Published online Apr 7, 2014. doi: 10.3748/wjg.v20.i13.3418
Revised: September 28, 2013
Accepted: November 28, 2013
Published online: April 7, 2014
Processing time: 226 Days and 13.8 Hours
Hepatitis C virus (HCV) infection affects about 170 million people worldwide and it is a major cause of liver cirrhosis and hepatocellular carcinoma. HCV is a hepatotropic non-cytopathic virus able to persist in a great percentage of infected hosts due to its ability to escape from the immune control. Liver damage and disease progression during HCV infection are driven by both viral and host factors. Specifically, adaptive immune response carries out an essential task in controlling non-cytopathic viruses because of its ability to recognize infected cells and to destroy them by cytopathic mechanisms and to eliminate the virus by non-cytolytic machinery. HCV is able to impair this response by several means such as developing escape mutations in neutralizing antibodies and in T cell receptor viral epitope recognition sites and inducing HCV-specific cytotoxic T cell anergy and deletion. To impair HCV-specific T cell reactivity, HCV affects effector T cell regulation by modulating T helper and Treg response and by impairing the balance between positive and negative co-stimulatory molecules and between pro- and anti-apoptotic proteins. In this review, the role of adaptive immune response in controlling HCV infection and the HCV mechanisms to evade this response are reviewed.
Core tip: In the last few years, the knowledge about the role of adaptive immune response in hepatitis C pathogenesis has increased exponentially. This review summarizes our current understanding of the role of antigen-specific responses in hepatitis C virus (HCV) control and liver damage and discusses recent findings that identify costimulatory molecules modulation, apoptosis induction and chemokine regulation as major HCV mechanisms to evade immune control.
- Citation: Larrubia JR, Moreno-Cubero E, Lokhande MU, García-Garzón S, Lázaro A, Miquel J, Perna C, Sanz-de-Villalobos E. Adaptive immune response during hepatitis C virus infection. World J Gastroenterol 2014; 20(13): 3418-3430
- URL: https://www.wjgnet.com/1007-9327/full/v20/i13/3418.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i13.3418
Hepatitis C virus (HCV) is a hepatotropic non-cytopathic virus which is able to evade immune system efficiently as mechanism to persist in infected hosts. To fight against a viral infection the host displays two kinds of immune responses, the innate and the adaptive immune response. The innate response is the first immunological barrier and it is essential in controlling cytopathic viruses but not enough in non-cytopathic infections. This primary response limits viral spreading but also acts as adaptive response activator through antigen presentation to viral specific cells. Adaptive response is the second line in the immunological defense and it plays a major role in non-cytopathic viral infections due to the ability of this kind of infections to remain occult to the innate system. The current knowledge about the role of the adaptive response role in viral control and pathogenesis during HCV infection will be reviewed in the following paragraphs.
Non-cytopathic viruses have developed evolutionary mechanisms to remain hidden to the immune system, which is an advantage for their persistence. They are usually not highly infectious but produce long-lasting diseases that allow them to spread the infection over time. The host/non-cytopathic-virus relationship is a dynamic process in which the virus tries to decrease its visibility, whereas the host attempts to prevent and eradicate infection with minimal collateral damage to itself[1].
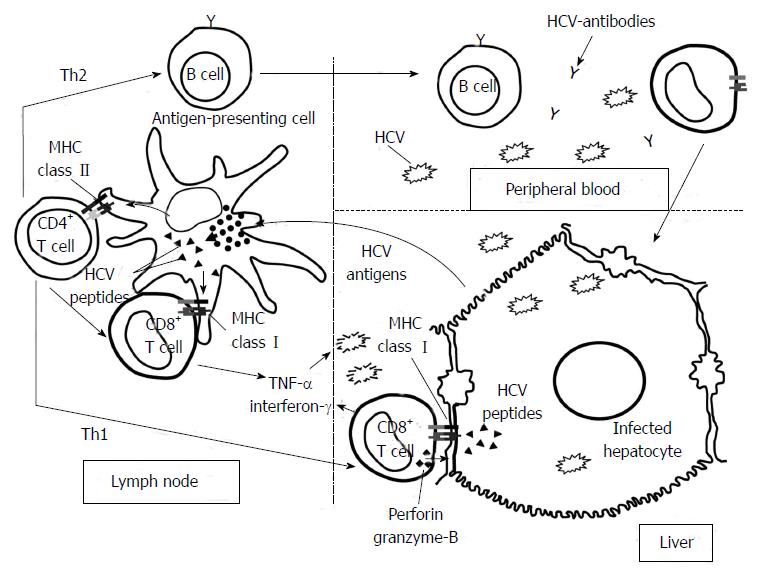
To control non-cytopathic viral infections, the activation of the adaptive immune system, especially the cellular immune response, is necessary (Figure 1). Naïve specific CD4+ and CD8+ T cells are primed by dendritic cells in the lymph nodes. Once these cells become activated, they change their phenotype into effector cells and migrate to the infected tissue attracted by the chemokines produced by the parenchymal cells. Primed specific CD4+ cells are essential to allow the adequate activation of specific cytotoxic T cells by secretion of T helper (Th)-1 cytokines[2]. Subsequently, these specific cytotoxic T lymphocytes (CTL) play a major role in resolution of spontaneous infection because they are able to recognize the infected cells and destroy them by cytolytic mechanisms. On the other hand, they also produce type-1 cytokines that eliminate the virus without tissue damage. Both CD4+ and CD8+ cell activation depends on the engagement between T cell receptor and the Major Histocompatibility Complex (MHC)/epitope complex as well as the interaction between co-stimulatory molecules with their ligands and the adequate cytokine milieu[3]. When these cells have finished their effector task, they express negative co-stimulatory molecules and pro-apoptotic factors to switch-off their activity, and a subsequent constriction in the specific T cell population is produced. After this event, a memory T cell population is maintained for years to come to respond faster to a new infection and in certain cases to keep under control occult viral infection[4].
The definitive barrier to control HCV infection is the adaptive immunity. This response has two arms to fight against pathogens; humoral and cellular immune response. Humoral immune response that means neutralizing and non-neutralizing antibodies (Non-nAbs), can endorse antiviral activity[5]. Cellular immune response shows antiviral immunity by means of virus specific CTLs and CD4 T helper cells, which play key effector and regulatory roles respectively. These T cells take part in viral clearing and pathogenesis of HCV by direct killing of infected cells or producing soluble factors that are able to clear the virus in a non-cytolytic manner, it also can lead to HCV pathogenic events, favoring direct liver damage and attracting non-specific inflammatory cells to perpetuate the liver inflammation[5].
nAbs generally play a critical role for controlling initial viremia and protecting from re-infection in viral infections. However, the role of the humoral immune response in the clearance of HCV infection is a controversial issue and there are still gaps in the knowledge of the interplay between HCV and nAbs. Antibodies generated during acute infection may be targeted against epitopes within structural and non-structural viral proteins; however, the majority of nAb have been mapped to the envelope glycoproteins E1 and E2[6].
HCV can be controlled in patients with hypogammaglobulinemia[7], suggesting this fact as a minor role for nAb in HCV control. In this situation, HCV-specific T cells may compensate for lack of neutralizing antibodies to obtain HCV clearance. Few studies supports the idea that HCV clearance occurs mostly in the absence of nAbs and these Abs alone are inadequate to eradicate HCV[8-12]. therefore, these data also confer a minor role to nAb in HCV control, although other studies propose, at least in some cases, a more important role for nAb in HCV clearing. In fact, there are also proofs supporting a maior role of nAb to neutralize virus infectivity during acute infection. The earliest studies in chimpanzees showed that hyperimmune serum agaist hypervariable region-1 induced protection against homologous HCV infection[13]. Evidence that nAb could protect from natural infection in humans arose form a cohort of women accidentally exposed to the same HCV-strain. In this cohort rapid induction of nAb during the early phase of infection contributed to HCV control[14]. Therefore, rapid induction of nAb early during infection is associated with spontaneous recovery and these antibodies appear to be more cross-neutralizing[15]. Nevertheless, these nAb are not detected in most of acute infections, while delayed appearance of high titer cross reactive nAbs in chronically infected patients suggests that selective mechanisms may operate to prevent the appearance of these Abs during acute infection[11]. Perhaps, the long-term persistence of these nAbs in chronically infected patients may regulate viral replication. In any case, delayed and inefficient neutralizing antibody response during chronic infection induces HCV escape mutations at Abs recognition sites[16], causing the selection of viral mutants.
Moreover, it has been proposed that HCV stimulates B cells in a B cell receptor-independent manner during chronic infection[17] and may favor the development of lymphoproliferative and autoimmune diseases[5]. Immune complexes induced during HCV infection are believed to play a pathogenetic role in the development of manifestations such as cryoglobulinemia, glomerulonephritis, porphyria cutanea tarda, and necrotizing cutaneous vasculitis[18-20].
A strong, multispecific and long-lasting T-cell immune response plays an important role for control of viral infection[8,21]. This efficient response has been observed both in patients as well as experimental models controlling HCV infection[10,12]. Efficiency of antiviral CTL response depends on where these cells are primed. Efficient antiviral CTL response is observed when it is primed in lymphoid organs, whereas within the liver, priming tends to induce T cell inactivation, tolerance or apoptosis[5]. Effector T cells fail to control persistant HCV due to multiple causes, such as: HCV escape mutation at T cell receptor recognition site, immunosuppressive effects exertion, Tregs induction, or T effector exhaustion or deletion[22-24].
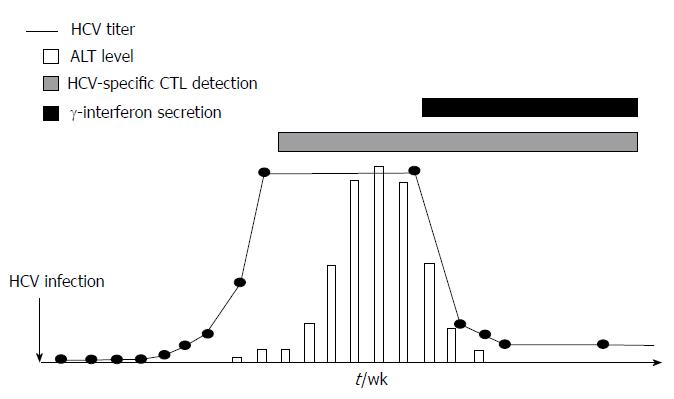
Adaptive cellular response during acute HCV infection: Vigorous CD4+ and CD8+ T cell responses targeting multiple HCV regions with intrahepatic production of interferon (IFN)-γ emerge in acute hepatitis C infection[10,24-26]. The appearance of HCV-specific T cells can be detectable in the peripheral blood or in the liver compartment several weeks after infection in humans or in experimental chimpanzee models[8,27], in association to primary peak of transaminases. Moreover, HCV titer is reduced when these cells start producing IFN-γ and correlates with transaminases level decrease[27] (Figure 2).
The protective function of CD4+ T cells appears to be due to the production of antiviral cytokines, but also due to their helping nature to antiviral B and CD8+ T cells. The HCV clearance has been observed and correlated with vigorous proliferation of specific CD4+ T cells[28,29] with concurrent interleukin (IL)-2 and IFN-γ production[30,31]. The early, sustained development of CD4+ T cell response needs to be successful for viral clearance[31], whereas HCV-specific CD4+ T cell responses are not observed in chronic HCV infection. Moreover, the recurrent viremia has been correlated with loss of previous strong CD4+ T cell responses after several months of viral clearance[32,33]. CD4 help during acute HCV infection is essential to develop a spontaneous recovery[34], since CTL priming in presence of CD4 help is a critical factor in developing a protective response[31].
On the other hand, the magnitude of CD8+ T cells response in acute HCV infection does not correlate with the clinical or viral outcome[31,35,36]. Expression of a dysfunctional phenotype with weak proliferation, IFN-γ production, cytotoxicity and increased levels of well known exhausted phenotype- programmed death-1 receptor (PD-1) are found in HCV infection, irrespective of infection progression[37-41]. Antigen-dependent reactivity of HCV-specific CD8+ T cells has been proved during the early phase of acute infection associated with subsequent rapid decay of CD8+ T cell responses in treatment responders, when these patients were submitted to antiviral therapy[42]. However, the development of self-sustaining memory T cells (CD127+ HCV-specific CD8+ T and CD4+ T cells) are associated with HCV infection control[10,24,31]. In fact, years following sustained virologic response after anti-HCV treatment; it is possible to find HCV traces in association with HCV-specific T cell reactivity. These data suggest that HCV-specific memory T cells are essential to control HCV reservoirs completely in treated patients after the initial treatment induced viral control[43].
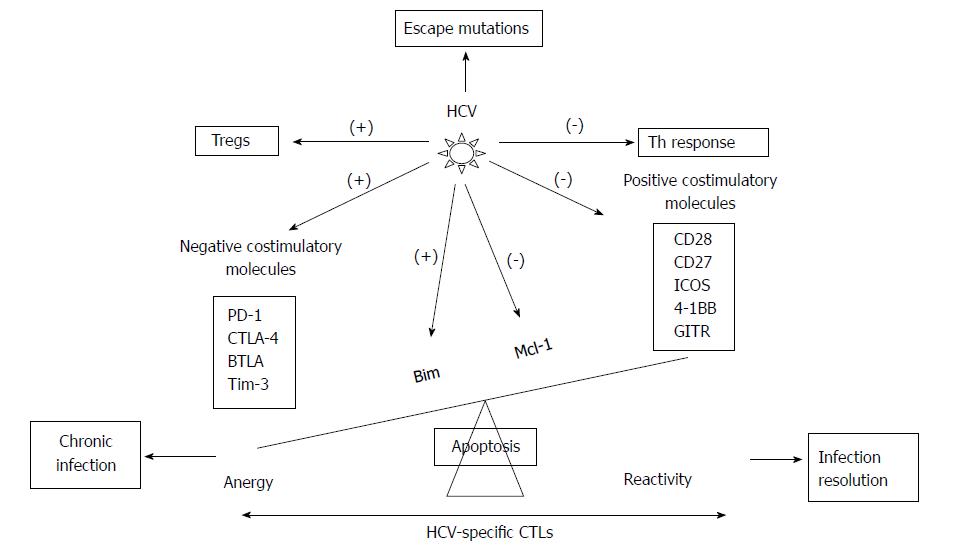
Adaptive cellular response during chronic HCV infection: Patients controlling HCV infection exhibit broader CTL responses with higher functional avidity and wider cross-recognition ability than patients with persistent HCV infection[44]. The main reasons for HCV persistence are the appearance of rapid HCV escape mutations[45,46], T cell exhaustion[47], and deletion[48], immune regulatory cytokines secretion[49] and immune modulatory T reg cell induction[50] (Figures 3 and 4).
HCV polymerase has high replication rate and lack of proof-reading capacity, which permits a rapid viral escape from emerging humoral and cellular immune responses, leading to persistent infection[45,51]. Mutation development in MHC class I restricted epitopes targeted by CD8+ T cells are associated with persistence[52,53], which proved indirectly that MHC-restricted CD8+ T cells exert selection pressure. Selection of viral escape mutations could occur early in acute infection and remained fixed thereafter, indicating that viral escape may indeed be a premature causative mechanism of CD8+ T cell failure and viral persistence[54,55]. Furthermore, the MHC alleles can influence infection outcome; protective T cell responses target epitopes that can not allow escape mutations due to high costs for viral replicative fitness[56-59]. In fact, there is an association between expression of some MHC class-I molecules, such as HLA-B27, and protection following HCV infection. This is explained because mutations in immunodominant epitopes recognized by these MHC class-I molecules lead to substantial viral loss of fitness or because a cluster of mutations is needed to impair T cell recognition.
The secretion of certain immune-regulatory cytokines is also related with HCV persistence. IL-10 cytokine is found to increase in chronic HCV infection[60]. In chronic HCV patients, the suppression of IFN-γ production and proliferation of virus-specific CD4+ and CD8+ T cells have been observed in livers with IL-10-producing HCV-specific CD8+ T cells[61]. IL-10 produced by monocytes or natural killer (NK) cells also downregulates effector T cell responses. For instance, monocytes secrete IL-10 in response to HCV core-mediated Toll like receptor (TLR) 2 stimulation, in vitro[62]. IL-10 producing HCV-specific CD8+ T cells inhibit IFN-α production[63], but also promote apoptosis of plasmocytoid dendritic cells[62]. In addition, intrahepatic HCV-specific IL-10 producing CD8+ T cells prevent liver damage during chronic infection[64]. Moreover, tumor growth factor (TGF)-β is also involved in antiviral immune suppression and chronic HCV infection evolution[49]. Finally, HCV infection can also shift the ratio between T cell-sustaing cytokines such as IL-2[65]. To sum up these data, regulatory cytokines such as IL-10 or TGF-β develop a dual task. First of all, they impair T cell responses to allow viral persistence but also decrease liver damage to extend host survival.
Regulatory T cells (Tregs) are important to control the balance between host damage and viral control produced by specific immune response. In cases of excessive immune response that could be harmful for the host, these cells can induce immune-tolerance to the viral epitopes. The T cell subset with suppressive function, CD4+ CD25+ FoxP3+ regulatory T (Treg) cells, engages in the control of auto-immunity and immune responses through various mechanisms including the inhibition of antigen presenting cell maturation and T-cell activation[66]. HCV infection increases the frequency of Treg cells and the extent of suppression irrespective of the outcome of the infection[67]. However, higher Tregs frequency has been observed in chronic HCV infected patients than in resolved cases[50,68-70]. Interestingly, depletion of CD25+ cells enhance in vitro responsiveness of the remaining HCV-specific effector cells[50,68,69], which suggests a fundamental role of Tregs in the establishment of chronic HCV infection. Moreover, Treg cells are induced and proliferated in chronic HCV infection and appeared to alter liver inflammation[70]. Conversely, Programmed Death Ligand-1 mediated inhibition limits the expansion of Tregs by controlling STAT-5 phosphorylation (pSTAT-5)[71], which can diminish suppressive function of Tregs, leading to viral load control and ultimately ensuring long-lasting host survival.
On the other hand, HCV is able to induce the up-regulation of different negative co-stimulatory molecules in order to provoke an anergic status on HCV-specific T cells. These effector cells are featured by their inability to kill infected hepatocytes, and by the impairment of proliferation and production of type-1 cytokines after antigen recognition. These features are more intense in the intrahepatic comparment due to the higher antigenemia and the tolerogenic hepatic enviorment[72]. Overall, T cell exhaustion follows a predictable pattern. T cells that undergo exhaustion first lose their capacity to produce IL-2, a cytokine that supports proliferation. IL-2 is predominantly produced by CD4+ T cells, whereas CD8+ T cells produce little IL-2 themselves and depend on CD4+ T cell help. This is followed by sequential loss of cytotoxicity and TNF-α and IFN-γ production[73].
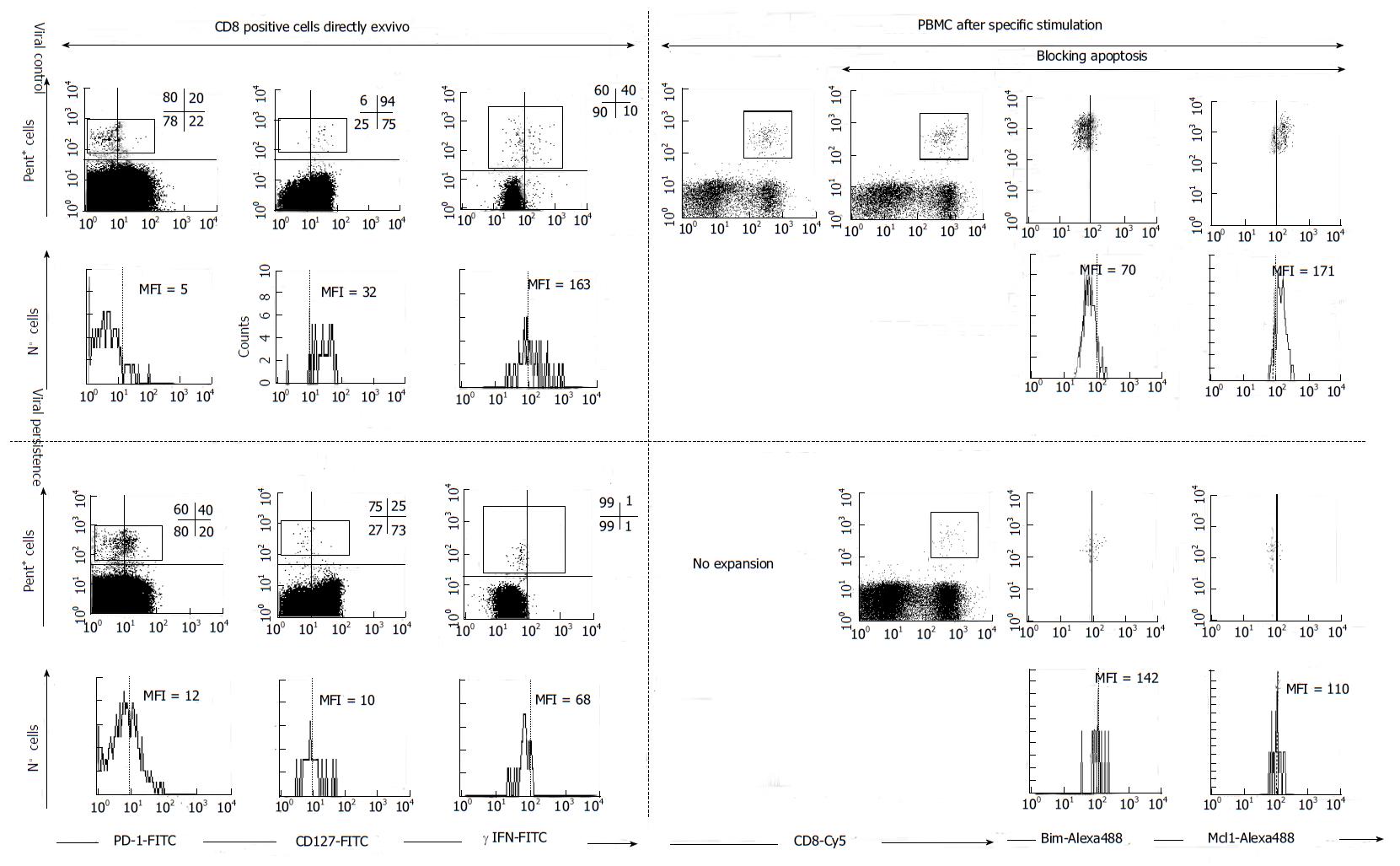
PD-1 is one of that molecules involved in the generation of a state of exhaustion on HCV-specific CD8+ T cells during chronic HCV infection[74]. Importance of PD-1 expression on HCV-specific T cell effector dysfunction has been described[75-77]. In addition, blocking of PD-1 signaling results in the functional restoration of blood-derived HCV-specific CD8+ T cell responses in chronic infection[47,77]. However, to restore function of more exhausted HCV-specific T cells isolated from liver biopsies of infected patients, there is need of Cytotoxic T-Lymphocyte Antigen 4 (CTLA4) blockade in addition to PD-1 blockade[78]. On top of this, the co-expression of other inhibitory receptors, besides PD-1 and CTLA-4, such as 2B4, CD160, KLRG1[79] and, T cell immunoglobulin and mucin domain containing molecule 3 (Tim-3)[80] occurred in about half of HCV-specific CD8+ T cell responses and correlate with low or intermediate level of CD127 expression, impaired proliferative capacity, an intermediate T cell differentiation stage[81] (Figures 3 and 4). These data indicate that HCV infection modulates different negative co-stimulatory molecules to favor the development of HCV-specific CD8+ T cell exhaustion. On the other hand, HCV infection is also able to regulate pro-apoptotic pathways to induce HCV-specific T cell deletion, in order to escape from immune response[82] (Figures 3 and 4). HCV-specific CTLs from chronic patients targeting the virus express an exhausted phenotype associated to the up-regulation of the pro-apoptotic molecule Bcl-2-interacting mediator (Bim) and down-regulation of induced myeloid leukemia cell differentiation protein (Mcl-1). Bim activity is blocked by the anti-apoptotic molecule Mcl-1. The reactivity of these cells is impaired due to the imbalance between Mcl-1 and Bim during chronic infection but can be restored by blocking apoptosis[48,75] (Figures 3 and 4). In Table 1 the phenotype and reactivity of peripheral HCV-specific CTLs according to viral control after modulating apoptosis and different co-stimulatory molecules is summarized.
| PD-1ex vivo | CD127ex vivo | Mcl-1ex vivo/after TCR triggering | Bimex vivo/after TCR triggering | Expansion1 | Expansion(αPD-L1)2 | Expansion(zVAD-fmk)3 | Expansion(αPD-L1 andαCTLA-4)4 | Expansion(αPD-L1 andα41BB)5 | |
| Persistent infection | |||||||||
| PBMC | (+) | (-) | Low/low | Low/high | Impaired | Restored | Restored | Restored | Restored |
| IHMC | (++) | (-) | Impaired | Restored | Impaired | ||||
| Resolved infection | |||||||||
| PBMC | (-) | (++) | High/low | Low/low | Positive | Positive | Positive | ||
Persistent HCV infection also affects CD4+ T cell responses but can be only partially restored by PD-1/PD-L1 blockade or by antigen removal[83,84]. In chronic HCV infection, NK cells develop a regulatory function against CD4+ T cells that may be involved in the antigen-specific defective T helper immunity[85,86].
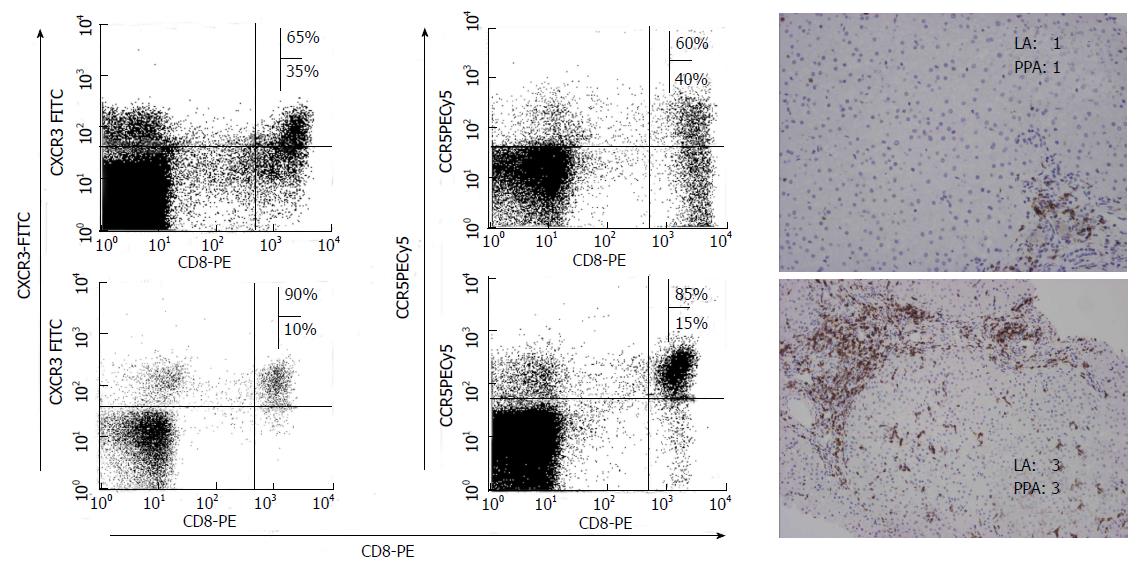
As previously commented, specific CTLs play a central role in HCV immunopathogenesis. These cells are not only able to kill some infected hepatocytes inducing a minor liver damage, but also they secrete type-I cytokines responsible for non-cytopathic virus clearing. To attract these cells into the liver, the infected hepatocytes secrete different chemokines. The migration of lymphocytes to the liver is a complex process including adhesion, rolling, and transendothelial migration. Chemokines and their receptors play an essential role in this multistep pathway[87]. During primo infection, when the adaptive immune system is not able to control infection, the infected hepatocytes continue secreting chemokines to try to attract more defensive cells. In viral chronic hepatitis, the expression of different chemokines in the liver has been described. CXCL-10 is increased in the liver and peripheral blood during chronic viral hepatitis[88,89]. This molecule is produced by hepatocytes and sinusoidal endothelial cells. Moreover, CXCL9 and CXCL11 are also increased in serum and liver of subjects with chronic viral hepatitis[90]. CXCL9 is detected primarily on sinusoidal endothelial cells, while CXCL-11 is produced mainly by hepatocytes[91]. CCL5 intrahepatic expression is also elevated in viral chronic hepatitis and is produced by hepatocytes, sinusoidal endothelial cells and biliary epithelium. Finally, several studies have reported an increased level of CCL3 and CCL4 either in the liver or in serum. These molecules are detected on endothelial cells, on some hepatocytes and biliary epithelial cells[92]. The expression of all these chemokines in the liver can be induced directly by viral proteins. Previous reports have shown a high hepatocyte synthesis of CXCL10, CXCL9 and CCL5, induced by some HCV proteins such as NS5A and core[93], although a recent in vitro study suggests that HCV proteins could also decrease CCL5 and CXCL10 genes expression[94]. All these chemokines recruit T cells with a Th1/Tc1 phenotype, expressing specific chemokine receptors such as CCR5 and CXCR3. The non-ELR-CXC chemokine attracts CXCR3 expressing T cells while CC chemokine attract CCR5 expressing T cells to the liver. Consequently, in viral chronic hepatitis, an intrahepatic enrichment of CCR5 and CXCR3 expressing T cells, located in hepatic lobule and portal tracts has been shown, while these populations are very infrequent in uninfected subjects[95] (Figure 5).
Persistent HCV infection is characterized by a non-specific inflammatory infiltrate in the liver, mainly of CD8+ cells[96], responsible for liver damage. These cells are attracted by the interaction between the intrahepatic secreted chemokines and the chemokine receptors expressed on T cells. Actually, previous reports have shown a correlation between liver inflammation and liver infiltrating CXCR3/CCR5 expressing T cells. The frequency of these cells was positively correlated with portal and lobular inflammation. These data suggest that CCR5 and CXCR3 could play an important role in chronic liver damage by means of inflammatory T cells recruitment into the liver. Obviously, several previous studies have also shown a correlation between liver inflammation and chemokine levels. Intrahepatic CXCL10 mRNA levels are associated with intralobular inflammation[97] and also CXCL9 and CXCL11 correlate with the grade of liver inflammation[98]. Furthermore, CC chemokines are also correlated with the intrahepatic inflammatory activity[99]. Clearly, intrahepatic CCL5 level correlates with the inflammatory activity but not with liver fibrosis. Bearing in mind all the previous data, it is possible to speculate that chemokines are secreted in the infected liver to attract an adaptive immune response able to clear the virus. Unfortunately, when the specific response fails, these chemokines also attract non-specific inflammatory cells, which are not able to remove the virus but produce by-standard liver inflammation. Therefore, as chemokines are nonspecific chemoattractants, intrahepatic inflammatory infiltrate during chronic infection is mainly non-virus-specific and consequently unable to eliminate the infection, but able to produce cytokines capable of initiating and perpetuating hepatic damage and fibrogenesis[95,100,101] (Figure 6).
Taking into account that specific T cell responses are essential to control HCV during natural immune response, several studies have been performed to analyze the role of different therapeutic approaches on T cell response to know whether it is possible to reverse dysfunction of these cells. One possible mechanism to restore T cell function could be to decrease exhaustion by viral load reduction. During HCV infection, a clear HCV-specific cytotoxic T cell response restoration during anti-HCV therapy has not been shown, as could be expected after treatment induced HCV load decrease[102,103], although specific T helper response restoration in sustained viral responders has been described[104]. Nevertheless, patients presenting a better HCV-specific CD8 cell proliferative potential at baseline, are more likely to present a rapid and sustained viral response. Therefore, currently there are contradictory data about the effect of HCV titter reduction on specific T cell response during chronic hepatitis treatment.
In any case, several pre-clinical studies using different strategies have been performed to try to restore HCV-specific responses in vitro. Modulation of co-stimulatory molecules (Table 1), in addition to blocking immunosuppressive cytokines could be promising strategies to restore an effective T cell response. The blockade of negative co-stimulatory molecules, such as PD-1, CTLA-4, Tim-3, has shown in vitro to increase specific-T cell reactivity. This strategy can be combined with the stimulation of positive co-stimulatory molecules such as 4-1BB[105]. Finally, after restoring a T cell response could be necessary to boost that response using a therapeutic vaccine[106]. Moreover, redirecting CD8+ T cells to recognize HCV-epitopes by HCV-specific Vβ and Vα T cell receptor gene transduction has been demonstrated in vitro and it could be a future strategy to induce a new HCV-specific T cell population in chronic patients with deletion of these responses[107]. Although all these results seem to be quite promising, the blockade of negative co-stimulatory pathways, stimulation of positive ones and generation of new specific T cells could lead to the development of autoimmune or lymphoproliferative diseases, which could prevent the use of this strategy as a therapeutic tool in humans in the near future. Therefore, more research is necessary in this field before these strategies are suitable for the treatment of chronic viral infections[75,78,106].
HCV is a hepatotropic non-cytopathic virus able to produce a chronic liver disease. HCV clearing resides in the efficacy of adaptive immune response and, particularly HCV specific CTL response plays a central role in viral control through cytopathic and non-cytopathic mechanisms. Nevertheless, during persistent infection, specific-CTL response is impaired due to its exhaustion and deletion, emergence of HCV escape mutations and lack of T helper cooperation. Several in vitro strategies have shown to be effective in T cell response restoration but it is necessary to perform more research before these approaches can be applied to clinical practice. Finally, when the virus is not controlled by adaptive response a non-specific inflammatory infiltrate is attracted to the liver which is responsible for the persistent low-grade liver damage, allowing the generation of liver fibrosis and disease progression.
P- Reviewers: Ciotti M, El Din NGB, Marcelo LL, Maggi F S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Nowak MA, Bangham CR. Population dynamics of immune responses to persistent viruses. Science. 1996;272:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 458] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 2. | Larrubia JR, Benito-Martínez S, Miquel-Plaza J, Sanz-de-Villalobos E, González-Mateos F, Parra T. Cytokines - their pathogenic and therapeutic role in chronic viral hepatitis. Rev Esp Enferm Dig. 2009;101:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Choudhuri K, Kearney A, Bakker TR, van der Merwe PA. Immunology: how do T cells recognize antigen? Curr Biol. 2005;15:R382-R385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 562] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 5. | Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 594] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 6. | Edwards VC, Tarr AW, Urbanowicz RA, Ball JK. The role of neutralizing antibodies in hepatitis C virus infection. J Gen Virol. 2012;93:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Semmo N, Lucas M, Krashias G, Lauer G, Chapel H, Klenerman P. Maintenance of HCV-specific T-cell responses in antibody-deficient patients a decade after early therapy. Blood. 2006;107:4570-4571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Dustin LB, Rice CM. Flying under the radar: the immunobiology of hepatitis C. Annu Rev Immunol. 2007;25:71-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 235] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 9. | Lechner F, Gruener NH, Urbani S, Uggeri J, Santantonio T, Kammer AR, Cerny A, Phillips R, Ferrari C, Pape GR. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J Immunol. 2000;30:2479-2487. [PubMed] |
| 10. | Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499-1512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1003] [Cited by in RCA: 1000] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 11. | Logvinoff C, Major ME, Oldach D, Heyward S, Talal A, Balfe P, Feinstone SM, Alter H, Rice CM, McKeating JA. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci USA. 2004;101:10149-10154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 335] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 12. | Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, Govindarajan S, Purcell RH, Chisari FV. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci USA. 2002;99:15661-15668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 477] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 13. | Farci P, Shimoda A, Wong D, Cabezon T, De Gioannis D, Strazzera A, Shimizu Y, Shapiro M, Alter HJ, Purcell RH. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc Natl Acad Sci USA. 1996;93:15394-15399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 454] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 14. | Pestka JM, Zeisel MB, Bläser E, Schürmann P, Bartosch B, Cosset FL, Patel AH, Meisel H, Baumert J, Viazov S. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci USA. 2007;104:6025-6030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 434] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 15. | Dowd KA, Netski DM, Wang XH, Cox AL, Ray SC. Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology. 2009;136:2377-2386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Zeisel MB, Cosset FL, Baumert TF. Host neutralizing responses and pathogenesis of hepatitis C virus infection. Hepatology. 2008;48:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Racanelli V, Frassanito MA, Leone P, Galiano M, De Re V, Silvestris F, Dammacco F. Antibody production and in vitro behavior of CD27-defined B-cell subsets: persistent hepatitis C virus infection changes the rules. J Virol. 2006;80:3923-3934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Agnello V, De Rosa FG. Extrahepatic disease manifestations of HCV infection: some current issues. J Hepatol. 2004;40:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Amarapurkar DN, Amarapurkar AD. Extrahepatic manifestations of viral hepatitis. Ann Hepatol. 2002;1:192-195. [PubMed] |
| 20. | Manns MP, Rambusch EG. Autoimmunity and extrahepatic manifestations in hepatitis C virus infection. J Hepatol. 1999;31 Suppl 1:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Zhang P, Zhong L, Struble EB, Watanabe H, Kachko A, Mihalik K, Virata-Theimer ML, Alter HJ, Feinstone S, Major M. Depletion of interfering antibodies in chronic hepatitis C patients and vaccinated chimpanzees reveals broad cross-genotype neutralizing activity. Proc Natl Acad Sci USA. 2009;106:7537-7541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Bassett SE, Thomas DL, Brasky KM, Lanford RE. Viral persistence, antibody to E1 and E2, and hypervariable region 1 sequence stability in hepatitis C virus-inoculated chimpanzees. J Virol. 1999;73:1118-1126. [PubMed] |
| 23. | Thimme R, Lohmann V, Weber F. A target on the move: innate and adaptive immune escape strategies of hepatitis C virus. Antiviral Res. 2006;69:129-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395-1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 925] [Cited by in RCA: 900] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 25. | Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 576] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 26. | Shin EC, Seifert U, Kato T, Rice CM, Feinstone SM, Kloetzel PM, Rehermann B. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J Clin Invest. 2006;116:3006-3014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 418] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 28. | Diepolder HM, Zachoval R, Hoffmann RM, Wierenga EA, Santantonio T, Jung MC, Eichenlaub D, Pape GR. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346:1006-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 522] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 29. | Missale G, Bertoni R, Lamonaca V, Valli A, Massari M, Mori C, Rumi MG, Houghton M, Fiaccadori F, Ferrari C. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J Clin Invest. 1996;98:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 490] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 30. | Kapadia SB, Barth H, Baumert T, McKeating JA, Chisari FV. Initiation of hepatitis C virus infection is dependent on cholesterol and cooperativity between CD81 and scavenger receptor B type I. J Virol. 2007;81:374-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 31. | Urbani S, Amadei B, Fisicaro P, Tola D, Orlandini A, Sacchelli L, Mori C, Missale G, Ferrari C. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology. 2006;44:126-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Gerlach JT, Diepolder HM, Jung MC, Gruener NH, Schraut WW, Zachoval R, Hoffmann R, Schirren CA, Santantonio T, Pape GR. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 1999;117:933-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 526] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 33. | Nascimbeni M, Mizukoshi E, Bosmann M, Major ME, Mihalik K, Rice CM, Feinstone SM, Rehermann B. Kinetics of CD4+ and CD8+ memory T-cell responses during hepatitis C virus rechallenge of previously recovered chimpanzees. J Virol. 2003;77:4781-4793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Smyk-Pearson S, Tester IA, Klarquist J, Palmer BE, Pawlotsky JM, Golden-Mason L, Rosen HR. Spontaneous recovery in acute human hepatitis C virus infection: functional T-cell thresholds and relative importance of CD4 help. J Virol. 2008;82:1827-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Francavilla V, Accapezzato D, De Salvo M, Rawson P, Cosimi O, Lipp M, Cerino A, Cividini A, Mondelli MU, Barnaba V. Subversion of effector CD8+ T cell differentiation in acute hepatitis C virus infection: exploring the immunological mechanisms. Eur J Immunol. 2004;34:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Kaplan DE, Sugimoto K, Newton K, Valiga ME, Ikeda F, Aytaman A, Nunes FA, Lucey MR, Vance BA, Vonderheide RH. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology. 2007;132:654-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 37. | Bowen DG, Shoukry NH, Grakoui A, Fuller MJ, Cawthon AG, Dong C, Hasselschwert DL, Brasky KM, Freeman GJ, Seth NP. Variable patterns of programmed death-1 expression on fully functional memory T cells after spontaneous resolution of hepatitis C virus infection. J Virol. 2008;82:5109-5114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 334] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 39. | Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, Shaked A, Olthoff K, Gostick E, Price DA. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927-1937, 1937e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 40. | Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1019] [Cited by in RCA: 1203] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 41. | Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398-11403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 453] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 42. | Rahman F, Heller T, Sobao Y, Mizukoshi E, Nascimbeni M, Alter H, Herrine S, Hoofnagle J, Liang TJ, Rehermann B. Effects of antiviral therapy on the cellular immune response in acute hepatitis C. Hepatology. 2004;40:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 43. | Veerapu NS, Raghuraman S, Liang TJ, Heller T, Rehermann B. Sporadic reappearance of minute amounts of hepatitis C virus RNA after successful therapy stimulates cellular immune responses. Gastroenterology. 2011;140:676-685.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Yerly D, Heckerman D, Allen TM, Chisholm JV, Faircloth K, Linde CH, Frahm N, Timm J, Pichler WJ, Cerny A. Increased cytotoxic T-lymphocyte epitope variant cross-recognition and functional avidity are associated with hepatitis C virus clearance. J Virol. 2008;82:3147-3153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Chang KM, Rehermann B, McHutchison JG, Pasquinelli C, Southwood S, Sette A, Chisari FV. Immunological significance of cytotoxic T lymphocyte epitope variants in patients chronically infected by the hepatitis C virus. J Clin Invest. 1997;100:2376-2385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 241] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 46. | Timm J, Li B, Daniels MG, Bhattacharya T, Reyor LL, Allgaier R, Kuntzen T, Fischer W, Nolan BE, Duncan J. Human leukocyte antigen-associated sequence polymorphisms in hepatitis C virus reveal reproducible immune responses and constraints on viral evolution. Hepatology. 2007;46:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, Missale G, Ferrari C. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 229] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 48. | Larrubia JR, Lokhande MU, García-Garzón S, Miquel J, González-Praetorius A, Parra-Cid T, Sanz-de-Villalobos E. Persistent hepatitis C virus (HCV) infection impairs HCV-specific cytotoxic T cell reactivity through Mcl-1/Bim imbalance due to CD127 down-regulation. J Viral Hepat. 2013;20:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Alatrakchi N, Graham CS, van der Vliet HJ, Sherman KE, Exley MA, Koziel MJ. Hepatitis C virus (HCV)-specific CD8+ cells produce transforming growth factor beta that can suppress HCV-specific T-cell responses. J Virol. 2007;81:5882-5892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 50. | Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 2003;38:1437-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 51. | Tester I, Smyk-Pearson S, Wang P, Wertheimer A, Yao E, Lewinsohn DM, Tavis JE, Rosen HR. Immune evasion versus recovery after acute hepatitis C virus infection from a shared source. J Exp Med. 2005;201:1725-1731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 52. | Ray SC, Fanning L, Wang XH, Netski DM, Kenny-Walsh E, Thomas DL. Divergent and convergent evolution after a common-source outbreak of hepatitis C virus. J Exp Med. 2005;201:1753-1759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 53. | Timm J, Lauer GM, Kavanagh DG, Sheridan I, Kim AY, Lucas M, Pillay T, Ouchi K, Reyor LL, Schulze zur Wiesch J. CD8 epitope escape and reversion in acute HCV infection. J Exp Med. 2004;200:1593-1604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 241] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 54. | Cox AL, Mosbruger T, Mao Q, Liu Z, Wang XH, Yang HC, Sidney J, Sette A, Pardoll D, Thomas DL. Cellular immune selection with hepatitis C virus persistence in humans. J Exp Med. 2005;201:1741-1752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 232] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 55. | Cox AL, Mosbruger T, Lauer GM, Pardoll D, Thomas DL, Ray SC. Comprehensive analyses of CD8+ T cell responses during longitudinal study of acute human hepatitis C. Hepatology. 2005;42:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 56. | Dazert E, Neumann-Haefelin C, Bressanelli S, Fitzmaurice K, Kort J, Timm J, McKiernan S, Kelleher D, Gruener N, Tavis JE. Loss of viral fitness and cross-recognition by CD8+ T cells limit HCV escape from a protective HLA-B27-restricted human immune response. J Clin Invest. 2009;119:376-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 57. | Neumann-Haefelin C, McKiernan S, Ward S, Viazov S, Spangenberg HC, Killinger T, Baumert TF, Nazarova N, Sheridan I, Pybus O. Dominant influence of an HLA-B27 restricted CD8+ T cell response in mediating HCV clearance and evolution. Hepatology. 2006;43:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 58. | Kim AY, Kuntzen T, Timm J, Nolan BE, Baca MA, Reyor LL, Berical AC, Feller AJ, Johnson KL, Schulze zur Wiesch J. Spontaneous control of HCV is associated with expression of HLA-B 57 and preservation of targeted epitopes. Gastroenterology. 2011;140:686-696.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 59. | Fitzmaurice K, Petrovic D, Ramamurthy N, Simmons R, Merani S, Gaudieri S, Sims S, Dempsey E, Freitas E, Lea S. Molecular footprints reveal the impact of the protective HLA-A*03 allele in hepatitis C virus infection. Gut. 2011;60:1563-1571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 60. | Piazzolla G, Tortorella C, Schiraldi O, Antonaci S. Relationship between interferon-gamma, interleukin-10, and interleukin-12 production in chronic hepatitis C and in vitro effects of interferon-alpha. J Clin Immunol. 2000;20:54-61. [PubMed] |
| 61. | Accapezzato D, Francavilla V, Paroli M, Casciaro M, Chircu LV, Cividini A, Abrignani S, Mondelli MU, Barnaba V. Hepatic expansion of a virus-specific regulatory CD8(+) T cell population in chronic hepatitis C virus infection. J Clin Invest. 2004;113:963-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 62. | Dolganiuc A, Chang S, Kodys K, Mandrekar P, Bakis G, Cormier M, Szabo G. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol. 2006;177:6758-6768. [PubMed] |
| 63. | Duramad O, Fearon KL, Chan JH, Kanzler H, Marshall JD, Coffman RL, Barrat FJ. IL-10 regulates plasmacytoid dendritic cell response to CpG-containing immunostimulatory sequences. Blood. 2003;102:4487-4492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 64. | Abel M, Sène D, Pol S, Bourlière M, Poynard T, Charlotte F, Cacoub P, Caillat-Zucman S. Intrahepatic virus-specific IL-10-producing CD8 T cells prevent liver damage during chronic hepatitis C virus infection. Hepatology. 2006;44:1607-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 65. | Radziewicz H, Ibegbu CC, Hon H, Bédard N, Bruneau J, Workowski KA, Knechtle SJ, Kirk AD, Larsen CP, Shoukry NH. Transient CD86 expression on hepatitis C virus-specific CD8+ T cells in acute infection is linked to sufficient IL-2 signaling. J Immunol. 2010;184:2410-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1257] [Cited by in RCA: 1343] [Article Influence: 83.9] [Reference Citation Analysis (0)] |
| 67. | Manigold T, Shin EC, Mizukoshi E, Mihalik K, Murthy KK, Rice CM, Piccirillo CA, Rehermann B. Foxp3+CD4+CD25+ T cells control virus-specific memory T cells in chimpanzees that recovered from hepatitis C. Blood. 2006;107:4424-4432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 68. | Boettler T, Spangenberg HC, Neumann-Haefelin C, Panther E, Urbani S, Ferrari C, Blum HE, von Weizsäcker F, Thimme R. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79:7860-7867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 329] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 69. | Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, Nelson DR. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 430] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 70. | Rushbrook SM, Ward SM, Unitt E, Vowler SL, Lucas M, Klenerman P, Alexander GJ. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol. 2005;79:7852-7859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 226] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 71. | Franceschini D, Paroli M, Francavilla V, Videtta M, Morrone S, Labbadia G, Cerino A, Mondelli MU, Barnaba V. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J Clin Invest. 2009;119:551-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 256] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 72. | Protzer U, Maini MK, Knolle PA. Living in the liver: hepatic infections. Nat Rev Immunol. 2012;12:201-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 429] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 73. | Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2481] [Cited by in RCA: 3031] [Article Influence: 216.5] [Reference Citation Analysis (0)] |
| 74. | Larrubia JR, Benito-Martínez S, Miquel J, Calvino M, Sanz-de-Villalobos E, Parra-Cid T. Costimulatory molecule programmed death-1 in the cytotoxic response during chronic hepatitis C. World J Gastroenterol. 2009;15:5129-5140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Larrubia JR, Benito-Martínez S, Miquel J, Calvino M, Sanz-de-Villalobos E, González-Praetorius A, Albertos S, García-Garzón S, Lokhande M, Parra-Cid T. Bim-mediated apoptosis and PD-1/PD-L1 pathway impair reactivity of PD1(+)/CD127(-) HCV-specific CD8(+) cells targeting the virus in chronic hepatitis C virus infection. Cell Immunol. 2011;269:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 76. | Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249-9258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 308] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 77. | Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545-2553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 380] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 78. | Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 299] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 79. | Schlaphoff V, Lunemann S, Suneetha PV, Jaroszewicz J, Grabowski J, Dietz J, Helfritz F, Bektas H, Sarrazin C, Manns MP. Dual function of the NK cell receptor 2B4 (CD244) in the regulation of HCV-specific CD8+ T cells. PLoS Pathog. 2011;7:e1002045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 80. | Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122-9130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 377] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 81. | Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, Pircher H, Thimme R. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 305] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 82. | Larrubia JR, Lokhande MU, García-Garzón S, Miquel J, Subirá D, Sanz-de-Villalobos E. Role of T cell death in maintaining immune tolerance during persistent viral hepatitis. World J Gastroenterol. 2013;19:1877-1889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 83. | Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha SJ, Barber DL, Ye L, Sharpe AH, Freeman GJ, Ahmed R. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci USA. 2011;108:21182-21187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 84. | Han S, Asoyan A, Rabenstein H, Nakano N, Obst R. Role of antigen persistence and dose for CD4+ T-cell exhaustion and recovery. Proc Natl Acad Sci USA. 2010;107:20453-20458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 85. | Welsh RM, Waggoner SN. NK cells controlling virus-specific T cells: Rheostats for acute vs. persistent infections. Virology. 2013;435:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 86. | Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481:394-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 415] [Cited by in RCA: 485] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 87. | Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5269] [Cited by in RCA: 5166] [Article Influence: 166.6] [Reference Citation Analysis (0)] |
| 88. | Larrubia JR, Calvino M, Benito S, Sanz-de-Villalobos E, Perna C, Pérez-Hornedo J, González-Mateos F, García-Garzón S, Bienvenido A, Parra T. The role of CCR5/CXCR3 expressing CD8+ cells in liver damage and viral control during persistent hepatitis C virus infection. J Hepatol. 2007;47:632-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 89. | Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163:6236-6243. [PubMed] |
| 90. | Bièche I, Asselah T, Laurendeau I, Vidaud D, Degot C, Paradis V, Bedossa P, Valla DC, Marcellin P, Vidaud M. Molecular profiling of early stage liver fibrosis in patients with chronic hepatitis C virus infection. Virology. 2005;332:130-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 91. | Apolinario A, Majano PL, Alvarez-Pérez E, Saez A, Lozano C, Vargas J, García-Monzón C. Increased expression of T cell chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. Am J Gastroenterol. 2002;97:2861-2870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 92. | Apolinario A, Diago M, Lo Iacono O, Lorente R, Pérez C, Majano PL, Clemente G, García-Monzón C. Increased circulating and intrahepatic T-cell-specific chemokines in chronic hepatitis C: relationship with the type of virological response to peginterferon plus ribavirin combination therapy. Aliment Pharmacol Ther. 2004;19:551-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 93. | Apolinario A, Majano PL, Lorente R, Núñez O, Clemente G, García-Monzón C. Gene expression profile of T-cell-specific chemokines in human hepatocyte-derived cells: evidence for a synergistic inducer effect of cytokines and hepatitis C virus proteins. J Viral Hepat. 2005;12:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 94. | Sillanpää M, Kaukinen P, Melén K, Julkunen I. Hepatitis C virus proteins interfere with the activation of chemokine gene promoters and downregulate chemokine gene expression. J Gen Virol. 2008;89:432-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 95. | Larrubia JR, Benito-Martínez S, Calvino M, Sanz-de-Villalobos E, Parra-Cid T. Role of chemokines and their receptors in viral persistence and liver damage during chronic hepatitis C virus infection. World J Gastroenterol. 2008;14:7149-7159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 96. | Sprengers D, van der Molen RG, Kusters JG, Kwekkeboom J, van der Laan LJ, Niesters HG, Kuipers EJ, De Man RA, Schalm SW, Janssen HL. Flow cytometry of fine-needle-aspiration biopsies: a new method to monitor the intrahepatic immunological environment in chronic viral hepatitis. J Viral Hepat. 2005;12:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 97. | Harvey CE, Post JJ, Palladinetti P, Freeman AJ, Ffrench RA, Kumar RK, Marinos G, Lloyd AR. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol. 2003;74:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 194] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 98. | Helbig KJ, Ruszkiewicz A, Semendric L, Harley HA, McColl SR, Beard MR. Expression of the CXCR3 ligand I-TAC by hepatocytes in chronic hepatitis C and its correlation with hepatic inflammation. Hepatology. 2004;39:1220-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 99. | Kusano F, Tanaka Y, Marumo F, Sato C. Expression of C-C chemokines is associated with portal and periportal inflammation in the liver of patients with chronic hepatitis C. Lab Invest. 2000;80:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Bertoletti A, Maini MK. Protection or damage: a dual role for the virus-specific cytotoxic T lymphocyte response in hepatitis B and C infection? Curr Opin Microbiol. 2000;3:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 101. | Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1292] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 102. | Barnes E, Harcourt G, Brown D, Lucas M, Phillips R, Dusheiko G, Klenerman P. The dynamics of T-lymphocyte responses during combination therapy for chronic hepatitis C virus infection. Hepatology. 2002;36:743-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 103. | Pilli M, Zerbini A, Penna A, Orlandini A, Lukasiewicz E, Pawlotsky JM, Zeuzem S, Schalm SW, von Wagner M, Germanidis G. HCV-specific T-cell response in relation to viral kinetics and treatment outcome (DITTO-HCV project). Gastroenterology. 2007;133:1132-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 104. | Kamal SM, Fehr J, Roesler B, Peters T, Rasenack JW. Peginterferon alone or with ribavirin enhances HCV-specific CD4 T-helper 1 responses in patients with chronic hepatitis C. Gastroenterology. 2002;123:1070-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 138] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 105. | Fisicaro P, Valdatta C, Massari M, Loggi E, Ravanetti L, Urbani S, Giuberti T, Cavalli A, Vandelli C, Andreone P. Combined blockade of programmed death-1 and activation of CD137 increase responses of human liver T cells against HBV, but not HCV. Gastroenterology. 2012;143:1576-1585.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 106. | Ferrari C. Therapeutic vaccination for hepatitis C: can protective T-cell responses be restored after prolonged antigen exposure? Gastroenterology. 2008;134:1601-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 107. | Zhang Y, Liu Y, Moxley KM, Golden-Mason L, Hughes MG, Liu T, Heemskerk MH, Rosen HR, Nishimura MI. Transduction of human T cells with a novel T-cell receptor confers anti-HCV reactivity. PLoS Pathog. 2010;6:e1001018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |