INTRODUCTION
Inflammatory bowel diseases (IBD) are chronic disorders of the intestinal tract characterized by relapsing and remitting intestinal inflammation; Crohn’s disease (CD) and ulcerative colitis (UC) are the best-recognized of these entities. The etiology of IBD is not clear, but it is thought to occur when the intestinal flora induces an exaggerated immune response in the context of genetic predisposition. Most therapeutic regimens focus on immunosupression as the hallmark of treatment with medications including corticosteroids, purine anti-metabolites, and newer biologic agents that target specific molecules of the inflammatory cascade like tumor necrosis factor (TNF). Dramatic advances in the treatment of IBD have been seen with the availability of new drugs and our ability to tailor the treatment strategy for each patient. Drug monitoring is relevant not only when trying to achieve treatment efficacy, but also when trying to mitigate toxic side effects and optimize costs. In medicine, we usually measure levels in drugs with a narrow therapeutic range and that pose significant deleterious side effects with overdosing (e.g., theophylline, cyclosporine, and lithium). With some antibiotics (e.g., vancomycin), we measure levels in order to assure the patient receives the required dose to induce a therapeutic effect. Accordingly, the treatment of IBD has evolved with advances in laboratory medicine. We will review the latest evidence concerning measurement of genomic risk for metabolic side effects and levels of metabolites, drugs and antibodies used in the treatment of IBD, including thiopurines (purine anti-metabolites) and anti tumor necrosis factor (anti-TNF) agents.
THIOPURINE ANALOGS
In IBD patients, thiopurines have been successfully employed for decades. The thiopurine analogs with activity against IBD include mercaptopurine (MP) and its pro-drug azathioprine (AZA). The general consensus is that the thiopurine medications are effective at preventing relapse of quiescent disease, but not at inducing remission[1]. They also exert a beneficial effect when combined with biologic drugs such as infliximab (an anti-TNF agent), improving rates of clinical remission and mucosal healing[2].
These medications antagonize endogenous purines, interfering with the synthesis of DNA. They were first developed to treat leukemia in pediatric patients, but because of their observed anti-proliferative effect on T-cells, they have been successfully used to treat autoimmune disease and to prevent graft rejection after solid organ transplantation. While the exact molecular mechanism by which thiopurines exert their immunosuppressive effect is not well-comprehended, several theories have been proposed. One hypothesis suggests that 6-thioguanine (6-TGN), a metabolite of AZA, accumulates in lymphocytes and blocks the expression of inflammatory-related cytokines, including TNF-related apoptosis-inducing ligand, TNF receptor S7, and alpha-4-integrins, ultimately inhibiting the inflammatory response induced by T-cells in the intestinal lamina propria in patients with IBD[3].
Drug metabolism
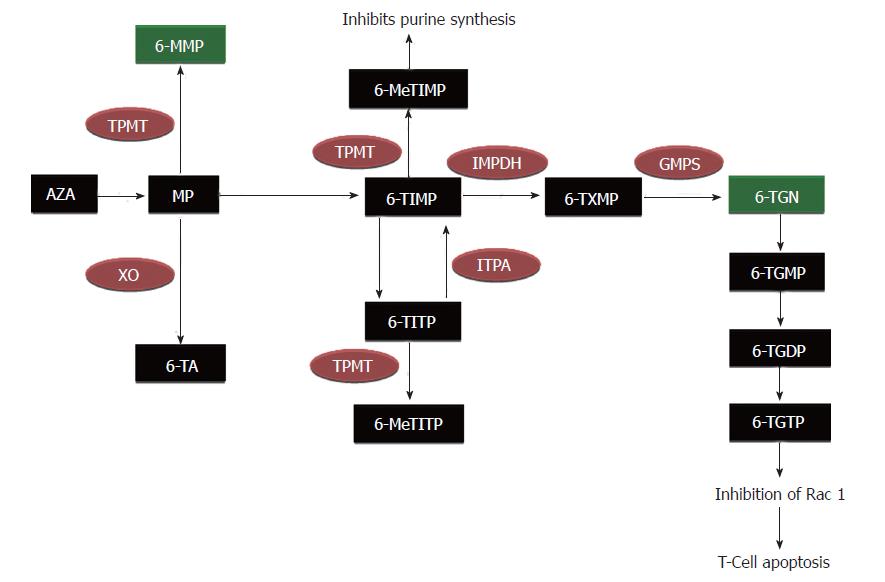
The metabolism of AZA is quite complex, and involves many enzymatic pathways producing active, inactive, and potentially toxic metabolites (Figure 1). After getting absorbed from the gastrointestinal tract, 88% of AZA is converted to MP and methyl-nitro-thioimidazole in red blood cells (RBC)[4]. MP can then be metabolized through three of the following competing pathways: conversion to 6-thiouric acid by xanthine oxidase (XO), methylation by thiopurine methyltransferase (TPMT) into 6-methyl mercaptopurine (6-MMP) (which is associated with potential hepatotoxicity), or conversion to thioinosine monophosphate (TIMP) by hypoxanthine phosphoribosyltransferase (HGPRT)[5]. TIMP is subsequently converted into 6-TGN, which are thought to be desirable therapeutic metabolites of AZA/MP[4,6]. Though 6-TGN is a favorable metabolite, high levels of this agent can result in life-threatening myelosuppression.
Figure 1 Metabolic pathway of azathioprine/mercaptopurine metabolism.
AZA: Azathioprine; MP: Mercaptopurine; TPMT: Thiopurine methyltransferase; XO: Xanthine oxidase; IMPDH: Inosine-5-monophosphate dehydrogenase; GMPS: Guanidine- 5-monophosphate synthetase; 6-TA: 6-Thiouric acid; 6-MMP: 6-Methyl- mercaptopurine; 6-TIMP, 6-Thioinosine monophosphate; 6-MeTIMP: 6-Methylthioinosine monophosphate; 6-MeTITP: 6-Methyl thioinosine triphosphate pyrophosphate; 6-TITP: 6-Thioinosine triphos- phate pyrophosphate; TXMP: Thioxanthosine monophosphate; 6-TGN: 6-Thioguanine nucleotides; 6-TGMP: 6-Thioguanine monophosphate; 6-TGDP: 6-Thioguanine diphosphate; 6-TGTP: 6-Thioguanine triphosphate.
Measuring the thiopurine methyltransferase phenotype/genotype
Thiopurine drugs have a complex metabolism, and some metabolites may cause life-threatening toxicity, including myelosuppression and hepatotoxicity. TPMT catalyzes one of the rate-limiting pathways in AZA/MP metabolism. Because TPMT has variable activity among patients, checking TPMT activity prior to initiation of thiopurine therapy is routinely used in clinical practice and is recommended. TPMT induces the metabolism of MP to 6-MMP, “competing” with the HGPRT and XO enzymatic pathways (Figure 1). TPMT also metabolizes TIMP to 6-methyl-thioinosine monophosphate (6-MeTIMP); thus, low TPMT activity results in greater conversion of MP to 6-TGN, as less TIMP is converted to 6-MeTIMP. Measuring TPMT before prescribing AZA/MP can help predict those patients who will accumulate high levels of 6-TGN relative to 6-MMP, a profile which increases the risk of leukopenia early in the treatment course. The TPMT status of a patient may be identified by requesting the genotype or the phenotype from a commercial laboratory, with the phenotype testing being in general more clinically useful.
TPMT genotype: Studies in different racial and ethnic backgrounds have shown that most of the population (89%) are homozygous for the wild type (WT) TPMT gene (high TPMT metabolism), 10% are heterozygous for the WT and a low metabolic polymorphism (intermediate TPMT metabolism), and 1 in 300 are homozygous for low TPMT metabolic polymorphism (low TPMT metabolism)[7]. While higher 6-TGN levels are associated with a better clinical response, they also increase risk of myelotoxicity with AZA/MP; therefore, determining TPMT phenotype/genotype is currently used to predict early leukopenia[6]. Further research in this area has resulted in the identification of fourteen single nucleotide polymorphisms for the TPMT gene that cause a diminished or absent enzymatic activity.
TPMT phenotype: As with genotype, enzyme activity (or phenotype) may also be measured and sub-divided into three major groups (high, intermediate, and low TPMT metabolizers). The correlation between TPMT genotype and phenotype varies between 65 and 89%[8,9]. The cause of this variance is unclear, but measuring phenotype has a better predictive value for myelosuppression when compared to genotype[8].
Select situations exist where the genotype can theoretically be more reliable than the phenotype. Because TPMT is measured in erythrocytes and uremia may affect the assay, measuring TPMT genotype and not phenotype may be reasonable when a patient has had a recent transfusion of red blood cells or has a high blood urea nitrogen, (usually in patients requiring dialysis)[10]. Also, some medications including azathioprine itself and some diuretics may increase TPMT activity, but the clinical significance of this effect is not clear[11]. Conversely, mesalamines and sulfasalazine inhibit TPMT, theoretically increasing the risk of leukopenia, though this claim is unproven[12].
Monitoring thiopurine metabolites
Once the decision has been made to treat patients and at a particular dose, monitoring thiopurine metabolite levels is a clinical option. Measuring metabolites has two important applications, increasing the likelihood of treatment efficacy and reducing the risk of treatment-related toxicities. The two metabolites that are commercially available are 6-TGN and 6-MMP.
6-TGN has been the metabolite most associated with treatment efficacy; as such, its measurement has been proposed as a strategy to optimize treatment in patients with IBD receiving AZA/MP. 6-TGN is a metabolite of TIMP, which goes through a series of phosphorylation events resulting in 6-thioguanine diphosphate. A 6-TGN level > 230 pmol/8 × 108 RBC has been correlated with clinical remission in both adults and children with IBD[6,13]. Another study using a different assay that included only adult patients failed to show a relation between 6-TGN levels and clinical activity[14].
The need to follow 6-TGN levels during treatment has not been well-established. In a prospective cohort study, Wright et al[15] found that patients on a stable dose of azathioprine present with variable levels of 6-TGN over time, bringing into question the value of interpreting any single 6-TGN level. The difference in outcomes among studies is unclear, but could be related to the heterogeneity in the instrument used to determine IBD activity and the use of different assays to measure the 6-TGN levels. Another added potential use for metabolite measurement is to assess adherence to medical therapy. If both 6-TGN and 6-MMP are low, it is likely the patient is not ingesting or absorbing the medication. Randomized controlled trials looking at the role of serial measurements of thiopurine metabolites and the effect of subsequent dose adjustment on outcomes are needed.
AZA metabolite measurement can also be used to help prevent drug-related toxicity. 6-MMP is a metabolite produced from MP by TPMT. Higher 6-MMP levels have been found to correlate with a higher risk of hepatotoxicity. Even though patients with 6-MMP levels > 5700 pmol/8 × 108 RBC have a three-fold increased risk of hepatotoxicity, not all patients with a high 6-MMP level will develop elevated liver enzymes, and having a low 6-MMP level does not preclude the development of hepatotoxicity[6,16]. As with 6-MMP, some patients with very high 6-TGN levels do not develop myelotoxicity while some with low 6-TGN levels may still develop this abnormality. Thus, measuring 6-TGN and 6-MMP levels do not replace monitoring liver enzymes and blood counts. 6-TGN level measurements can also be useful to identify those patients who will not experience clinical benefit despite an optimal AZA/MP dose. Patients with normal TPMT activity and 6-TGN levels > 400 pmol/8 × 108 RBC who do not achieve clinical remission will likely remain refractory to treatment even if the treatment is continued for 6 more months or the dose is increased[17].
Patients resistant to AZA/MP therapy and the use of allopurinol
Patients who fail therapy with AZA/MP may not always benefit from dose escalation. The metabolic pathways in some patients favor an increased conversion of medication into 6-MMP rather than 6-TGN. Metabolite levels confirm this phenomenon when increasing thiopurine dose in patients who are clinically not responding[18]. This finding supports the metabolic heterogeneity that exists among the IBD population and how some patients preferentially favor particular AZA/MP metabolic pathways. The identification of these patients represents another potential use for metabolite measurement. Up to 24% of patients preferentially produce 6-MMP while maintaining low levels of 6-TGN despite increasing the dose[18]; these patients usually have high TPMT activity, which is genetically determined and are usually labeled as “thiopurine resistant”.
In this setting, one option is to switch to another therapeutic class (methotrexate or anti-TNFs), but manipulation of the known pathways provides an alternative option to optimize therapy in these so-called “shunters”. Inhibition of TPMT could reduce the production of 6-MMP, but drugs with this effect (e.g., mesalamines/sulfasalazine) have failed to reliably optimize the metabolic profile, likely due to their weak inhibitory effect on the enzyme. Another potential target is XO. This enzyme converts MP into 6-thiouric acid, which has no known biologic effect. When this enzyme is inhibited, much of the MP is shunted down the 6-TGN pathway. Allopurinol, a XO-inhibitor used in the treatment of gout, has been successfully used to shunt metabolism of AZA/MP down this more favorable 6-TGN pathway. The mechanism by which this occurs is not completely understood, as the sole inhibition of XO would not explain the rise in 6-TGN. Sparrow et al[19] selected a group of patients who had a preferable metabolism towards 6-MMP and initiated therapy with 100 mg of allopurinol while reducing the AZA/MP dose by 25%-50%. After 2-4 wk on this regimen, they found that the level of 6-TGN had significantly increased while the 6-MMP level had decreased. A subsequent study by the same group looking at clinical response in AZA/MP non-responders when starting allopurinol found that the allopurinol-immunomodulator regimen also improved disease activity[20]. This beneficial effect was not only observed for disease activity, but also reversed hepatotoxicity, presumably by reducing the 6-MMP levels.
However, there are several issues that must be addressed when starting patients on a combination of a thiopurine and allopurinol. First, we recommend serial blood counts, checking a complete blood count (CBC) with differential weekly for the first month and monthly thereafter. Thiopurine metabolites may be checked one month after initiating therapy in order to ensure the goal of increased 6-TGN and decreased 6-MMP is achieved. If leukopenia develops, the dose of AZA/MP can be reduced.
Another proposed strategy to overcome the shunting of MP towards 6-MMP is splitting the thiopurine into twice daily dosing. A retrospective study in patients with preferential 6-MMP metabolism showed that dividing the daily dose of the thiopurine (MP or AZA) modifies how the drug is metabolized, resulting in a significant reduction in 6-MMP levels and decreasing toxicity without having an adverse impact on clinical disease activity or 6-TGN levels[21]. The exact mechanism explaining the beneficial outcomes seen when implementing this strategy is unknown, and further studies are needed to confirm the results.
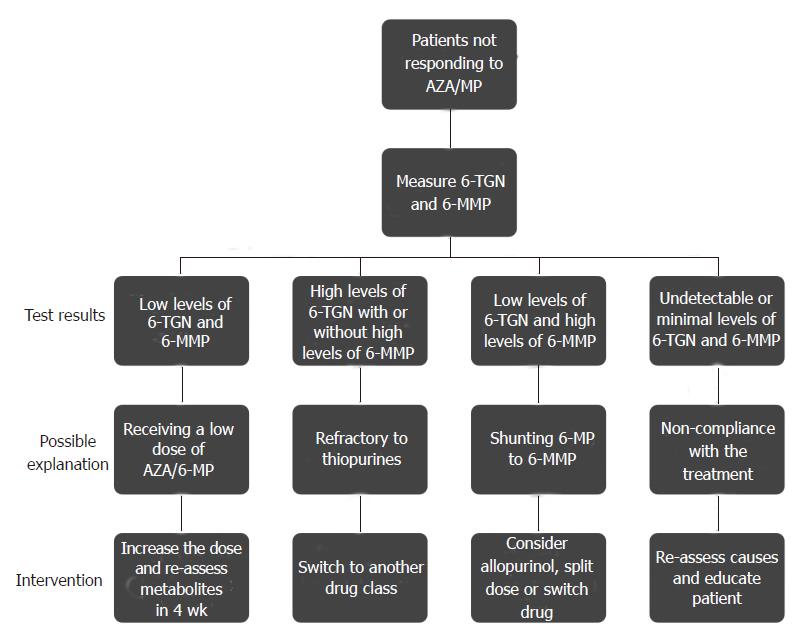
In summary, measuring TPMT activity prior to thiopurine initiation has improved the clinical care for IBD patients, both by improving treatment efficacy and by ameliorating iatrogenic toxicities. Many clinicians, including our group, also follow thiopurine metabolite levels with the intent of positively impacting patient care. The enclosed algorithm represents a logical approach to the care of IBD patients on thiopurine therapy (Figure 2).
Figure 2 Algorithm with recommendation on how to manage patients that fail therapy with Azathioprine/Mercaptopurine.
AZA: Azathioprine; MP: Mercaptopurine; 6-TGN: 6-Thioguanine nucleotides; 6-MMP: 6-Methyl-mercaptopurine.
ANTI-TUMOR NECROSIS FACTOR
Anti-TNF agents have dramatically changed the management of CD and UC. Though four anti-TNF agents are currently approved to treat IBD, we will focus on infliximab (IFX) and adalimumab (ADA), as these are the two anti-TNF drugs with commercially available assays to measure drug levels and anti-drug antibodies. IFX is a chimeric IgG1 (human-constant and murine-variable regions) monoclonal antibody consisting of human constant and murine variable regions; it is indicated for induction and maintenance of clinical remission in patients with moderate-to-severely active CD and UC[22,23]. ADA is a recombinant fully human IgG1 monoclonal antibody, also approved for the induction and maintenance of remission in patients with CD and UC[24-26].
These biologic therapies are of proven benefit, reducing rates of hospitalization and surgery rates while improving quality of life[27,28]. Unfortunately, up to 50% of patients lose response to treatment (secondary non-responders) and up to 30% do not respond at all (primary non-responders)[29]. The rationale for lack or loss of response is multi-factorial, but is likely related to the molecular structures and complex pharmacokinetics (PK) and pharmacodynamics of the medications, including the development of anti-drug antibodies. One of the proposed strategies when patients lose response to an anti-TNF is switching to another drug in the same or different class. Another strategy is to empirically increase the dose, hoping to overcome increased drug clearance. Unfortunately, this can lead to significant adverse events including hypersensitivity reactions. A third potential tactic is to measure drug levels and antibodies, trying to identify those patients who will benefit from dose escalation and those who will be best served by switching to an alternate drug class. This latter strategy has been proven to be more cost effective when compared to the former and highlights the importance and usefulness of measuring levels in these patients[30]. Most of our collective experience has been measuring IFX levels and antibodies to IFX (ATI), but the measurement of ADA and antibodies to ADA (ATA) has recently become available commercially.
PHARMACOKINETICS OF ANTI-TNF
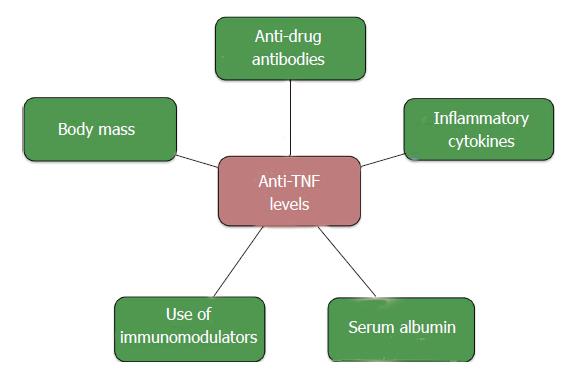
Within an individual patient, a linear relationship exists between anti-TNF dose and serum medication levels[31]. However, inter-patient PK variability complicates the reliable prediction of medication levels among patients[32,33]. Some have theorized that this inconsistency occurs as a result of differences in body mass, but variable levels are observed with drugs with weight-based dosing like IFX. The clearance of TNF-inhibitors likely also plays a role.
Anti-TNFs are monoclonal antibodies with a high molecular weight, and as is the case with other with IgGs, they are metabolized through many pathways. One of the primary mechanisms by which anti-TNFs are cleared involves the reticuloendothelial system (RES). The main component of IgG metabolism is the Brambell receptor (FcRn), which is responsible for internalizing an antibody intra-cellularly, degrading the antigen, and then “recycling” it by releasing it back into the circulation for excretion[31]. High circulating IgG levels may saturate the FcRn, translating into an inverse association between IgG levels and anti-TNF clearance.
Many of the mechanisms underlying variation in drug levels and clearance of TNF-inhibitors are summarized in Figure 3.
Figure 3 Variables associated with Anti-tumor necrosis factor levels.
TNF: Tumor necrosis factor.
Anti-drug antibodies
The human immune system recognizes these biologic drugs as foreign antigens, creating specific antibodies that neutralize their effect. This phenomenon, known as immunogenicity, increases drug clearance and ultimately may contribute to treatment failure. Immunogenicity was first described with IFX, a chimeric antibody. Originally, it was believed that ADA would demonstrate less immunogenicity as it is a fully human antibody, but this theory was not substantiated by clinical practice findings[33,34].
In patients with IBD, the negative effect that antibodies to IFX (ATI) and antibodies to ADA (ATA) have on clinical outcomes has been well established[32,35-38]. ATI and ATA bind to the medication, forming an immune complex that induces drug clearance via the RES. The ability to routinely measure drug levels and anti-drug antibodies has led to an understanding of why some patients lose response to therapy.
Currently, there are several methods to measure IFX and ADA. Most studies have been performed using a solid-phase, double-antigen, enzyme-linked immunosorbent assay (ELISA). Unfortunately, this assay cannot measure the presence of antibodies when there are detectable levels of anti-TNF. Another method is a fluid-phase radioimmunoassay, which can report antibodies irrespective of the presence of drug. A recently developed assay is a homogeneous mobility shift assay using size-exclusion high-performance liquid chromatography, which has a high sensitivity and specificity and can potentially detect all isotypes of immunoglobulin[39]. This modality permits the measurement of antibodies to anti-TNF medications, even in the presence of detectable drug. However, most clinical studies have used the ELISA assay, which could have implications in how we interpret the available data.
Role of immunomodulators with biologic therapy
Observations from randomized controlled trials have shown that the concomitant use of immunomodulators (AZA, MP or methotrexate) and biologic medications increases anti-TNF levels[36]. It is generally believed that this occurs by diminishing immunogenicity (decreasing the formation of anti-TNF antibodies) while simultaneously reducing the amount of systemic inflammation which, as we describe below, will negatively affect IgG levels.
The presence of ATI has consistently been associated with lower IFX levels[36]. In an exploratory analysis of a randomized controlled trial looking at efficacy of IFX monotherapy, AZA monotherapy, and the two biologic drugs combined with immunomodulators, the authors found that at week 30 the IFX levels were 1.6 μg per milliliter for patients in the IFX group and 3.5 μg per milliliter for those in the combination therapy group (P < 0.001)[40]. They also found that only 1 of 116 patients (0.9%) receiving combination therapy (compared to 15 of 103 patients (14.6%) receiving IFX monotherapy) had ATI[40]. Studies looking at the effect of ATA on ADA levels have yielded similar results[33,37].
Even though most studies have shown a clear association between the presence of anti-TNF antibodies and drug levels, some data have failed to support this observation. Lichtenstein et al looked at the influence that concomitant immunomodulators and IFX therapy had on drug levels[41]. They reviewed the ACCENT I and II trials (effect on induction and maintenance of remission that IFX have in CD) as well as ACT 1 and 2 (effect on induction and maintenance of remission that IFX have in UC). In contrast to the previously mentioned studies, they found that IFX levels were similar when comparing patients who did and did not receive immunomodulators, even though those patients that received immunomodulators had a higher incidence of ATI antibodies to infliximab[41]. Of note, this report was based on a post-hoc analysis and the results should be interpreted in that context.
Disease activity and systemic inflammation
Patients with severe disease have been found to have lower levels of anti-TNF. The exact mechanisms by which disease activity and systemic inflammation affect drug clearance are not completely understood. Systemic inflammation induces the RES to increase its catabolic activity, reducing levels of IgG and albumin. Direct and indirect markers of inflammation have been used to predict IFX efficacy. For example, higher levels of CRP correlate with poor response to IFX and may predict loss of response[32,42,43]. Also, low albumin levels are associated with lower IFX levels, which suggests that increased catabolic activity in systemic inflammation induces the Brambell receptor[44]. As with IFX, preliminary studies looking at ADA levels showed that higher CRP levels are associated with lower ADA levels[33,34]. In one study, a minimum ADA cut-point of 5 μg/mL best predicted elevation of CRP levels[33].
These studies cannot prove causation but do show an association between low drug levels, high CRP, and low albumin. Severe inflammation of the mucosa also induces a protein-losing enteropathy, with a loss of drug through the intestinal lumen. A recent study that included patients going through IFX induction therapy found that even though all patients had detectable IFX in the stool, those who did not respond to treatment had a significantly higher level of fecal IFX[45].
Body mass and composition may influence anti-TNF drug levels
Many other variables have been proposed to affect the levels of anti-TNF. One study found that body mass and gender influence the central volume of distribution of IFX (increasing with weight and male gender)[46]. While one might suspect these results were due to the collinearity of gender and mass (as men weigh more than women), the authors tested each variable individually and concluded that each had independent influence on the central volume of distribution; this finding was attributed to the fact plasma volume is lower in women than in men. The influence of body mass on ADA levels in patients with IBD is unknown, but might play a key role as ADA does not have weight-based dosing; this also applies to other anti-TNFs approved for IBD (certolizumab pegol and golimumab).
An important distinction must be made between total body mass and body composition. For example, Bultman et al[47] found that patients on ADA who required dose escalation had a higher body mass index (BMI) despite equivalent dosing of ADA given per kg of body weight. The component responsible for the increased BMI is unclear, but it may be related to mesenteric fat, an important contributor to the inflammatory response in CD[48].
CLINICAL IMPLICATIONS OF MEASURING ANTI-TNF LEVELS AND ANTIBODIES
Many studies have shown that higher IFX levels are associated with better outcomes in patients with IBD. A detectable IFX trough level is associated with a higher rate of clinical remission, lower serum CRP levels, and an improvement in endoscopic disease activity[32,49].
The presence of ATI has been associated with treatment failure and lower IFX levels[36]. In a landmark study, Baert et al[35] studied the immunogenicity of a group of patients with CD. They found that 61% had detectable ATI after the 5th infusion, which did not increase after subsequent infusions. This high prevalence of ATI can be explained by the fact those patients received episodic dosing (when they relapsed) and not scheduled (every 8 wk) infusions. They found that those patients with ATI >8.0 μg/mL before an IFX infusion had a shorter duration of response and a higher risk of infusion reactions[35]. Afif et al[50] looked at the clinical utility of measuring IFX levels and ATI in patients with loss of response or an incomplete response to IFX. They found that most patients with ATI did not respond to IFX dose escalation, but those with no ATIs and sub-therapeutic concentrations did benefit from a higher dose[50].
With ADA, the experience is more limited. In an observational study, Karmiris et al[37] found that lower ADA serum trough levels were associated with drug discontinuation and the presence of ATA was associated with lower ADA levels. Recent studies have found that detectable ATA and ADA levels < 5 μg/mL are associated with higher CRP serum levels, increased endoscopic inflammatory activity, and use of steroids[33,34]. Another recent study measuring ADA trough levels by ELISA assay showed similar results; an ADA trough of 4.85-4.90 μg/mL was the optimal cut-off value for predicting clinical remission and mucosal healing[51].
ROLE OF ANTI-TNF DRUG MONITORING IN CLINICAL PRACTICE
Commercial assays to measure anti-TNF drug and antibody levels have been available for a relatively short time; direct cost implications for patients and/or insurers have limited their widespread clinical use despite documented efficacy in directing patient management. Knowledge of drug levels and measurements of anti-drug antibodies can help identify those patients who will benefit from dose escalation versus those who are unlikely to respond to this strategy (high titers of anti-drug antibodies or those with high drug levels but persistent intestinal inflammation). A recent study modeled both strategies and found that testing for drug levels and antibodies in patients with secondary loss of response is more cost-effective when compared to empiric drug escalation[52].
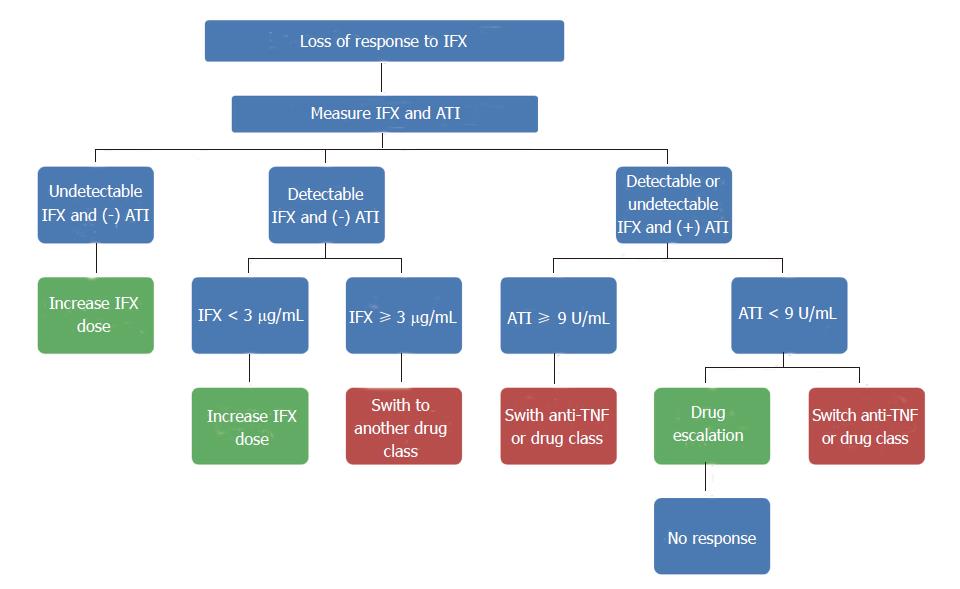
The titer of ATI may also predict response to dose escalation, as demonstrated by a cohort of 90 patients undergoing serial IFX and ATI levels with at least one ATI positive measurement throughout the follow-up period. In 15 of those patients (28%), ATI disappeared overtime[53]. Only 2 of those 15 patients (13%) required discontinuation of therapy, which is quite significant as ATI may be transient and do not necessarily lead to treatment failure. However, they also found that ATI > 7.95 U/mL was associated with IFX discontinuation, which may suggest that patients with high ATI titers would not benefit from dose adjustment[53]. Most of the available evidence includes patients on IFX, so further studies are needed with the other medications. A proposed algorithm to guide therapy in patients receiving IFX who lose response to treatment is shown in Figure 4.
Figure 4 Approaches to patients on infliximab in the setting of lose of response.
ATI: Anti-infliximab antibody; IFX: Infliximab; TNF: Tumor necrosis factor.
An unanswered question is the potential benefit of measuring drug levels in all patients and adjusting the dose accordingly to maintain adequate levels, thereby avoiding immunogenicity. Considering low trough IFX levels are associated with immunogenicity and the fact that the dose of IFX and serum levels are not always linear, measuring drug levels even in patients with clinical response could improve outcomes. The study cited above also showed that an IFX trough level at week 14 of < 2.2 μg/mL correlated with IFX discontinuation[53]. These results are based on a retrospective analysis and future prospective controlled studies are needed, especially considering anti-TNF levels are influenced by several variables that can fluctuate over time and among individuals.
The repeated demonstration of clinical utility and evidence for cost-effective patient care have made routine measurement of anti-TNF drug and antibody levels a regular part of our group’s clinical practice. For UC, when patients tend to have a lower starting albumin level and a markedly elevated CRP, our practice has been to give IFX at a higher dose (10 mg/kg) and then check levels after induction. While this practice has yet to be validated, we have had anecdotal success in achieving remission in patients who we feel clinically merit higher induction doses. Monitoring trough drug levels after induction allows for a tapered return to lower doses and a sustained remission.