, Vitetta E, Ferrara E, Strangio G, Zullo A, Hassan C. A retrospective analysis of early and late outcome of biodegradable stent placement in the management of refractory anastomotic colorectal strictures. Surg Endosc. 2013;27:2487-2491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Fazio VW, Ziv Y, Church JM, Oakley JR, Lavery IC, Milsom JW, Schroeder TK. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg. 1995;222:120-127. [PubMed] |
| 41. | Prudhomme M, Dozois RR, Godlewski G, Mathison S, Fabbro-Peray P. Anal canal strictures after ileal pouch-anal anastomosis. Dis Colon Rectum. 2003;46:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Shen B, Lian L, Kiran RP, Queener E, Lavery IC, Fazio VW, Remzi FH. Efficacy and safety of endoscopic treatment of ileal pouch strictures. Inflamm Bowel Dis. 2011;17:2527-2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 43. | Shen B. Diagnosis and management of postoperative ileal pouch disorders. Clin Colon Rectal Surg. 2010;23:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Swain BT, Ellis CN. Fibrin glue treatment of low rectal and pouch-anal anastomotic sinuses. Dis Colon Rectum. 2004;47:253-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Wu XR, Wong RC, Shen B. Endoscopic needle-knife therapy for ileal pouch sinus: a novel approach for the surgical adverse event (with video). Gastrointest Endosc. 2013;78:875-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Hellers G, Bergstrand O, Ewerth S, Holmström B. Occurrence and outcome after primary treatment of anal fistulae in Crohn’s disease. Gut. 1980;21:525-527. [PubMed] |
| 47. | Tang LY, Rawsthorne P, Bernstein CN. Are perineal and luminal fistulas associated in Crohn’s disease? A population-based study. Clin Gastroenterol Hepatol. 2006;4:1130-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | Wise PE, Schwartz DA. The evaluation and treatment of Crohn perianal fistulae: EUA, EUS, MRI, and other imaging modalities. Gastroenterol Clin North Am. 2012;41:379-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Schratter-Sehn AU, Lochs H, Vogelsang H, Schurawitzki H, Herold C, Schratter M. Endoscopic ultrasonography versus computed tomography in the differential diagnosis of perianorectal complications in Crohn’s disease. Endoscopy. 1993;25:582-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Orsoni P, Barthet M, Portier F, Panuel M, Desjeux A, Grimaud JC. Prospective comparison of endosonography, magnetic resonance imaging and surgical findings in anorectal fistula and abscess complicating Crohn’s disease. Br J Surg. 1999;86:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 126] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 51. | Schwartz DA, Wiersema MJ, Dudiak KM, Fletcher JG, Clain JE, Tremaine WJ, Zinsmeister AR, Norton ID, Boardman LA, Devine RM. A comparison of endoscopic ultrasound, magnetic resonance imaging, and exam under anesthesia for evaluation of Crohn’s perianal fistulas. Gastroenterology. 2001;121:1064-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 341] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 52. | Schreiber S, Lawrance IC, Thomsen OØ, Hanauer SB, Bloomfield R, Sandborn WJ. Randomised clinical trial: certolizumab pegol for fistulas in Crohn’s disease - subgroup results from a placebo-controlled study. Aliment Pharmacol Ther. 2011;33:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 53. | Björnsson E, Lindqvist-Ottosson J, Asztely M, Olsson R. Dominant strictures in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2004;99:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 54. | Okolicsanyi L, Fabris L, Viaggi S, Carulli N, Podda M, Ricci G. Primary sclerosing cholangitis: clinical presentation, natural history and prognostic variables: an Italian multicentre study. The Italian PSC Study Group. Eur J Gastroenterol Hepatol. 1996;8:685-691. [PubMed] |
| 55. | Gotthardt D, Stiehl A. Endoscopic retrograde cholangiopancreatography in diagnosis and treatment of primary sclerosing cholangitis. Clin Liver Dis. 2010;14:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | van Milligen de Wit AW, van Bracht J, Rauws EA, Jones EA, Tytgat GN, Huibregtse K. Endoscopic stent therapy for dominant extrahepatic bile duct strictures in primary sclerosing cholangitis. Gastrointest Endosc. 1996;44:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 94] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | van Milligen de Wit AW, Rauws EA, van Bracht J, Mulder CJ, Jones EA, Tytgat GN, Huibregtse K. Lack of complications following short-term stent therapy for extrahepatic bile duct strictures in primary sclerosing cholangitis. Gastrointest Endosc. 1997;46:344-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Ponsioen CY, Lam K, van Milligen de Wit AW, Huibregtse K, Tytgat GN. Four years experience with short term stenting in primary sclerosing cholangitis. Am J Gastroenterol. 1999;94:2403-2407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 59. | Kaya M, Petersen BT, Angulo P, Baron TH, Andrews JC, Gostout CJ, Lindor KD. Balloon dilation compared to stenting of dominant strictures in primary sclerosing cholangitis. Am J Gastroenterol. 2001;96:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 136] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 60. | Michaels A, Levy C. Endoscopic and surgical management of primary sclerosing cholangitis. Medscape J Med. 2008;10:242. [PubMed] |
| 61. | Baluyut AR, Sherman S, Lehman GA, Hoen H, Chalasani N. Impact of endoscopic therapy on the survival of patients with primary sclerosing cholangitis. Gastrointest Endosc. 2001;53:308-312. [PubMed] [DOI] [Full Text] |
| 62. | Gluck M, Cantone NR, Brandabur JJ, Patterson DJ, Bredfeldt JE, Kozarek RA. A twenty-year experience with endoscopic therapy for symptomatic primary sclerosing cholangitis. J Clin Gastroenterol. 2008;42:1032-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 63. | Stiehl A, Rudolph G, Klöters-Plachky P, Sauer P, Walker S. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: outcome after endoscopic treatment. J Hepatol. 2002;36:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 64. | Claessen MM, Vleggaar FP, Tytgat KM, Siersema PD, van Buuren HR. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009;50:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 275] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 65. | Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005;50:1734-1740. [PubMed] |
| 66. | Charatcharoenwitthaya P, Enders FB, Halling KC, Lindor KD. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 267] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 67. | Tischendorf JJ, Krüger M, Trautwein C, Duckstein N, Schneider A, Manns MP, Meier PN. Cholangioscopic characterization of dominant bile duct stenoses in patients with primary sclerosing cholangitis. Endoscopy. 2006;38:665-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 68. | Ramchandani M, Reddy DN, Gupta R, Lakhtakia S, Tandan M, Darisetty S, Sekaran A, Rao GV. Role of single-operator peroral cholangioscopy in the diagnosis of indeterminate biliary lesions: a single-center, prospective study. Gastrointest Endosc. 2011;74:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 69. | Heif M, Yen RD, Shah RJ. ERCP with probe-based confocal laser endomicroscopy for the evaluation of dominant biliary stenoses in primary sclerosing cholangitis patients. Dig Dis Sci. 2013;58:2068-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 70. | Hoffman A, Kiesslich R, Bittinger F, Galle PR, Neurath MF. Methylene blue-aided cholangioscopy in patients with biliary strictures: feasibility and outcome analysis. Endoscopy. 2008;40:563-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 71. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2077] [Article Influence: 86.5] [Reference Citation Analysis (1)] |
| 72. | Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn’s disease with colonic involvement. Lancet. 1990;336:357-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 448] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 73. | Lashner BA, Turner BC, Bostwick DG, Frank PH, Hanauer SB. Dysplasia and cancer complicating strictures in ulcerative colitis. Dig Dis Sci. 1990;35:349-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Karlén P, Kornfeld D, Broström O, Löfberg R, Persson PG, Ekbom A. Is colonoscopic surveillance reducing colorectal cancer mortality in ulcerative colitis? A population based case control study. Gut. 1998;42:711-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 196] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 75. | Choi PM, Nugent FW, Schoetz DJ, Silverman ML, Haggitt RC. Colonoscopic surveillance reduces mortality from colorectal cancer in ulcerative colitis. Gastroenterology. 1993;105:418-424. [PubMed] |
| 76. | Hurlstone DP, Sanders DS, Lobo AJ, McAlindon ME, Cross SS. Indigo carmine-assisted high-magnification chromoscopic colonoscopy for the detection and characterisation of intraepithelial neoplasia in ulcerative colitis: a prospective evaluation. Endoscopy. 2005;37:1186-1192. [PubMed] |
| 77. | Kiesslich R, Fritsch J, Holtmann M, Koehler HH, Stolte M, Kanzler S, Nafe B, Jung M, Galle PR, Neurath MF. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 557] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 78. | Marion JF, Waye JD, Present DH, Israel Y, Bodian C, Harpaz N, Chapman M, Itzkowitz S, Steinlauf AF, Abreu MT. Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol. 2008;103:2342-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 79. | Rutter MD, Saunders BP, Schofield G, Forbes A, Price AB, Talbot IC. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut. 2004;53:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 353] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 80. | Wallace MB, Kiesslich R. Advances in endoscopic imaging of colorectal neoplasia. Gastroenterology. 2010;138:2140-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 81. | Ignjatovic A, East JE, Subramanian V, Suzuki N, Guenther T, Palmer N, Bassett P, Ragunath K, Saunders BP. Narrow band imaging for detection of dysplasia in colitis: a randomized controlled trial. Am J Gastroenterol. 2012;107:885-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 82. | Pellisé M, López-Cerón M, Rodríguez de Miguel C, Jimeno M, Zabalza M, Ricart E, Aceituno M, Fernández-Esparrach G, Ginès A, Sendino O. Narrow-band imaging as an alternative to chromoendoscopy for the detection of dysplasia in long-standing inflammatory bowel disease: a prospective, randomized, crossover study. Gastrointest Endosc. 2011;74:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 83. | Neumann H, Kiesslich R, Wallace MB, Neurath MF. Confocal laser endomicroscopy: technical advances and clinical applications. Gastroenterology. 2010;139:388-392, 392.e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 84. | Kiesslich R, Goetz M, Lammersdorf K, Schneider C, Burg J, Stolte M, Vieth M, Nafe B, Galle PR, Neurath MF. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007;132:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 376] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 85. | Hurlstone DP, Sanders DS, Atkinson R, Hunter MD, McAlindon ME, Lobo AJ, Cross SS, Thomson M. Endoscopic mucosal resection for flat neoplasia in chronic ulcerative colitis: can we change the endoscopic management paradigm? Gut. 2007;56:838-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |