Published online Mar 21, 2014. doi: 10.3748/wjg.v20.i11.3050
Revised: December 4, 2013
Accepted: January 3, 2014
Published online: March 21, 2014
Processing time: 167 Days and 17.7 Hours
Extrahepatic bile duct (EHBD) cancer may occur metachronously, and these cancers are resectable with a favorable prognosis. We aimed to identify the pattern of metachronous EHBD cancer. We classified the cases of metachronous EHBD cancer reported in the literature thus far and investigated two new cases of metachronous EHBD cancer. A 70-year-old female underwent R0 bile duct resection for a type 1 Klatskin tumor (pT1N0M0). A 70-year-old male patient underwent R0 bile duct resection for a middle bile duct cancer (pT2N1M0). Imaging studies of both patients taken at 14 and 24 mo after first surgery respectively revealed a metachronous cholangiocarcinoma that required pancreaticoduodenectomy (PD). Histopathology of the both tumors after PD revealed cholangiocarcinoma invading the pancreas (pT3N0M0). Both patients have been free from recurrence for 6 years and 16 mo respectively after the second surgery. Through a review of the literature on these cases, we classified the pattern of metachronous EHBD cancer according to the site of de novo neoplasia. The proximal remnant bile duct was most commonly involved. Metachronous EHBD cancer should be distinguished from an unresectable recurrent tumor. Classifying metachronous EHBD cancer may be helpful in identifying rare metachronous tumors.
Core tip: The cases of metachronous double bile duct cancer described emphasize the importance of differentiating this condition from a loco-regional recurrence. It is not a progression of the initial tumor but rather a new tumor. Therefore, it may have a good prognosis. The cases that have been reported thus far have shown a favorable prognosis. Tumor classification may be valuable during the long-term follow up after the initial surgery because it allows prediction of the potential site and proper surgical methods for the new tumors. Diagnosing these tumors and classifying their patterns may allow physicians the opportunity to cure the disease.
-
Citation: Kwon HJ, Kim SG, Chun JM, Hwang YJ. Classifying extrahepatic bile duct metachronous carcinoma by
de novo neoplasia site. World J Gastroenterol 2014; 20(11): 3050-3055 - URL: https://www.wjgnet.com/1007-9327/full/v20/i11/3050.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i11.3050
Bile duct cancer may arise from anywhere along the biliary tree. Pathological factors, such as the site of tumor, the extent of tumor spreading, and multicentricity, are critical factors for selecting the type of surgery and achieving an R0 resection.
Multicentricity is one of the pathological characteristics of EHBD cancer. Synchronous bile duct and gallbladder cancer has been reported in 5.0%-7.4% of cases, but the occurrence of metachronous cancer in EHBD has rarely been reported[1-4]. Warren and Gates established a set of criteria for multiple primary malignant neoplasms: Each tumor must have definite features of malignancy; each tumor should be distinct and separate excluding the probability of one tumor being a metastasis of the other tumor[5]. Moertel proposed a definition for synchronous cancers as those occurring within 6 mo of the first primary cancer and for metachronous cancers as those occurring more than 6 mo later[6].
If synchronous multifocal EHBD cancer is diagnosed preoperatively, it usually requires aggressive resections, such as hepatectomy and pancreaticoduodenectomy, to achieve an R0 resection. Similarly, when metachronous EHBD cancer is diagnosed after the first surgery, additional resections are required to achieve an R0 resection, although the incidence, risk factors, and clinical behavior of metachronous EHBD cancer are not well known. One might assume that metachronous de novo neoplasia of the remnant bile duct could be more easily cured than can loco-regional recurrent diseases, as the former may be in an early stage. For this reason, it is clinically important to distinguish this rare metachronous cancer from loco-regional recurrences. Using a literature review, we summarized these cases and report two cases of metachronous carcinomas of the extrahepatic bile duct, both of which were successfully resected using pylorus preserving pancreaticoduodenectomy.
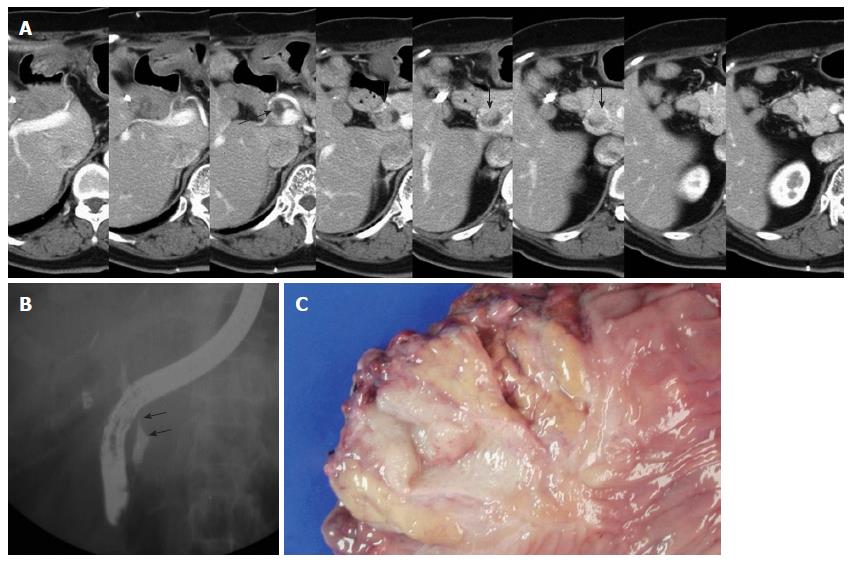
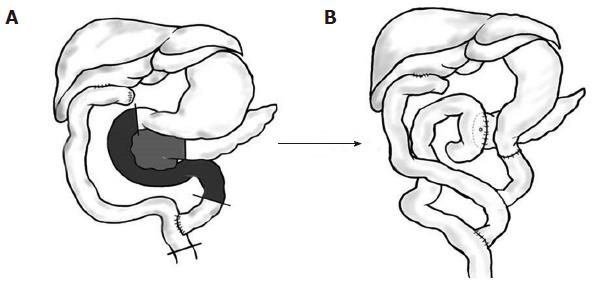
A 70-year-old female patient presented with jaundice. Abdominal CT revealed a mass lesion on the proximal common hepatic duct. Endoscopic retrograde cholangio-pancreatography (ERCP) revealed a complete occlusion of the bile duct at the proximal common hepatic duct. Upon the diagnosis of a type 1 Klatskin tumor, we resected the extrahepatic bile duct using a Roux-en-Y hepaticojejunostomy. Histopathology revealed a T1N0M0 tumor of the bile duct with a tumor-free resection margin. The patient had been free from recurrence for 14 mo until a new lesion on the distal remnant bile duct was detected from an abdominal CT scan during a routine follow up (Figure 1A). The PET-CT also suggested a metachronous bile duct cancer of the distal remnant bile duct, and ERCP revealed mild dilatation of the intrapancreatic remnant bile duct with a filling defect (Figure 1B). We decided to perform pancreaticoduodenectomy on the newly developed lesion using the method illustrated in Figure 2. The frozen histopathology of the resected bile duct stump and the neighboring connective tissue around superior mesenteric vein were free of carcinoma. The final pathological diagnosis of the specimen confirmed a cholangiocarcinoma with a polypoid configuration (Figure 1C) arising from the remnant bile duct with invasion into the pancreas (pT3N0M0). She was discharged 14 d after surgery without complications. The patient has been free of recurrence for 6 years after the second surgery.
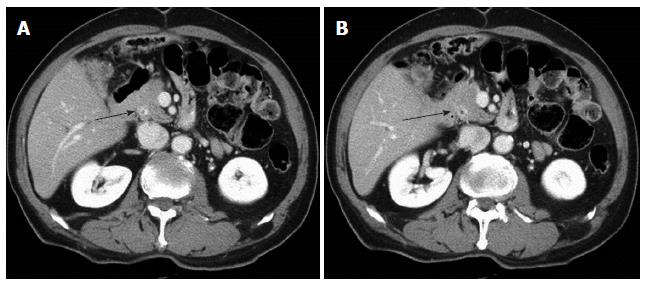
A 70-year-old male patient presented with jaundice. Abdominal CT revealed a mass lesion at the middle common bile duct. ERCP revealed a complete occlusion at the middle common bile duct. We performed a resection of the extrahepatic bile duct using a Roux-en-Y hepaticojejunostomy. Histopathology revealed a T1N1M0 tumor of the bile duct with a tumor-free resection margin. He had been free from recurrence for 24 mo until a new lesion with slight contrast enhancement at distal remnant bile duct was detected from an abdominal CT scan during a routine follow up (Figure 3). The PET-CT also suggested a metachronous bile duct cancer of the distal remnant bile duct. Pancreaticoduodenectomy was performed on the newly developed lesion using the method illustrated in Figure 3. The final pathological diagnosis of the specimen confirmed a bile duct cancer invading the pancreas (pT3N0M0). The patient was discharged 15 days after surgery. He has been free of recurrence for 16 mo after the second surgery.
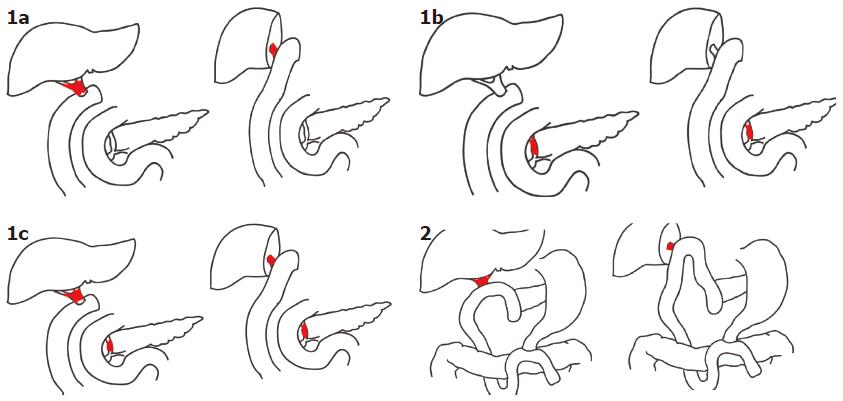
The potential site of metachronous bile duct cancer depends on the type of first surgery. After a bile duct resection with or without concomitant hepatectomy, both the proximal and distal bile ducts remain (Type 1), and metachronous bile duct cancer can occur at one or both remnant bile ducts. We applied the term Type 1a to describe new tumors at the proximal remnant bile duct; Type 1b to describe those at the distal remnant bile duct; Type 1c to describe those at both the proximal and distal remnant bile ducts. After pancreatoduodenectomy with or without concomitant hepatectomy, only the proximal bile duct remains for de novo neoplasia. We applied the term Type 2 metachronous bile duct cancer to describe these tumors (Figure 4). Out of 13 cases that have been reported thus far, eight patients (Type 1) underwent bile duct resection with or without a concomitant liver resection. The remaining 5 patients underwent pancreaticoduodenectomy (Type 2), which removed the potential site for future neoplasia. In the eight Type 1 patients who were left with remnant proximal and distal bile ducts, there were two patients of Type 1a who required liver resection, five patients of Type 1b who required pancreaticoduodenectomy, and one patient of Type 1c who required hepatectomy and pancreaticoduodenectomy. In all of the remaining 5 patients of Type 2, whose distal bile duct was removed by a previous pancreaticoduodenectomy, liver resection was performed to resect the metachronous bile duct tumor. The most frequent type was type 2. Our present cases would be classified as type 1b metachronous bile duct cancer.
Although resection rates have increased, the prognosis of EHBD cancer (even after R0 resection) remains unsatisfactory[7,8]. Loco-regional recurrence is a common problem. In these cases, curative re-resection is usually impossible due to the invasiveness of the tumor and the anatomical complexity near the hepatic hilum. Furthermore, it is difficult to clarify the role and optimal regimen of adjuvant treatment in the setting of locally recurrent bile duct cancer[9].
Unlike loco-regional recurrence, metachronous de novo bile duct cancer is often curable because it is a less advanced tumor compared to a loco-regional recurrence. Some bile duct cancers are synchronously or metachronously multifocal[1-3]. However, metachronous tumors arising from the remnant bile duct epithelium have rarely been reported compared to those of the gastrointestinal tract, such as the stomach, colon, and liver[4]. The small amount of remnant bile duct tissue remaining after curative resection and the poor prognosis of this tumor may explain the rarity.
Discriminating metachronous bile duct tumor from other loco-regional recurrence is clinically important because the de novo neoplasia presents a different progression from that of the prior tumor. All of the cases of metachronous bile duct cancer that have been reported thus far have been resectable with curative intent, and notably, all but two patients are alive at the time of publication (Table 1).
| Ref. | Year published | Age (yr) | Sex | Site of | Type of | Time to | Site of | Type of | Type of | Status |
| 1st tumor | 1st surgery1 | 2nd tumor | 2nd tumor1 | 2nd surgery2 | meta-BDCA | |||||
| Todoroki et al[11] | 1993 | 67 | F | Perihilar | LH with RYHJ | 13 Y | IHBD | RPMS | Type 1a | Alive, 3 Y 5 M |
| Seki et al[12] | 1998 | 68 | M | Dist. BD | PD | 12.8 Y | Prox. BD. | ERH+α | Type 2 | Dead, 6 M |
| 1998 | 69 | F | Dist. BD | PD | 5.8 Y | Prox. BD | RH | Type 2 | Alive, 17 M | |
| Saiura et al[10] | 1999 | 75 | F | IHBD | LH extended | 7 Y | Dist. BD | PD | Type 1a | Alive, 36 M |
| Nakakubo et al[13] | 2001 | 49 | F | BD | PD | 4 Y | Prox. BD. | LH ext. | Type 2 | Dead, 14 M |
| Okamoto et al[14] | 2003 | 42 | F | BD | Excision of cyst | 3 Y | Right HD | RH | Type 1a | Alive 11.2 Y |
| 2003 | 62 | M | BD | PD | 3.5 Y | Left HD | LH | Type 2 | Alive 2 Y | |
| Yoon et al[15] | 2005 | 50 | F | Diffuse | BDR | 5.5 Y | Prox. and Dist. BD | HPD | Type 1c | Alive, 46 M |
| 2005 | 29 | F | Prox. BD | RH with RYHJ | 2.3 Y | Dist. BD | PD | Type 1b | Alive, 9 M | |
| Hibi et al[16] | 2006 | 65 | M | Mid. to Dist. BD | PD | 3 Y | Prox. BD | RH | Type 2 | Alive, 8 M |
| Merenda et al[4] | 2007 | 65 | M | Middle BD. | BDR | 2.3 Y | Dist. BD | PD | Type 1b | Alive, 6 M |
| Present Case 1 | 2013 | 70 | F | Prox. BD | BDR | 14 M | Dist. BD | PD | Type 1b | Alive, 6 Y |
| Present Case 2 | 2013 | 70 | M | Middle BD | BDR | 24 M | Dist. BD | PD | Type 1b | Alive, 16 M |
In the English literature, Saiura et al[10] reported the first case of metachronous bile duct cancer occurring sequentially in the intrahepatic and extrahepatic bile ducts. Todoroki et al[11] and Seki et al[12] also reported three cases. We reviewed thirteen cases recently reported by Nakakudo[13], Okamoto[14], Yoon et al[15], Hibi et al[16], and Merenda et al[4], including our present cases (Table 1).
In these reports, abdominal CT scan was most commonly utilized as imaging method during the follow up period. In our cases, we regularly followed up the patients with the interval of 6 mo and the abdominal CT scan was useful to detect the suspicious de novo lesions of remnant bile duct. The findings of PET-CT were consistent with the findings of abdominal CT scan. We preformed the second surgery in both cases without tissue confirmation preoperatively.
In our cases, the prior lesions were a type 1 Klatskin tumor and a middle common bile duct cancer. Fortunately, bile duct resection only allowed an R0 resection. The abdominal CT scans taken at 14 and 24 mo after the initial bile duct resection in cases 1 and 2 revealed new lesions confined to the distal remnant bile duct. These imaging findings prompted us to consider metachronous bile duct cancer rather than a loco-regional recurrence. The present two cases meet the criteria for multiple metachronous tumors proposed by Warren and Gates[5] for the following reasons: (1) abdominal CT and FDG PET-CT showed no evidence of a later lesion in the distal bile duct at the time of first surgery in both cases; (2) 14 and 24 mo elapsed until the new lesions were found on imaging studies in both cases; and (3) the first and second tumors occurred at different sites of EHBD that were far from each other with normal intervening bile duct epithelium, as confirmed by the tumor-free margins in both cases.
From our review of the cases reported thus far in the literature, we classified metachronous bile duct cancers according to the site of the tumor. Metachronous bile duct cancer depends on the remnant bile duct and must therefore fall into one of the types listed here (Figure 4). The remnant proximal bile duct was usually involved. Being aware of this rare metachronous tumor and its classification may be clinically valuable during long-term follow up after initial surgery because we can predict the potential site and proper second surgical methods needed to treat the metachronous bile duct.
In conclusion, because metachronous bile duct cancer can be successfully treated with a second surgery, patients with EHBD cancer should be followed up closely to avoid losing the opportunity to treat this curable condition.
A 70-year-old male and a 70-year-old female respectively presented a new lesion in remnant bile duct without symptoms which was discovered during the regular follow up after first surgery for extrahepatic bile duct cancer.
Loco-regional recurrences.
Laboratory test such as complete blood counts, serum amylase and liver function test in both patients were within normal limits.
Abdominal computed tomography scan of the female patient showed dilatation of the remnant distal bile duct and the polypoid mass whereas the abdominal CT scan of the male patient showed a new lesion with slight contrast enhancement at the distal remnant bile duct without definite mass.
Histopathology of the resected specimen of both patients confirmed adenocarcinoma of remnant bile duct invading into pancreas.
Both patients underwent pancreaticoduodenectomy for the newly developed lesions.
Unlike loco-regional recurrences which are usually unresectable with poor prognosis, the metachronous bile duct cancer may be resectable with favorable prognosis reported thus far.
Metachronous tumor is defined as tumors occurring more than 6 mo after the first primary cancer by Moertel.
As show in these two cases, metachronous bile duct cancer can be successfully treated with a second surgery so the patients with extrahepatic bile duct cancer should be followed up closely to avoid losing the opportunity to treat the curable condition.
This article shows the importance of differentiating curable the metachronous cholangiocellular carcinoma from loco regional recurrence of cholangiocellular carcinoma.
P- Reviewers: Montiel-Jarquín A, Rustemovic N, Zavoral M S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Gertsch P, Thomas P, Baer H, Lerut J, Zimmermann A, Blumgart LH. Multiple tumors of the biliary tract. Am J Surg. 1990;159:386-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Chijiiwa K. Synchronous carcinoma of the gall-bladder in patients with bile duct carcinoma. Aust N Z J Surg. 1993;63:690-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Kozuka S, Tsubone M, Hachisuka K. Evolution of carcinoma in the extrahepatic bile ducts. Cancer. 1984;54:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Merenda R, Portale G, Sturniolo GC, Marciani F, Faccioli AM, Ancona E. A rare surgical case of metachronous double carcinoma of the biliary tract. Scand J Gastroenterol. 2007;42:1265-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Warren S, Gates O. Multiple primary malignant tumors. A survey of the literature and a statistical study. Am J Cancer. 1932;16:1358-1414. |
| 6. | Moertel CG. Multiple primary malignant neoplasms: historical perspectives. Cancer. 1977;40:1786-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Sakamoto Y, Kosuge T, Shimada K, Sano T, Ojima H, Yamamoto J, Yamasaki S, Takayama T, Makuuchi M. Prognostic factors of surgical resection in middle and distal bile duct cancer: an analysis of 55 patients concerning the significance of ductal and radial margins. Surgery. 2005;137:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Todoroki T, Kawamoto T, Koike N, Fukao K, Shoda J, Takahashi H. Treatment strategy for patients with middle and lower third bile duct cancer. Br J Surg. 2001;88:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Ramírez-Merino N, Aix SP, Cortés-Funes H. Chemotherapy for cholangiocarcinoma: An update. World J Gastrointest Oncol. 2013;5:171-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 85] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Saiura A, Takayama T, Sano K, Toyoda H, Abe H, Kubota K, Mori M, Makuuchi M. Metachronous bile duct cancer in a patient surviving for a decade and undergoing curative surgery twice. Jpn J Clin Oncol. 1999;29:353-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Todoroki T, Fukuda Y, Kawamoto T, Saida Y, Ohara K, Iwasaki Y, Matsuzaki O. Long-term survivors after salvage surgery combined with radiotherapy for recurrence of stage IV main hepatic duct cancer--report of two cases. Hepatogastroenterology. 1993;40:285-293. [PubMed] |
| 12. | Seki H, Miyagawa S, Kobayashi A, Kawasaki S. Surgical treatment for biliary carcinoma arising after pancreatoduodenectomy. HPB Surg. 1998;10:395-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Nakakubo Y, Kondo S, Omi M, Hirano S, Ambo Y, Morikawa T, Okaushiba S, Kato H, Shimizu M. A case of heterochromic development of extrahepatic bile duct carcinoma and cholangiocellular carcinoma. Nihon Syokakigeka Gakkaizasshi. 2001;34:1429-1432. |
| 14. | Okamoto A, Tsuruta K, Matsumoto G, Takahashi T, Kamisawa T, Egawa N, Funata N. Papillary carcinoma of the extrahepatic bile duct: characteristic features and implications in surgical treatment. J Am Coll Surg. 2003;196:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Yoon YS, Kim SW, Jang JY, Park YH. Curative reoperation for recurrent cancer of the extrahepatic bile duct: report of two cases. Hepatogastroenterology. 2005;52:381-384. [PubMed] |
| 16. | Hibi T, Sakamoto Y, Tochigi N, Ojima H, Shimada K, Sano T, Kosuge T. Extended right hemihepatectomy as a salvage operation for recurrent bile duct cancer 3 years after pancreatoduodenectomy. Jpn J Clin Oncol. 2006;36:176-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |