Published online Mar 21, 2014. doi: 10.3748/wjg.v20.i11.2995
Revised: September 28, 2013
Accepted: November 2, 2013
Published online: March 21, 2014
Processing time: 239 Days and 19.3 Hours
AIM: To assess the endoscopic activity before and after a one-year period of biological therapy and to evaluate the frequency of relapses and need for retreatment after stopping the biologicals in patients with Crohn’s disease (CD) and ulcerative colitis (UC).
METHODS: The data from 41 patients with CD and 22 patients with UC were assessed. Twenty-four CD patients received infliximab, and 17 received adalimumab. The endoscopic severity of CD was quantified with the simplified endoscopic activity score for Crohn’s disease in CD and with the Mayo endoscopic subscore in UC.
RESULTS: Mucosal healing was achieved in 23 CD and 7 UC patients. Biological therapy had to be restarted in 78% of patients achieving complete mucosal healing with CD and in 100% of patients with UC. Neither clinical remission nor mucosal healing was associated with the time to restarting the biological therapy in either CD or UC.
CONCLUSION: Mucosal healing did not predict sustained clinical remission in patients in whom the biological therapies had been stopped.
Core tip: Mucosal healing has become a subject of renewed interest, as tumor necrosis factor-α blockers have proven their efficacy in inducing and maintaining clinical and endoscopic remission. In this study, mucosal healing was observed in 56% and 32% of Crohn’s disease (CD) and ulcerative colitis (UC) patients. Retreatment with biological therapies was required in 78% of CD patients and in 100% of UC patients, despite achieving mucosal healing within 12 mo. Our results showed that mucosal healing after 12 mo of treatment was not associated with sustained clinical remission.
- Citation: Farkas K, Lakatos PL, Szűcs M, Pallagi-Kunstár &, Bálint A, Nagy F, Szepes Z, Vass N, Kiss LS, Wittmann T, Molnár T. Frequency and prognostic role of mucosal healing in patients with Crohn’s disease and ulcerative colitis after one-year of biological therapy. World J Gastroenterol 2014; 20(11): 2995-3001
- URL: https://www.wjgnet.com/1007-9327/full/v20/i11/2995.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i11.2995
Colonoscopies still play an important role in the diagnosis, management and monitoring of inflammatory bowel disease (IBD) - a chronic, relapsing inflammatory condition of the gut. IBD comprises Crohn’s disease (CD) and ulcerative colitis (UC). Mucosal healing is now regarded as one of the most important goals in the treatment of IBD, considering that mucosal healing can alter the course of the disease due to its association with sustained clinical remission and reduced rates of hospitalization and surgery[1]. Although endoscopy provides a direct evaluation of the mucosal lesions in IBD and intestinal activity may be quantified by indices of endoscopic activity, a clear definition of mucosal healing is still lacking. Most of the clinical trials on CD define mucosal healing as the total disappearance of mucosal ulcerations[2]. The simplified endoscopic activity score for Crohn’s disease (SES-CD), developed by Daperno et al[3] has become a relatively easy tool for the assessment of mucosal lesions in CD, although it is not widely used in practice. In UC, several endoscopic indices have been used in clinical trials to evaluate the endoscopic activity[4]; however, the Mayo endoscopic score remains the most commonly used, not only in trials but also in clinical practice[5]. The weaknesses of these endoscopic activity indices include the absence of a clear definition and the lack of validation of mucosal healing[6].
Mucosal healing has become a subject of renewed interest, as TNF-α blockers have proven their efficacy in inducing and maintaining clinical and endoscopic remission in both CD and UC. In CD, the effect of infliximab on mucosal healing has been examined in substudies of larger clinical trials. In the substudy of the ACCENT I trial[7], 50% of patients receiving scheduled infliximab achieved mucosal healing at week 54.
Routine endoscopic follow-up is recommended for all CD patients who have achieved clinical remission with medical therapy; for those with persistent complaints, in order to rule out post-inflammatory irritable bowel syndrome; for those still within their first year after surgery; and for those who are stopping biological therapies but continuing immunosuppressants[8,9]. Combined immunosuppression is seems to be associated with higher rates of mucosal healing therefore treatment intensification is not recommended. However, mucosal healing at the time of treatment withdrawal may predict better outcomes in CD[2].
According to the Hungarian reimbursement regulations (National Health Insurance Fund Administration), biological therapies (infliximab approved for the treatment of CD and UC and adalimumab approved for the treatment of CD) have to be discontinued after a one-year treatment period. The endoscopic healing of the mucosa is commonly evaluated at the end of the one-year treatment period with anti-TNF blockers in the Hungarian biological centers. In the present study, our aim was to assess the endoscopic activity and the rate of mucosal healing after a one-year period of biological therapy and to evaluate how the endoscopic findings of the mucosa predict the frequency of relapses and the need for restarting biological therapy after stopping in patients with CD and UC.
This was a prospective observational study conducted at 2 Hungarian tertiary referral biological centers in the First Department of Medicine, University of Szeged and First Department of Medicine, Semmelweis University between January 2010 and December 2011. The study was approved by the Semmelweis University Regional and Institutional Committee of Science and Research Ethics and by the Regional and Institutional Human Medical Biological Research Ethics Committee of the University of Szeged. The analysis focused on patients who underwent an ileocolonoscopy before and after the one-year biological therapy and in whom anti-TNFs were discontinued at the end of the year. Endoscopies were performed by four experienced gastroenterologists (Molnár T, Lakatos PL, Nagy F, and Szepes Z) after stopping the one-year biological therapy.
Forty-one consecutive CD patients (25 females, 16 males, mean disease duration at the beginning of biological therapy: 5 years) and 22 UC patients (14 females, 8 males, mean disease duration at the beginning of biological therapy: 9.1 years) were prospectively followed up in this study. All patients received maintenance infliximab or adalimumab therapy for one year in accordance with Hungarian regulations. Diagnosis was based on the Lennard-Jones Criteria[10]. CD disease phenotypes were determined according to the Montreal Classification[11]. Twenty-four CD patients received infliximab, and 17 received adalimumab. All of the UC patients received infliximab. These patients received the last dose of biological therapy at least 3 mo before the repeated one-year therapy. Twenty-eight patients were naive to biological therapy (did not receive biological therapy before the one-year treatment period analyzed in the study) in the CD group, and 21 patients, in the UC group. The concomitant immunosuppression during the induction therapy was steroids in 42 and azathioprine in 51 patients. The clinical characteristics of the patients are presented in Table 1. Patient data regarding smoking status, previous appendectomy, perianal involvement, presence of extraintestinal manifestation, concomitant immunosuppressive medications, outcome of induction therapy, previous surgical procedures, and previous biological therapy were collected. Biopsy samples were not taken routinely.
| CD patients (n = 41) | UC patients (n = 22) | |
| Female/male | 25/16 | 14/8 |
| Mean age at diagnosis (yr) | 28 (15-59) | 33 (14-53) |
| Mean age at the beginning of biological therapy (yr) | 32 (20-62) | 43 (17-66) |
| Age at diagnosis | ||
| < 16 yr (A1) | 4 | 2 |
| 17-40 yr (A2) | 31 | 14 |
| > 40 yr (A3) | 6 | 6 |
| Location | ||
| Ileal (L1) | 6 | - |
| Colonic (L2) | 7 | - |
| Ileocolonic (L3) | 28 | - |
| Upper GI (L4) | 0 | - |
| Proctitis | - | 0 |
| Left-sided colitis | - | 14 |
| Extensive colitis | - | 8 |
| Behaviour | ||
| Inflammatory (B1) | 12 | - |
| Stricturing (B2) | 8 | - |
| Penetrating (B3) | 21 | - |
| Perianal manifestation | 23 | - |
| Extraintestinal manifestation | 26 | 9 |
| Concomitant medications | ||
| Corticosteroids | 32 | 10 |
| Azathioprine | 35 | 16 |
| Surgery before the biological therapy | 19 | 3 |
| Previous biological therapy | 13 | 1 |
| Median CDAI/pMayo at the start of biological therapy | 340 | 8 |
| Median CRP level at the start of biological therapy (mg/L) | 10 | 9.5 |
| Current smokers | 18 | 0 |
| Appendectomy | 7 | 0 |
Clinical activities, as determined by the Crohn’s Disease Activity Index (CDAI - with hematocrit value)[12] in CD and by the Mayo score[4] in UC, were calculated at the end of the biological therapy when the endoscopic assessment was performed, while partial Mayo scores were calculated when biological therapy needed to be restarted. Clinical remission was defined as a CDAI of < 150 points and a Mayo score of < 2 points. Sustained clinical remission was defined as a stable, steroid-free clinical remission during the 1-year follow-up period. The definition of relapse and indication for restarting biologicals were an increase of > 100 points in CDAI and a CDAI of > 150 points and a partial Mayo score of > 3 points.
The endoscopic severity of CD was quantified with SES-CD in CD[2] and with Mayo endoscopic subscore in UC[4]. The endoscopic scores were prospectively assessed by two investigators (Molnár T, Lakatos PL). Mucosal healing was defined using the endoscopic indices as SES-CD between 0 and 3 and Mayo endoscopic subscore as 0.
Data collection and analysis were performed at the 1st Department of Medicine at the University of Szeged. The primary endpoint of the study was the proportion of mucosal healing in IBD after the one-year period of biological therapy. The secondary endpoint was the frequency of relapses in the next year after achieving mucosal healing.
Variables were tested for normality using Shapiro-Wilk’s W test. The χ2-test and χ2-test with Yates correction and logistic regression analysis were used to assess the association between categorical clinical variables and clinical/endoscopic outcomes. The variables analyzed were gender, disease duration, active smoking, appendectomy, location/extent, behavior, associated perineal disease, type of anti-TNF agent, extraintestinal manifestations, steroid and azathioprine therapy during the induction period, previous surgery, previous biological therapy, clinical activities, CRP levels (mg/L), and outcomes of induction therapy. The difference between patients with mucosal healing and those who failed to achieve endoscopic remission was assessed by chi-square or Fisher’s exact tests. Kaplan-Meier survival curves were plotted for analysis with the Log-Rank and Breslow tests. A P value < 0.05 was considered significant. For the statistical analysis, SPSS15.0 (SPSS Inc., Chicago, IL) was used.
The median CDAI was 60 (interquartile range: 39.3-96) (P < 0.001) and the partial Mayo score was 0 (interquartile range: 0-4) (P < 0.001) at the end of the treatment period. A total of 35/41 patients with CD (85%) and 12/22 with UC (55%) achieved clinical remission at the end of the year of biological therapy.
Colonoscopies reached the terminal ileum in every case. The median values of the SES-CD and the Mayo endoscopic subscores significantly improved after the anti-TNF therapy [16 (interquartile range: 12-23) vs 5 (interquartile range: 3-9), P < 0.001, and 3 (interquartile range: 2-3) vs 1 (interquartile range: 0-2), P < 0.001]. Mucosal healing was achieved in 23 CD (56%) and 7 UC (32%) patients. At the end of one year of treatment, clinical remission was achieved in 96% of CD patients with mucosal healing and in 71% of UC patients with mucosal healing. Deep remission - both mucosal healing and clinical remission - was achieved in 22 CD and 5 UC patients.
During the one-year follow up period, 11 patients in the clinical remission group and 18 patients in the deep remission group had to be retreated. In CD, biological therapy was restarted due to clinical relapse in 32 (78%) patients after a median 5 mo (interquartile range: 3.5-6 mo). The median CDAI was 332 (interquartile range: 121-371) at the time of relapse. In UC, biological therapy needed to be restarted in 13 patients (59%) after a median 7.5 mo (interquartile range: 4-11 mo). The median partial Mayo score was 6.5 (interquartile range: 5.3-7) at the time of retreatment. Of note, therapy was restarted in the two patients who achieved mucosal healing, and 5 of the 7 patients who achieved clinical remission and all 5 patients who achieved deep remission had to be retreated within one year. Endoscopic activity was not assessed in every patient when the biological therapy was restarted. The response rates for retreatment were 81% in CD and 54% in UC within an average of 8 wk after the reintroduction of the therapy.
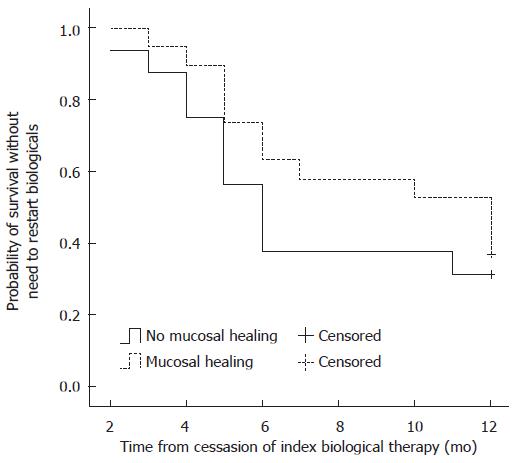
In a univariate or Kaplan-Meier analysis using the Log-Rank and Breslow tests, neither clinical remission nor mucosal healing was associated with the time to restarting biological therapy in either CD (Figure 1) or UC. In univariate analysis, none of the investigated parameters (e.g., gender, disease duration, smoking status, history of appendectomy, location/extent, behavior, the type of anti TNF-α therapy, extraintestinal manifestations, steroid therapy at inclusion, previous surgery, previous biological therapy, CRP level, or the effect of induction therapy) was associated with the need to restart biological therapy in either CD or UC (Table 2). No association was found between combined immunomodulator therapy and mucosal healing (Table 2).
| Factor | CD-P value | UC-P value |
| Gender | 0.98 | 0.57 |
| Disease duration | 0.09 | 0.12 |
| Smoking status | 0.99 | 0.37 |
| Appendectomy | 0.99 | - |
| Location/extent | 0.99 | 0.81 |
| Behaviour | 0.99 | - |
| Type of anti TNF-α therapy | 0.73 | - |
| Extraintestinal manifestations | 0.35 | - |
| Steroid therapy at inclusion | 0.15 | 0.09 |
| Previous surgery | 0.99 | - |
| Previous biological therapy | 0.38 | - |
| Elevated CRP level | 0.47 | 0.97 |
| Combined immunomodulator use | 0.22 | 0.59 |
| Outcome of induction therapy | 0.30 | 0.29 |
In this prospective observational study conducted in patients with CD and UC receiving biological therapy for one year, mucosal healing was observed in 56% and 32% of the patients, respectively. Deep remission, including both clinical and endoscopic remission, was detected in 54% and 23% of patients with CD and UC. Retreatment with biological therapy was necessary in 78% of CD patients and in 100% of UC patients, despite their achieving mucosal healing within 12 mo. Our results showed that mucosal healing after 12 mo of treatment was not associated with sustained clinical remission.
Mucosal healing seems to be associated with better outcomes (reduced rate of hospitalization, complications, surgery) in CD[13,14]. The study of Ananthakrishnan demonstrated that mucosal healing as an endpoint is cost effective in CD patients initiating infliximab therapy[15]. In a Norwegian study, mucosal healing was associated with lower colectomy rate in UC and decreased need for steroid treatment in CD[16]. The STORI trial suggested that one of the predictors of relapse after discontinuation of biological therapy was the absence of mucosal healing at the time of drug withdrawal[9]. The study of Baert et al[17] confirmed that complete mucosal healing after 2 years of therapy in patients with early stage CD predicted sustained steroid-free remission 3 and 4 years after therapy was initiated.
Regarding the therapeutic repertoire of IBD, biological agents proved to be the most effective in inducing mucosal healing. The ACCENT I study confirmed that scheduled infliximab therapy is more effective in achieving mucosal healing than episodic treatment[14] for CD. The beneficial effect of the combined use of infliximab and azathioprine on mucosal healing was proven by the SONIC trial, in which the achievement of mucosal healing occurred in 44% of patients receiving both therapies[18]. In the EXTEND trial, 24% of adalimumab-treated patients reached complete mucosal healing at week 52[19]. The ACT trials confirmed the efficacy of infliximab in inducing and maintaining mucosal healing in active UC[7]. The combined use of infliximab and azathioprine resulted in 63% mucosal healing in the SUCCESS trial[20]. In our study, anti TNF-α therapy proved to be more effective in achieving mucosal healing in CD than in UC (almost twice as many CD patients achieved endoscopic remission as UC patients). This result may be due to the difference in the sizes of the inflamed area, the enrolled patient number and the proportion of patients with previous biological therapy in the CD and UC groups. No differences were detected in the efficacy between infliximab and adalimumab; however, it should be noted that adalimumab was used in a lower number of patients.
In the future, an important question for discussion is exactly when mucosal healing should be established. International guidelines recommend assessing endoscopic healing after stopping the therapy with anti-TNF agents. Our results do not support this recommendation after one-year therapy because more than 80% of the patients with mucosal healing relapsed and needed retreatment. From another point of view, to date, there is no established guideline on when biological therapy can be discontinued. According to the recent London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD, the withdrawal of biological therapy is suggested in CD patients who have both complete mucosal healing and no biological evidence of inflammation[21]. In our study, 85% of patients with deep remission relapsed within a year. The response rates for retreatment were 81% in CD and 54% in UC. In the STORI study, infliximab therapy was terminated in 115 CD patients in clinical remission after treatment with a combination of scheduled infliximab and a stable dose of immunosuppressant for at least one year[9]. Forty-five percent of patients relapsed following withdrawal from infliximab. Re-treatment with infliximab was effective and well tolerated in 88% of patients who experienced a relapse. In a Danish single-center study, 24% of CD patients and 30% of UC patients discontinued infliximab while in clinical steroid-free remission[22]. The proportion of patients in remission declined steadily, with 61% of CD patients and 75% of UC patients remaining in remission after 1 year. Half of these patients maintained their remission after a median of 2 years. In total, 96% of CD patients and 71% of UC patients experienced complete clinical remission when retreated with infliximab after their relapses. In the longitudinal cohort study by Waugh et al[23] 50% of patients relapsed within 477 d, while 35% remained in sustained clinical remission for nearly 7 years.
There are some limitations of this study that should be mentioned. First, biopsy samples were not taken routinely to assess microscopic activity of IBD. Theoretically, the microscopic evaluation of the mucosa reflects the therapeutic response more accurately than an endoscopy, but it should be noted that the histological assessment of biopsy samples demonstrates only mucosal abnormalities. In CD, the transmural pattern is difficult to evaluate[24] in this way. The recently published paper by Bessissow et al[25] demonstrated that the presence of basal plasmacytosis predicts UC clinical relapse in patients with complete mucosal healing (OR = 5.13, 95%CI: 1.32-19.99).
Moreover, the study of Laharie et al[26] did not find any correlation between histologically confirmed microscopic inflammation and endoscopic activity indices. Therefore, the need for microscopic evaluation in the assessment of mucosal healing may be worth reassessing. Second, the sample size is a bit small to draw significant conclusions, although we think that the tendency of these results is interesting and worth considering. Third, there is no universal agreement regarding an acceptable definition of mucosal healing. In this study, mucosal healing was evaluated on the basis of validated endoscopic activity indices; however, in clinical practice, the disappearance of mucosal ulcers and erosions may be used more frequently.
Currently, the primary goals of treatment in IBD are not only the induction and maintenance of clinical remission but also the induction of mucosal healing in an attempt to alter the course of the disease. Endoscopy is still the gold standard method of assessing changes of the mucosa. Considering that the macroscopic findings of the mucosa represent its real alterations after the initiation of a new therapy, mucosal healing represents a more reliable and objective marker in the assessment of therapeutic response than clinical activity indices. However, none of the studies mentioned above support that mucosal healing correlates with clinical activity. Our results also revealed a high proportion of patients who relapsed after mucosal healing. The higher relapse rates in patients who achieved mucosal healing may be explained by the shorter duration of their biological therapy and less frequent use of combined immunosuppressive therapy than in previous similar studies. In this respect, our results call into question the routine endoscopic examinations at the end of the one-year period of biological therapy. Stopping or continuing the biological therapy may be determined by assessing the patient’s general condition and the clinical activity. Certainly, large controlled clinical trials are required to confirm these results. However, based on our observations, we conclude that the long-term advantages of mucosal healing can be achieved only if we continue previous effective therapies, even after the endoscopic examination.
Mucosal healing is now regarded as one of the most important goals in the treatment of inflammatory bowel disease. Mucosal healing is associated with a lower colectomy rate and a decreased need for steroid treatment. Tumor necrosis factor (TNF)-α blockers have proven their efficacy in inducing and maintaining clinical and endoscopic remission in both Crohn’s disease (CD) and ulcerative colitis (UC). In Hungarian biological centers, the endoscopic healing of the mucosa is commonly evaluated at the end of the one-year treatment period with anti-TNF blockers.
Previous studies have suggested that the absence of mucosal healing at the time of discontinuation of biological therapy predicted a relapse. Scheduled infliximab therapy is more effective in achieving mucosal healing than episodic treatment.
Mucosal healing can alter the course of the disease, as it is associated with sustained clinical remission and reduced rates of hospitalization and surgery. Exactly when mucosal healing should be established is an important question. The results call into question the routine endoscopic examinations at the end of a one-year period of biological therapy.
Anti TNF-α therapy proved to be more effective in achieving mucosal healing in CD than in UC. Retreatment with biological therapy was needed in 78% of CD patients and in 100% of UC patients, despite their achieving mucosal healing within 12 mo. The results call into question the routine endoscopic examinations performed at the end of the one-year period of biological therapy.
This is an interesting study with the important message that mucosal healing does not predict “sustained” clinical remission if the biologicals are stopped after one year of treatment.
P- Reviewers: Efthymiou A, Liu TC, Nielsen OH S- Editor: Wen LL L- Editor: A E- Editor: Zhang DN
| 1. | Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 354] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 2. | Armuzzi A, Van Assche G, Reinisch W, Pineton de Chambrun G, Griffiths A, Sladek M, Preiss JC, Lukas M, D’Haens G. Results of the 2nd scientific workshop of the ECCO (IV): therapeutic strategies to enhance intestinal healing in inflammatory bowel disease. J Crohns Colitis. 2012;6:492-502. [PubMed] |
| 3. | Daperno M, D’Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, Sostegni R, Rocca R, Pera A, Gevers A. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 999] [Cited by in RCA: 1322] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 4. | Dave M, Loftus EV. Mucosal healing in inflammatory bowel disease-a true paradigm of success? Gastroenterol Hepatol (N Y). 2012;8:29-38. [PubMed] |
| 5. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1958] [Cited by in RCA: 2252] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 6. | Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 664] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 7. | Rutgeerts P, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology. 2004;126:402-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 677] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 8. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2744] [Cited by in RCA: 2886] [Article Influence: 144.3] [Reference Citation Analysis (2)] |
| 9. | Louis E, Mary JY, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Dupas JL, Pillant H, Picon L, Veyrac M. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142:63-70.e5; quiz e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 459] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 10. | Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2-6; discussion 16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1445] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 11. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [PubMed] |
| 12. | Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439-444. [PubMed] |
| 13. | Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, Hoffman I, Van Steen K, Vermeire S, Rutgeerts P. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis. 2009;15:1295-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 533] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 14. | Rutgeerts P, Diamond RH, Bala M, Olson A, Lichtenstein GR, Bao W, Patel K, Wolf DC, Safdi M, Colombel JF. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn’s disease. Gastrointest Endosc. 2006;63:433-442; quiz 464. [PubMed] |
| 15. | Ananthakrishnan AN, Korzenik JR, Hur C. Can mucosal healing be a cost-effective endpoint for biologic therapy in Crohn’s disease? A decision analysis. Inflamm Bowel Dis. 2013;19:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Frøslie KF, Jahnsen J, Moum BA, Vatn MH. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 873] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 17. | Baert F, Moortgat L, Van Assche G, Caenepeel P, Vergauwe P, De Vos M, Stokkers P, Hommes D, Rutgeerts P, Vermeire S. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138:463-468; quiz e10-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 647] [Article Influence: 43.1] [Reference Citation Analysis (35)] |
| 18. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2376] [Article Influence: 158.4] [Reference Citation Analysis (1)] |
| 19. | Rutgeerts P, Van Assche G, Sandborn WJ, Wolf DC, Geboes K, Colombel JF, Reinisch W, Kumar A, Lazar A, Camez A. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology. 2012;142:1102-1111.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 452] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 20. | Panaccione R, Ghosh S, Middleton S. Infliximab, azathioprine, or infliximab azathioprine for treatment of moderate to severe ulcerative colitis. Gastroenterology. 2011;140:S134. |
| 21. | D’Haens GR, Panaccione R, Higgins PD, Vermeire S, Gassull M, Chowers Y, Hanauer SB, Herfarth H, Hommes DW, Kamm M. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2011;106:199-212; quiz 213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 301] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 22. | Steenholdt C, Molazahi A, Ainsworth MA, Brynskov J, Østergaard Thomsen O, Seidelin JB. Outcome after discontinuation of infliximab in patients with inflammatory bowel disease in clinical remission: an observational Danish single center study. Scand J Gastroenterol. 2012;47:518-527. [PubMed] |
| 23. | Waugh AW, Garg S, Matic K, Gramlich L, Wong C, Sadowski DC, Millan M, Bailey R, Todoruk D, Cherry R. Maintenance of clinical benefit in Crohn’s disease patients after discontinuation of infliximab: long-term follow-up of a single centre cohort. Aliment Pharmacol Ther. 2010;32:1129-1134. [PubMed] |
| 24. | Freeman HJ. Limitations in assessment of mucosal healing in inflammatory bowel disease. World J Gastroenterol. 2010;16:15-20. [PubMed] |
| 25. | Bessissow T, Lemmens B, Ferrante M, Bisschops R, Van Steen K, Geboes K, Van Assche G, Vermeire S, Rutgeerts P, De Hertogh G. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am J Gastroenterol. 2012;107:1684-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 26. | Laharie D, Filippi J, Roblin X, Nancey S, Chevaux JB, Hébuterne X, Flourié B, Capdepont M, Peyrin-Biroulet L. Impact of mucosal healing on long-term outcomes in ulcerative colitis treated with infliximab: a multicenter experience. Aliment Pharmacol Ther. 2013;37:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |