Published online Mar 21, 2014. doi: 10.3748/wjg.v20.i11.2888
Revised: November 15, 2013
Accepted: January 6, 2014
Published online: March 21, 2014
Processing time: 171 Days and 16.4 Hours
Hepatitis C virus (HCV) infection disrupts the normal metabolism processes, but is also influenced by several of the host’s metabolic factors. An obvious and significantly detrimental pathophysiological feature of HCV infection is insulin resistance in hepatic and peripheral tissues. Substantial research efforts have been put forth recently to elucidate the molecular mechanism of HCV-induced insulin resistance, and several cytokines, such as tumor necrosis factor-α, have been identified as important contributors to the development of insulin resistance in the distant peripheral tissues of HCV-infected patients and animal models. The demonstrated etiologies of HCV-induced whole-body insulin resistance include oxidative stress, lipid metabolism abnormalities, hepatic steatosis and iron overload. In addition, myriad effects of this condition have been characterized, including glucose intolerance, resistance to antiviral therapy, progression of hepatic fibrosis, development of hepatocellular carcinoma, and general decrease in quality of life. Metabolic-related conditions and disorders, such as visceral obesity and diabetes mellitus, have been shown to synergistically enhance HCV-induced metabolic disturbance, and are associated with worse prognosis. Yet, the molecular interactions between HCV-induced metabolic disturbance and host-associated metabolic factors remain largely unknown. The diet and lifestyle recommendations for chronic hepatitis C are basically the same as those for obesity, diabetes, and metabolic syndrome. Specifically, patients are suggested to restrict their dietary iron intake, abstain from alcohol and tobacco, and increase their intake of green tea and coffee (to attain the beneficial effects of caffeine and polyphenols). While successful clinical management of HCV-infected patients with metabolic disorders has also been achieved with some anti-diabetic (i.e., metformin) and anti-lipid (i.e., statins) medications, it is recommended that sulfonylurea and insulin be avoided.
Core tip: A specific pathophysiologic feature of hepatitis C virus (HCV) infection is whole-body insulin resistance, which is related to oxidative stress, lipid metabolism abnormalities, hepatic steatosis, and iron overload. Host metabolic factors synergistically enhance the HCV-induced metabolic disturbance, affectively deteriorating the clinical course in patients with chronic hepatitis C. Consequently, diet, lifestyle and medications appropriate for metabolic disorders are important for management of HCV-infected patients to improve their prognosis.
- Citation: Kawaguchi Y, Mizuta T. Interaction between hepatitis C virus and metabolic factors. World J Gastroenterol 2014; 20(11): 2888-2901
- URL: https://www.wjgnet.com/1007-9327/full/v20/i11/2888.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i11.2888
Epidemiological and clinical studies have shown that patients with chronic liver disease, especially those infected with the hepatitis C virus (HCV), have a higher prevalence of glucose intolerance than the general population[1-8]. Insulin resistance appears to be a central feature of the pathogenesis of HCV-induced glucose intolerance. Eradication of HCV by antiviral therapy has been shown to ameliorate insulin resistance, both in hepatic tissues[4] and whole body[9]; in addition, these patients with antiviral treatment response show significantly lower incidence rates of glucose metabolism abnormalities during the subsequent follow-up[10,11].
Insulin resistance has emerged as an important prognostic factor for the clinical course of HCV infection, due to its association with resistance to antiviral therapy[12-18], progression of hepatic fibrosis[13,19-24], development of hepatocellular carcinoma (HCC)[25], and poor quality of life[26]. In addition, insulin resistance, as well as oxidative stress, has been shown to contribute to the HCV-related disruptions in host metabolic factors, particularly lipids and iron[27-31]. Visceral obesity has been shown to enhance HCV-induced insulin resistance[32], and HCV infection in patients with both obesity and diabetes mellitus has been reported to strongly promote the development of HCC[33]. Thus, synergistic effects of viral and metabolic factors are hypothesized to contribute to hepatocarcinogenesis.
Liver cirrhosis, regardless of etiology, leads to marked metabolic disturbances in protein-energy malnutrition[34], whole-body insulin resistance[35,36], and peripheral hyperinsulinemia[37-40]. Thus, the pathophysiology of liver cirrhosis is not included in the present discussion of interactive and synergistic relationships between HCV-specific metabolic disturbances. Instead, we provide overviews of the following: (1) insulin signaling factors and pathways that play important roles in glucose and lipid metabolism; (2) mechanism of HCV-induced insulin resistance in multiple organs; (3) mechanisms of altered lipid metabolism and hepatic steatosis under conditions of HCV infection; (4) interactions between the iron metabolism and oxidative stress pathways in HCV infection; (5) impact of host-related metabolic factors on HCV-induced metabolic disturbance; and (6) recommendations for diet, lifestyle and medications aimed at protecting against or resolving metabolic disorders in HCV-infected non-cirrhosis patients.
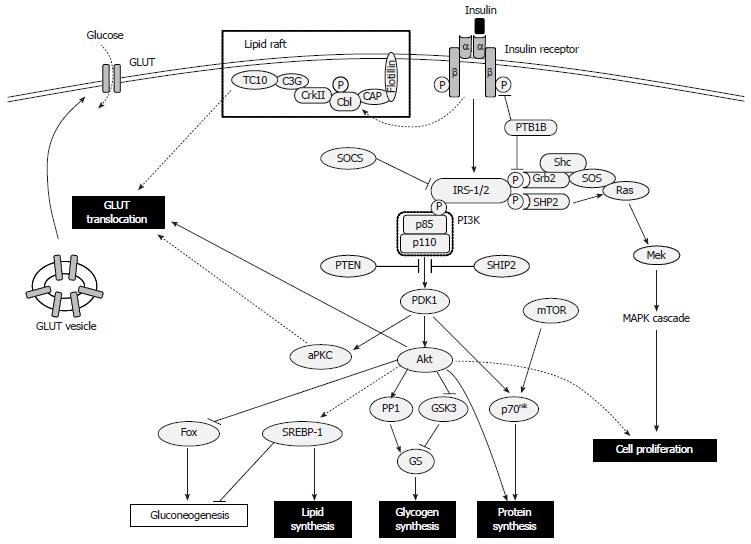
Insulin controls postprandial blood glucose levels by increasing glucose uptake in muscle and fat, and reducing hepatic glucose production. Insulin stimulates cellular synthesis of glycogen, proteins and lipids, and inhibits glycogenolysis, protein breakdown and lipolysis, thereby facilitating storage of these substrates. The uptake of glucose by muscle and fat cells is promoted through insulin’s stimulation of glucose transporter (GLUT) 4 translocation from the cytoplasm to the plasma membrane. Although insulin does not affect GLUT2 in hepatocytes, it blocks gluconeogenesis and glycogenolysis, and stimulates glycogen synthesis[41].
The biological action of insulin involves modulation of a cascade of intracellular signaling molecules in response to circulating insulin binding to its cognate cell surface receptor. The insulin receptor is a tetrameric complex, consisting of two extracellular insulin-binding α-subunits and two β-subunits transversing the cell membrane; these subunits function as allosteric enzymes, whereby the α-subunit inhibits the tyrosine kinase activity of the β-subunit[42]. Furthermore, insulin binding promotes its receptor’s autophosphorylation, which leads to tyrosine phosphorylation of the intracellular insulin receptor substrate (IRS)-1 and IRS-2, initiating a cascade of multifaceted events[41]. Conversely, serine phosphorylation of the IRS proteins attenuates insulin signaling by decreasing insulin-stimulated tyrosine phosphorylation; this action acts as a negative feedback signal under normal physiologic conditions, providing a crosstalk mechanism between pathways that are not directly modulated by insulin but which can produce insulin resistance[43-46]. Additional factors that suppress activation of IRS proteins have also been implicated in development of insulin resistance; these include the protein tyrosine phosphatases (PTPs), especially PTP1B, which dephosphorylate tyrosine residues on the insulin receptor or IRS-1/2[47], and the suppressor of cytokine signaling (SOCS) proteins, SOCS-1 and SOCS-3, which promote ubiquitin-mediated IRS-1 and IRS-2 degradation[48].
The phosphatidyl inositol 3-kinase (PI3K)-Akt pathway is a key transducer of the insulin-mediated metabolic signal[41,49]. PI3K itself consists of a p110 catalytic subunit and a p85 regulatory subunit. IRS proteins activate PI3K by phosphorylating two SH2 domains in the p85 component[50]. Subsequently, the p110 component of PI3K phosphorylates the membrane phospholipid phosphatidylinositol 4,5-bisphosphate at the 3’ position. The resultant phosphatidylinositol 3,4,5-triphosphate (PIP3) regulates the phosphoinositide-dependent kinase 1, which phosphorylates and activates Akt[51]. Overexpression of the phosphatase and tensin homolog[52] and the SH2 domain-containing inositol-5-phosphatase[53] leads to decreased levels of PIP3, resulting in inhibition of the PI3K-Akt pathway.
The following three pathways regulate glucose uptake: PI3K-Akt[54]; PI3K-atypical protein kinase C (aPKC, composed of PKC ζ/λ)[55]; and lipid raft-expressed CAP-Cbl-TC10[56,57]. For all, the PI3K-Akt pathway is critical for GLUT4 translocation. Upon activation, Akt inhibits glycogen synthase kinase-3[58] and activates protein phosphatase 1[59], thereby activating glycogen synthase by promoting its dephosphorylation. Insulin itself inhibits gluconeogenesis and glycogenolysis through its modulation of certain process-related transcription factors, such as hepatic nuclear factor-4, members of the forkhead protein family and peroxisome proliferator-activated receptor (PPAR)γ co-activator 1, and increases lipogenesis by modulating the sterol regulatory element binding protein (SREBP)-1[41]. Insulin-mediated PI3K pathway and mammalian target of rapamycin (mTOR) signaling activate p70 ribosomal S6 kinase, which synthesizes proteins and modulates the mammalian translation machinery[60]. Insulin can also stimulate cellular proliferation and differentiation by perturbing Ras activation by Grb2-SOS, which modulates the downstream mitogen-activated protein kinase (MAPK) signaling cascade[61,62] (Figure 1).
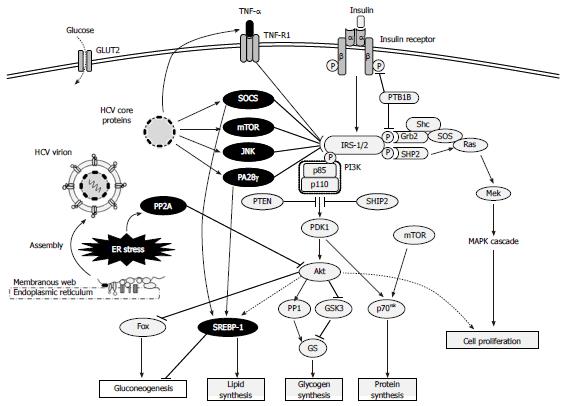
A study using liver biopsy specimens obtained from non-diabetic HCV-infected patients showed that HCV impaired the insulin-stimulated tyrosine phosphorylation of hepatic IRS-1, resulting in reduced PI3K-Akt activation without any alterations in the MAPK pathway[63]. Subsequent study of a transgenic mouse model expressing the HCV genotype 1 core protein indicated that the core protein was responsible for inducing hepatic insulin resistance via suppression of IRS-1 tyrosine phosphorylation, which was shown to ultimately lead to overt diabetes[64]. When human hepatoma cell lines were infected with HCV genotype 1, the core protein-mediated induction of SOCS-3 was shown to promote proteosomal degradation of IRS-1/2 through ubiquitination, resulting in inhibition of the PI3K-Akt pathway[65]. Although another study demonstrated that HCV genotype 2 did not mediate the same IRS-1/2 degradation via up-regulation of SOCS-3[66], both of the core proteins derived from genotype 2 and genotype 1 have been shown to increase serine phosphorylation of IRS-1 through the c-Jun N-terminal kinase (JNK) signaling pathway to decrease tyrosine phosphorylation of IRS-1 and impair the PI3K-Akt pathway[44]. In addition, the core protein of genotype 1 has been shown to promote IRS-1 degradation through mTOR activation[67] and to suppress phosphorylation of tyrosine on IRS-1, as well as the production of IRS-2, through modulation of a proteasome activator (PA)28γ-dependent pathway[68]. The core protein of HCV genotype 3 can also promote IRS-1 degradation, and this effect is mediating by its effects on PPARγ that lead to up-regulation of SOCS-7[67,69]. In general, HCV can also inhibit insulin signaling by dephosphorylation of Akt, and this mechanism has been shown to involve the endoplasmic reticulum (ER) stress signal inducing overexpression of protein phosphatase (PP)2A[70-72] (Figure 2).
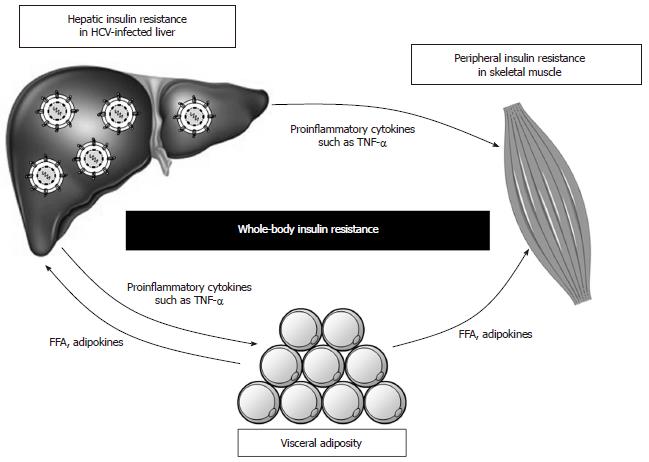
Under normal physiologic conditions, skeletal muscle accounts for up to 75% of insulin-dependent glucose disposal, while adipose tissue accounts for only a small fraction[41]. However, obese and diabetic patients simultaneously develop insulin resistance in liver, skeletal muscle, and fat. HCV-infected patients also develop insulin resistance in peripheral tissues (mainly skeletal muscle) as well as the liver, although the molecular mechanism remains unclear.
One clinical study using a euglycemic hyperinsulinemic clamp showed that HCV infection increased endogenous glucose production (reflecting hepatic insulin resistance) and decreased total glucose disposal (reflecting peripheral insulin resistance)[73]. Another clinical study showed that chronic hepatitis C was associated with peripheral, rather than hepatic, insulin resistance[74]. It is assumed that some humoral factors are necessary for the development of insulin resistance in peripheral tissues that are distant from the HCV-infected liver. A study using the Zucker (fa/fa) rat model of obesity and insulin resistance showed that tumor necrosis factor (TNF)-α impaired insulin signaling by inhibiting the function of insulin receptor or IRS-1 in the muscle and adipose tissues, but not in the liver[75]. Subsequent studies of the TNF-α induced insulin resistance indicated that the underlying mechanism involved up-regulation of SOCS-3 and consequent promotion of ubiquitin-mediated IRS-1/2 degradation[76], as well as activation of JNK and consequent phosphorylation of serine 307 on IRS-1[45,46]. Furthermore, the levels of TNF-α in patients with chronic hepatitis C were found to be strongly associated with both hepatic and systemic insulin resistance[9,32,64,68,77] and the development of diabetes[9,64,78]. While other cytokines, such as interleukin (IL)-6 and IL-18, have also been implicated in the development of insulin resistance[73,78-81], TNF-α appears to play a central role in obesity-related and HCV-induced insulin resistance (Figure 3).
Whether the pathogenesis of HCV-induced diabetes is the same as that of type 2 diabetes remains controversial, despite extensive studies of HCV effects on the main diabetes-related features of decreased islet mass and β-cell dysfunction. In vitro studies have demonstrated that HCV infection of human β-cells leads to a reduction in the cells’ glucose-stimulated insulin release[82] and induces a novel apoptosis-like death through an ER stress-involved, caspase 3-dependent, specific pathway[83]. In contrast, however, an in vivo study of the HCV core protein transgenic mouse model suggested no substantial effects on pancreas-related insulin, due to a compensatory increase of islet mass that occurred without infiltration of inflammatory cells[64].
These in vivo results agree with the reported clinical observations of up-regulated insulin secretion in HCV-infected patients[3,4,9,12-14,18-20,22-24,65,84,85]. Nonetheless, further studies are needed to elucidate the effects of HCV on pancreatic β-cells and their production and release of insulin.
The gastrointestinal tract plays pivotal roles in regulating glucose metabolism and energy homeostasis through the digestion and absorption of nutrients and the secretion of multiple gut hormones. The incretin hormones, glucagon-like peptide (GLP)-1 and glucose-dependent insulinotropic polypeptide, are produced mainly in the small intestine and promote insulin biosynthesis, insulin secretion, and β-cell survival, and are enzymatically inactivated by dipeptidyl peptidase (DPP)-IV[86]. Furthermore, GLP-1 inhibits glucagon secretion and gastric emptying, induces satiety, and activates glycogen synthesis in hepatocytes[86,87]. In contrast, the ghrelin hormone, which is produced in the stomach, inhibits insulin secretion and stimulates food intake[88]. The cholecystokinin and gastrin hormones both act to stimulate the formation of new β-cells by stimulating islet neogenesis[86].
Only a few reports to date have addressed the relationship between gut hormones and HCV infection. One study demonstrated that HCV could decrease serum GLP-1 through up-regulation of DPP-IV expression and suggested that this mechanism may explain HCV-induced glucose intolerance[89]. Another recent study showed that circulating levels of active ghrelin were positively correlated with serum albumin levels in HCV-infected patients[90]. However, the effect of ghrelin on glucose metabolism in HCV infection remains unclear.
Host lipids are manipulated by HCV to support its life cycle. Viral replication and assembly require close interactions with lipid droplets and factors of lipoprotein metabolism[91,92] in the host cell. Moreover, when mature virus is released from hepatocytes it is complexed with host lipoproteins[93]. Unfortunately, modulation of the lipid metabolism process in host cells by HCV can induce hepatic steatosis[27]. This fact is reflected by the higher prevalence of hepatic steatosis in HCV-infected patients compared to the general population or even patients with chronic hepatitis B[94].
Using the HCV core protein transgenic mouse model, the core protein was implicated as a key inducer of HCV-related hepatic steatosis[95] and the mechanism was determined to involve inhibition of both the microsomal triglyceride transfer protein activity and of very low density lipoprotein (VLDL) secretion[96]. HCV-induced oxidative stress via mitochondrial dysfunction has also been shown to cause lipid and protein peroxidation, resulting in impairment of VLDL export[27]. In addition, HCV core protein has been shown to impair the expression and function of PPARγ[97], thereby reducing mitochondrial long chain fatty acid β-oxidation[98], and induce hepatic gene expression and transcriptional activity of SREBP-1, thereby increasing fatty acid synthesis[99]. Activation of SREBP-1 is promoted via SOCS[100] or PA28γ[101] (Figure 2). Collectively, these results suggest that hepatic steatosis in HCV infection is induced by decreases in lipid export from the liver, reduction of fatty acid oxidation, and promotion of de novo fatty acid synthesis.
An in vitro study has shown that significant triglyceride accumulation occurs in cells upon transfection with HCV genotypes 1 and 3, but not with genotypes 2, 4 or 5. Furthermore, the HCV genotype 3 core protein was estimated to be three times more potent at inducing this triglyceride accumulation than the genotype 1 core protein[102]. Compared with genotype 1, HCV genotype 3 also induced greater SREBP-1-dependent fatty acid synthase promoter activity[103], impairment of PPARγ expression[104], and generation of larger lipid droplets in hepatocytes[105]. Clinical studies of hepatic steatosis in patients with HCV genotype 3 showed direct associations with serum[20,106,107] and intrahepatic[108] titers of HCV RNA, which disappeared after HCV eradication by antiviral therapy[107-110] and recurred in conjunction with HCV relapse[108]. These clinical phenomena have not been observed in studies of the other HCV genotypes, suggesting that only HCV genotype 3 possesses virus-specific cytopathic effects that may lead to steatosis.
A study using liver biopsy specimens from chronic hepatitis patients indicated that hepatic iron overload was a characteristic of HCV infection, and demonstrated that serum ferritin levels can reflect the extent of hepatic iron storage in a clinical setting[111]. Recently, a large cohort study in the United States showed that HCV infection was significantly associated with increases in serum iron and ferritin levels, and that serum ferritin levels were directly correlated with results of liver function tests[112]. In both in vitro and in vivo studies, the HCV core protein has been shown to cause mitochondrial oxidative stress manifests as liver injury[113]. Transgenic expression of HCV polyprotein in mice led to hepatic iron accumulation through a process that involved HCV-induced increases in reactive oxygen species (ROS) and subsequent down-regulation of hepcidin transcription leading to increased duodenal iron transport and macrophage iron release via ferroportin overexpression[31]. Patients with chronic hepatitis C have been shown to have up-regulated transferrin receptor 2, a type of hepatic iron transporter, as well as down-regulated hepcidin expression in liver[114,115]. Excess divalent iron is known to produce hydroxyl radicals, a type of ROS, through the Fenton reaction[116]; therefore, hepatic iron overload may also contribute to hepatic oxidative stress[117]. The above-mentioned vicious cycle between hepatic iron overload and oxidative stress has been implicated as an etiology of elevated serum alanine aminotransferase (ALT)[30,112,118], insulin resistance[28-30], and hepatocarcinogenesis[119].
Free fatty acids (FFAs) produced and secreted by visceral adipocytes can induce insulin resistance in skeletal muscle and liver[120]. Recent studies have provided substantial insights into the mechanism of lipid-induced insulin resistance[121]. In particular, intracellular accumulation of fatty acid metabolites was shown to trigger activation of novel PKC (δ, ε, η and θ), resulting in impairment of insulin signaling. In muscle, activated PKCθ is necessary for diacylglycerol (DAG)-mediated inhibition of GLUT4 transportation, and in the liver, activated PKCε is necessary for the DAG-mediated decrease in glycogen synthesis and increase in gluconeogenesis. In addition, visceral adipocytes have also been shown to promote insulin resistance by negatively modulating several adipokines, including TNF-α, adiponectin, leptin, and resistin[41]. The hypothesis suggested by these cumulated findings, that visceral obesity may cause whole-body insulin resistance and glucose intolerance via FFAs and adipokines, is supported by the clinical studies of chronic hepatitis C patients showing that visceral adiposity synergistically enhances HCV-induced insulin resistance[32] (Figure 3).
Hepatic and peripheral insulin resistance is strongly associated with response to pegylated interferon (peg-IFN)-α plus ribavirin combination therapy in patients with chronic hepatitis C[12,13-18]. Non-response to antiviral therapy in HCV-infected patients has also been shown to be associated with increased hepatic expression of SOCS-3[122], which is a physiological negative regulator of a key factor in the transduction of IFN-α signaling, the signal transducer and activator of transcription (STAT)-1[123]. HCV is known to escape from the host immune system by interfering with IFN signaling via up-regulation of PP2A and hypomethylation of STAT-1, both of which result in reduced transcriptional activation of IFN-stimulated genes[124]. Thus, factors related to IFN signaling may represent the molecular link between resistance to antiviral therapy and insulin resistance in patients with chronic hepatitis C. This theory is further supported by studies showing that SOCS proteins also play an important role in insulin resistance related to metabolic syndrome[100] and that obesity-related up-regulation of hepatic SOCS-3 expression is associated with a reduced biological response to IFN-α in HCV-infected patients[122].
The presence of visceral obesity in HCV-infected patients has been associated with decreased high-density lipoprotein cholesterol, hepatic insulin resistance, and steatosis, all of which affect serum ALT levels[125]. In contrast to HCV genotype 3, which causes viral hepatic steatosis, HCV genotype 1 can cause metabolic hepatic steatosis through its interactions with factors related to visceral adiposity[94]. Both insulin resistance and hepatic steatosis are related to hepatic inflammation and fibrosis, and might enhance these conditions when present as comorbidities[13,21-23,106]. Studies addressing the underlying molecular mechanisms of this phenomenon have indicated that hyperglycemia and hyperinsulinemia may directly affect hepatic stellate cells and increase connective tissue growth factor, stimulating production of extracellular matrix[126]. Moreover, in HCV-infected patients, the risk of HCC increases in proportion to body mass index (BMI)[127], and increased BMI is associated with younger onset of HCC[128]. The clinical observations of postprandial hyperglycemia[129] and hyperinsulinemia[130] accelerating development and progression of hepatocarcinogenesis were also confirmed by a large cohort study of Taiwanese HCV carriers that identified obesity and diabetes as significant risk factors (representing a > 100-fold increase in risk) of HCC[33]. Thus, the interactions of hepatitis virus and host metabolic factors appear to synergistically promote hepatocarcinogenesis.
The diet and lifestyle recommendations for managing chronic hepatitis C are basically the same as those for obesity, diabetes and metabolic syndrome, reflecting the potential negative effects of metabolic factors on the clinical course of HCV infection. A recent meta-analysis confirmed that diet and lifestyle modifications designed to address metabolic syndrome produced effective reductions in fasting blood glucose, waist circumference, blood pressure and triglycerides[131]. Exercise is a well-established behavioral modification that benefits metabolic disorders, and the molecular mechanism has been determined to involve exercise-stimulated glucose transport via activation of AMP-activated protein kinase (AMPK) in skeletal muscle[132]. Since the AMPK pathway is independent of insulin signaling, exercise is effective for improving hyperglycemia without influence from an insulin resistant milieu. Although the precise impact of diet and lifestyle modifications on outcomes of HCV infection remain to be fully elucidated, we have shown that appropriate diet and exercise intervention can increase insulin sensitivity in HCV-infected patients, as well as improve early viral response to antiviral therapy and decrease serum α-fetoprotein levels[84,85].
Considering the potential HCV-mediated effects on iron metabolism, it is recommended that HCV-infected patients reduce iron intake. Although the reported loads of HCV-induced iron accumulation in liver have not been extremely high[133,134], excessive iron intake may enhance the condition to a dangerous level[31,118,119]. Therefore, dietary iron restriction is important for HCV-infected patients. Intake of a low iron diet with appropriate nutrition has been shown to significantly decrease serum ALT and ferritin levels in patients with chronic hepatitis C[135]; in addition, reduction of hepatic iron by phlebotomy was also shown to improve serum ALT levels[30,136-138] and insulin resistance[30]. Long-term therapy of low iron diet in combination with phlebotomy further improved hepatic inflammation and fibrosis[138], and reduced the risk of hepatocarcinogenesis[139]. The collected results from these clinical studies led to the estimation of an ideal iron intake being < 7 mg/d for patients with chronic hepatitis C. The advantage of phlebotomy use in combination with dietary iron restriction may be explained by the fact that dietary iron absorption is enhanced under conditions of iron deficiency[140].
The practice of drinking alcohol is another important factor for any disease associated with the liver, such as hepatitis. Temperance is recommended for patients with chronic hepatitis C because HCV and alcohol metabolism have been shown to synergistically accelerate disease progression via the oxidative stress pathway, promoting HCV replication and suppressing the antiviral action of IFN[141]. Furthermore, alcoholics with HCV infection have been shown to develop more severe fibrosis and to have higher rates of both cirrhosis and HCC than their counterparts who are non-drinkers[142].
Tobacco smoking is another behavioral practice that is detrimental to both metabolic disorders and chronic pathogenic infections, in general. A meta-analysis of smoking and cancer[143] demonstrated a causal relationship between tobacco smoking and cancer of the liver [relative risk (RR) = 1.56, 95%CI: 1.29-1.87], as well as for lung (RR = 8.96, 95%CI: 6.63-12.11), larynx (RR = 6.98, 95%CI: 3.14-15.52), pharynx (RR = 6.76, 95%CI: 2.86-15.98), upper digestive tract (RR = 3.57, 95%CI: 2.63-4.84), oral cavity (RR = 3.43, 95%CI: 2.37-4.94), lower urinary tract (RR = 2.77, 95%CI: 2.17-3.54), esophagus (RR = 2.50, 95%CI: 2.00-3.13), nasal sinuses and nasopharynx (RR = 1.95, 95%CI: 1.31-2.91), pancreas (RR = 1.70, 95%CI: 1.51-1.91), uterine cervix (RR = 1.83, 95%CI: 1.51-2.21), stomach (RR = 1.65, 95%CI: 1.39-1.96), and kidney (RR = 1.52, 95%CI: 1.33-1.74). Although the effect of smoking on liver cancer was small compared to the other organs, smoking cessation is recommended for HCV-infected patients to avoid the synergistic effect on hepatocarcinogenesis.
Caffeine and polyphenols may be another dietary factor related to outcome of metabolic disorders and pathogenic infections. Some studies have shown that green tea and coffee may be beneficial to patients with chronic hepatitis C, with intake being associated with an improved clinical course. Green tea catechins have anti-inflammatory and antioxidant properties[144-148] and have been shown to ameliorate glucose metabolism[149]. In addition, data from several studies have indicated that this plant-based extract may be effective in preventing hepatocarcinogenesis[150-152]. The benefits of coffee consumption have been demonstrated by numerous studies, and include decreased risks of developing elevated ALT activity[153], hepatic fibrosis[154] and HCC[155], and improvements in glucose metabolism[156]. Furthermore, a recent meta-analysis showed that the RR of HCC was 0.80 (95%CI: 0.77-0.84) for an increment of one cup of coffee per day, regardless of sex, alcohol intake, or history of hepatitis or liver disease[157].
Although insulin resistance is strongly associated with resistance to IFN-based therapy in HCV-infected patients[12-18], the effect of insulin sensitizers on antiviral therapy seems to be restrictive. In one study, administration of metformin was shown to improve the rate of sustained viral response (SVR) to peg-IFN plus ribavirin therapy in patients with HCV genotype 1 infection and insulin resistance[158]; however, another study indicated that the metformin effect was limited to female patients[159]. Administration of pioglitazone was similarly reported to improve viral response to peg-IFN plus ribavirin therapy in patients with HCV genotype 4 and insulin resistance[160], but shown to provide no benefit to patients with HCV genotype 1 and insulin resistance[161].
In contrast, several studies have detected a harmful effect of sulfonylurea or insulin on HCC incidence in HCV-infected patients; however, the metformin appeared to provide a benefit in regard to this disease outcome[162,163]. A recent meta-analysis of observational studies confirmed an increased incidence of HCC in viral hepatitis patients with diabetes who were treated with sulfonylurea [odds ratio (OR) = 1.62; 95%CI: 1.16-2.24] or insulin (OR = 2.61; 95%CI: 1.46-4.65) and a reduced incidence for metformin treatment (OR = 0.50; 95%CI: 0.34-0.73)[164]. Therefore, the therapeutic strategy to address glucose intolerance in patients with chronic hepatitis C should aim to improve glucose metabolism as well as reduce serum insulin levels.
A recent meta-analysis showed that addition of statins (also known as HMG-CoA reductase inhibitors) to the combination therapy of IFN-α and ribavirin improved SVR without additional adverse events[165]. Although statins might still provide beneficial effects in the era of direct acting antivirals (DAAs)[166], close attention must be paid to potential drug-drug interactions. Co-administration of simvastatin or lovastatin with DAAs metabolized through cytochrome P450 (CYP) 3A is contraindicated[167]. CYP3A-independent statins can also affect the concentration of DAAs through interaction with organic anion transporter polypeptide 1B1, although to a lesser extent[167]. Nonetheless, a population-based cohort study of 260864 HCV-infected patients in Taiwan showed that statin use reduced the risk of HCC in a cumulative, dose-dependent manner[168] and a meta-analysis consisting of 1459417 patients confirmed the association of statin use with reduced risk of HCC (although the effect was stronger in Asian than Western populations)[169]. Therefore, statin use is considered beneficial for high-risk groups of HCC, such as HCV-infected patients, but regular monitoring is strongly recommended to readily detect the occurrence of any statin-related adverse effects.
Interactive and synergistic relationships exist between HCV-specific metabolic disturbances and host-associated metabolic factors. HCV can induce both hepatic and peripheral insulin resistance, and the myriad mechanisms involve oxidative stress pathways, lipid metabolism abnormalities, hepatic steatosis, and iron overload. The virus-host synergism ultimately promotes deterioration of the clinical course of HCV infection. Modifications to diet and lifestyle and application of the appropriate medications to address the metabolic disorder are important for the management of HCV-infected patients and help to improve response to antiviral therapy, inhibit progression of fibrosis, and prevent hepatocarcinogenesis.
P- Reviewers: Anand BS, Kawaguchi T S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
| 1. | Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 479] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 2. | Mehta SH, Brancati FL, Strathdee SA, Pankow JS, Netski D, Coresh J, Szklo M, Thomas DL. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 287] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault MP, Sobesky R, Martinot-Peignoux M, Maylin S, Nicolas-Chanoine MH. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 360] [Article Influence: 21.2] [Reference Citation Analysis (1)] |
| 4. | Kawaguchi T, Ide T, Taniguchi E, Hirano E, Itou M, Sumie S, Nagao Y, Yanagimoto C, Hanada S, Koga H. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135-1139. [PubMed] |
| 6. | Mason AL, Lau JY, Hoang N, Qian K, Alexander GJ, Xu L, Guo L, Jacob S, Regenstein FG, Zimmerman R. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328-333. [PubMed] |
| 7. | Caronia S, Taylor K, Pagliaro L, Carr C, Palazzo U, Petrik J, O’Rahilly S, Shore S, Tom BD, Alexander GJ. Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30:1059-1063. [PubMed] |
| 8. | Wang CS, Wang ST, Yao WJ, Chang TT, Chou P. Community-based study of hepatitis C virus infection and type 2 diabetes: an association affected by age and hepatitis severity status. Am J Epidemiol. 2003;158:1154-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Kawaguchi Y, Mizuta T, Oza N, Takahashi H, Ario K, Yoshimura T, Eguchi Y, Ozaki I, Hisatomi A, Fujimoto K. Eradication of hepatitis C virus by interferon improves whole-body insulin resistance and hyperinsulinaemia in patients with chronic hepatitis C. Liver Int. 2009;29:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Romero-Gómez M, Fernández-Rodríguez CM, Andrade RJ, Diago M, Alonso S, Planas R, Solá R, Pons JA, Salmerón J, Barcena R. Effect of sustained virological response to treatment on the incidence of abnormal glucose values in chronic hepatitis C. J Hepatol. 2008;48:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Kawamura Y, Yatsuji H, Sezaki H, Hosaka T, Hirakawa M. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49:739-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 200] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 12. | Mizuta T, Kawaguchi Y, Eguchi Y, Takahashi H, Ario K, Akiyama T, Oza N, Otsuka T, Kuwashiro T, Yoshimura T. Whole-body insulin sensitivity index is a highly specific predictive marker for virological response to peginterferon plus ribavirin therapy in chronic hepatitis C patients with genotype 1b and high viral load. Dig Dis Sci. 2010;55:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | D’Souza R, Sabin CA, Foster GR. Insulin resistance plays a significant role in liver fibrosis in chronic hepatitis C and in the response to antiviral therapy. Am J Gastroenterol. 2005;100:1509-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Romero-Gómez M, Del Mar Viloria M, Andrade RJ, Salmerón J, Diago M, Fernández-Rodríguez CM, Corpas R, Cruz M, Grande L, Vázquez L. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 539] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 15. | Khattab M, Eslam M, Sharwae MA, Shatat M, Ali A, Hamdy L. Insulin resistance predicts rapid virologic response to peginterferon/ribavirin combination therapy in hepatitis C genotype 4 patients. Am J Gastroenterol. 2010;105:1970-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Grasso A, Malfatti F, De Leo P, Martines H, Fabris P, Toscanini F, Anselmo M, Menardo G. Insulin resistance predicts rapid virological response in non-diabetic, non-cirrhotic genotype 1 HCV patients treated with peginterferon alpha-2b plus ribavirin. J Hepatol. 2009;51:984-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Dai CY, Huang JF, Hsieh MY, Hou NJ, Lin ZY, Chen SC, Hsieh MY, Wang LY, Chang WY, Chuang WL. Insulin resistance predicts response to peginterferon-alpha/ribavirin combination therapy in chronic hepatitis C patients. J Hepatol. 2009;50:712-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Poustchi H, Negro F, Hui J, Cua IH, Brandt LR, Kench JG, George J. Insulin resistance and response to therapy in patients infected with chronic hepatitis C virus genotypes 2 and 3. J Hepatol. 2008;48:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, McCaughan GW, George J. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected]. Gastroenterology. 2003;125:1695-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 523] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 20. | Fartoux L, Poujol-Robert A, Guéchot J, Wendum D, Poupon R, Serfaty L. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut. 2005;54:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 278] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 21. | Petta S, Cammà C, Di Marco V, Alessi N, Cabibi D, Caldarella R, Licata A, Massenti F, Tarantino G, Marchesini G. Insulin resistance and diabetes increase fibrosis in the liver of patients with genotype 1 HCV infection. Am J Gastroenterol. 2008;103:1136-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Petta S, Cammà C, Di Marco V, Macaluso FS, Maida M, Pizzolanti G, Belmonte B, Cabibi D, Di Stefano R, Ferraro D. Hepatic steatosis and insulin resistance are associated with severe fibrosis in patients with chronic hepatitis caused by HBV or HCV infection. Liver Int. 2011;31:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Cua IH, Hui JM, Kench JG, George J. Genotype-specific interactions of insulin resistance, steatosis, and fibrosis in chronic hepatitis C. Hepatology. 2008;48:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Muzzi A, Leandro G, Rubbia-Brandt L, James R, Keiser O, Malinverni R, Dufour JF, Helbling B, Hadengue A, Gonvers JJ. Insulin resistance is associated with liver fibrosis in non-diabetic chronic hepatitis C patients. J Hepatol. 2005;42:41-46. [PubMed] |
| 25. | El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 893] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 26. | Kuwashiro T, Mizuta T, Kawaguchi Y, Iwane S, Takahashi H, Oza N, Oeda S, Isoda H, Eguchi Y, Ozaki I. Impairment of health-related quality of life in patients with chronic hepatitis C is associated with insulin resistance. J Gastroenterol. 2014;49:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Syed GH, Amako Y, Siddiqui A. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol Metab. 2010;21:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 284] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 28. | Garrido Serrano A, Guerrero Igea FJ, Lepe Jiménez JA, Palomo Gil S, Grilo Reina A. Hepatitis C virus infection, increased serum ferritin and hyperinsulinemia. Rev Esp Enferm Dig. 2001;93:639-648. [PubMed] |
| 29. | Lecube A, Hernández C, Simó R. Glucose abnormalities in non-alcoholic fatty liver disease and chronic hepatitis C virus infection: the role of iron overload. Diabetes Metab Res Rev. 2009;25:403-410. [PubMed] |
| 30. | Mitsuyoshi H, Itoh Y, Sumida Y, Minami M, Yasui K, Nakashima T, Okanoue T. Evidence of oxidative stress as a cofactor in the development of insulin resistance in patients with chronic hepatitis C. Hepatol Res. 2008;38:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Nishina S, Hino K, Korenaga M, Vecchi C, Pietrangelo A, Mizukami Y, Furutani T, Sakai A, Okuda M, Hidaka I. Hepatitis C virus-induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology. 2008;134:226-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 32. | Eguchi Y, Mizuta T, Ishibashi E, Kitajima Y, Oza N, Nakashita S, Hara M, Iwane S, Takahashi H, Akiyama T. Hepatitis C virus infection enhances insulin resistance induced by visceral fat accumulation. Liver Int. 2009;29:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 423] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 34. | Caregaro L, Alberino F, Amodio P, Merkel C, Bolognesi M, Angeli P, Gatta A. Malnutrition in alcoholic and virus-related cirrhosis. Am J Clin Nutr. 1996;63:602-609. [PubMed] |
| 35. | Petrides AS, Vogt C, Schulze-Berge D, Matthews D, Strohmeyer G. Pathogenesis of glucose intolerance and diabetes mellitus in cirrhosis. Hepatology. 1994;19:616-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 185] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Jessen N, Buhl ES, Schmitz O, Lund S. Impaired insulin action despite upregulation of proximal insulin signaling: novel insights into skeletal muscle insulin resistance in liver cirrhosis. J Hepatol. 2006;45:797-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Johnson DG, Alberti KG, Faber OK, Binder C. Hyperinsulinism of hepatic cirrhosis: Diminished degradation or hypersecretion? Lancet. 1977;1:10-13. [PubMed] |
| 38. | Iwasaki Y, Ohkubo A, Kajinuma H, Akanuma Y, Kosaka K. Degradation and secretion of insulin in hepatic cirrhosis. J Clin Endocrinol Metab. 1978;47:774-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Bosch J, Gomis R, Kravetz D, Casamitjana R, Terés J, Rivera F, Rodés J. Role of spontaneous portal-systemic shunting in hyperinsulinism of cirrhosis. Am J Physiol. 1984;247:G206-G212. [PubMed] |
| 40. | Riggio O, Merli M, Cangiano C, Capocaccia R, Cascino A, Lala A, Leonetti F, Mauceri M, Pepe M, Rossi Fanelli F. Glucose intolerance in liver cirrhosis. Metabolism. 1982;31:627-634. [PubMed] |
| 41. | Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3583] [Cited by in RCA: 3655] [Article Influence: 152.3] [Reference Citation Analysis (0)] |
| 42. | Patti ME, Kahn CR. The insulin receptor--a critical link in glucose homeostasis and insulin action. J Basic Clin Physiol Pharmacol. 1998;9:89-109. [PubMed] |
| 43. | Tanti JF, Jager J. Cellular mechanisms of insulin resistance: role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr Opin Pharmacol. 2009;9:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 322] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 44. | Banerjee S, Saito K, Ait-Goughoulte M, Meyer K, Ray RB, Ray R. Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate-1 and impairs the downstream akt/protein kinase B signaling pathway for insulin resistance. J Virol. 2008;82:2606-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 45. | Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1886] [Cited by in RCA: 1842] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 46. | Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem. 2000;275:9047-9054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1069] [Cited by in RCA: 1110] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 47. | Koren S, Fantus IG. Inhibition of the protein tyrosine phosphatase PTP1B: potential therapy for obesity, insulin resistance and type-2 diabetes mellitus. Best Pract Res Clin Endocrinol Metab. 2007;21:621-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 48. | Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394-42398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 666] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 49. | Virkamaki A, Ueki K, Kahn CR. Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J Clin Invest. 1999;103:931-943. |
| 50. | Myers MG, Backer JM, Sun XJ, Shoelson S, Hu P, Schlessinger J, Yoakim M, Schaffhausen B, White MF. IRS-1 activates phosphatidylinositol 3’-kinase by associating with src homology 2 domains of p85. Proc Natl Acad Sci USA. 1992;89:10350-10354. [PubMed] |
| 51. | Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2076] [Cited by in RCA: 2218] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 52. | Ogg S, Ruvkun G. The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway. Mol Cell. 1998;2:887-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 323] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 53. | Wada T, Sasaoka T, Ishiki M, Hori H, Haruta T, Ishihara H, Kobayashi M. Role of the Src homology 2 (SH2) domain and C-terminus tyrosine phosphorylation sites of SH2-containing inositol phosphatase (SHIP) in the regulation of insulin-induced mitogenesis. Endocrinology. 1999;140:4585-4594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 54. | Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science. 2001;292:1728-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1417] [Cited by in RCA: 1467] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 55. | Tsuru M, Katagiri H, Asano T, Yamada T, Ohno S, Ogihara T, Oka Y. Role of PKC isoforms in glucose transport in 3T3-L1 adipocytes: insignificance of atypical PKC. Am J Physiol Endocrinol Metab. 2002;283:E338-E345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Chiang SH, Hou JC, Hwang J, Pessin JE, Saltiel AR. Cloning and functional characterization of related TC10 isoforms, a subfamily of Rho proteins involved in insulin-stimulated glucose transport. J Biol Chem. 2002;277:13067-13073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Chang L, Adams RD, Saltiel AR. The TC10-interacting protein CIP4/2 is required for insulin-stimulated Glut4 translocation in 3T3L1 adipocytes. Proc Natl Acad Sci USA. 2002;99:12835-12840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 58. | Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3826] [Cited by in RCA: 4055] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 59. | Brady MJ, Nairn AC, Saltiel AR. The regulation of glycogen synthase by protein phosphatase 1 in 3T3-L1 adipocytes. Evidence for a potential role for DARPP-32 in insulin action. J Biol Chem. 1997;272:29698-29703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 60. | Raught B, Gingras AC, Sonenberg N. The target of rapamycin (TOR) proteins. Proc Natl Acad Sci USA. 2001;98:7037-7044. [PubMed] |
| 61. | Sasaoka T, Draznin B, Leitner JW, Langlois WJ, Olefsky JM. Shc is the predominant signaling molecule coupling insulin receptors to activation of guanine nucleotide releasing factor and p21ras-GTP formation. J Biol Chem. 1994;269:10734-10738. [PubMed] |
| 62. | Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3924] [Cited by in RCA: 4008] [Article Influence: 167.0] [Reference Citation Analysis (33)] |
| 63. | Aytug S, Reich D, Sapiro LE, Bernstein D, Begum N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology. 2003;38:1384-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 187] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 64. | Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 543] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 65. | Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 432] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 66. | Persico M, Russo R, Persico E, Svelto M, Spano D, Andolfo I, La Mura V, Capasso M, Tiribelli C, Torella R. SOCS3 and IRS-1 gene expression differs between genotype 1 and genotype 2 hepatitis C virus-infected HepG2 cells. Clin Chem Lab Med. 2009;47:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Pazienza V, Clément S, Pugnale P, Conzelman S, Foti M, Mangia A, Negro F. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007;45:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 68. | Miyamoto H, Moriishi K, Moriya K, Murata S, Tanaka K, Suzuki T, Miyamura T, Koike K, Matsuura Y. Involvement of the PA28gamma-dependent pathway in insulin resistance induced by hepatitis C virus core protein. J Virol. 2007;81:1727-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 69. | Pazienza V, Vinciguerra M, Andriulli A, Mangia A. Hepatitis C virus core protein genotype 3a increases SOCS-7 expression through PPAR-{gamma} in Huh-7 cells. J Gen Virol. 2010;91:1678-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 70. | Bernsmeier C, Duong FH, Christen V, Pugnale P, Negro F, Terracciano L, Heim MH. Virus-induced over-expression of protein phosphatase 2A inhibits insulin signalling in chronic hepatitis C. J Hepatol. 2008;49:429-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Christen V, Treves S, Duong FH, Heim MH. Activation of endoplasmic reticulum stress response by hepatitis viruses up-regulates protein phosphatase 2A. Hepatology. 2007;46:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 72. | Tardif KD, Waris G, Siddiqui A. Hepatitis C virus, ER stress, and oxidative stress. Trends Microbiol. 2005;13:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 209] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 73. | Vanni E, Abate ML, Gentilcore E, Hickman I, Gambino R, Cassader M, Smedile A, Ferrannini E, Rizzetto M, Marchesini G. Sites and mechanisms of insulin resistance in nonobese, nondiabetic patients with chronic hepatitis C. Hepatology. 2009;50:697-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 74. | Milner KL, van der Poorten D, Trenell M, Jenkins AB, Xu A, Smythe G, Dore GJ, Zekry A, Weltman M, Fragomeli V. Chronic hepatitis C is associated with peripheral rather than hepatic insulin resistance. Gastroenterology. 2010;138:932-941.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 75. | Hotamisligil GS, Budavari A, Murray D, Spiegelman BM. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J Clin Invest. 1994;94:1543-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 562] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 76. | Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem. 2001;276:47944-47949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 316] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 77. | Lecube A, Hernández C, Genescà J, Simó R. Proinflammatory cytokines, insulin resistance, and insulin secretion in chronic hepatitis C patients: A case-control study. Diabetes Care. 2006;29:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 78. | Knobler H, Zhornicky T, Sandler A, Haran N, Ashur Y, Schattner A. Tumor necrosis factor-alpha-induced insulin resistance may mediate the hepatitis C virus-diabetes association. Am J Gastroenterol. 2003;98:2751-2756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 79. | Fernandez-Real JM, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, Ricart W. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab. 2001;86:1154-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 80. | Straczkowski M, Kowalska I, Nikolajuk A, Otziomek E, Adamska A, Karolczuk-Zarachowicz M, Gorska M. Increased serum interleukin-18 concentration is associated with hypoadiponectinemia in obesity, independently of insulin resistance. Int J Obes (Lond). 2007;31:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Chandrasekar B, Boylston WH, Venkatachalam K, Webster NJ, Prabhu SD, Valente AJ. Adiponectin blocks interleukin-18-mediated endothelial cell death via APPL1-dependent AMP-activated protein kinase (AMPK) activation and IKK/NF-kappaB/PTEN suppression. J Biol Chem. 2008;283:24889-24898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 82. | Masini M, Campani D, Boggi U, Menicagli M, Funel N, Pollera M, Lupi R, Del Guerra S, Bugliani M, Torri S. Hepatitis C virus infection and human pancreatic beta-cell dysfunction. Diabetes Care. 2005;28:940-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 83. | Wang Q, Chen J, Wang Y, Han X, Chen X. Hepatitis C virus induced a novel apoptosis-like death of pancreatic beta cells through a caspase 3-dependent pathway. PLoS One. 2012;7:e38522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 84. | Kawaguchi Y, Mizuta T, Eguchi Y, Sakurai E, Motomura Y, Isoda H, Kuwashiro T, Oeda S, Iwane S, Takahashi H. Whole-body insulin resistance is associated with elevated serum α-fetoprotein levels in patients with chronic hepatitis C. Intern Med. 2013;52:2393-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Kawaguchi Y, Mizuta T, Fukuyama M, Matsuda N, Kuwashiro T, Oeda S, Oza N, Takahashi H, Eguchi Y, Ushirokawa M. Impact of diet and exercise intervention on insulin resistance and early virological response to pegylated interferon plus ribavirin in patients with chronic hepatitis C. Gastroenterology. 2009;136 Suppl 1:A791. [DOI] [Full Text] |
| 86. | Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 445] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 87. | Redondo A, Trigo MV, Acitores A, Valverde I, Villanueva-Peñacarrillo ML. Cell signalling of the GLP-1 action in rat liver. Mol Cell Endocrinol. 2003;204:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 88. | Verhulst PJ, Depoortere I. Ghrelin’s second life: from appetite stimulator to glucose regulator. World J Gastroenterol. 2012;18:3183-3195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (1)] |
| 89. | Itou M, Kawaguchi T, Taniguchi E, Sumie S, Oriishi T, Mitsuyama K, Tsuruta O, Ueno T, Sata M. Altered expression of glucagon-like peptide-1 and dipeptidyl peptidase IV in patients with HCV-related glucose intolerance. J Gastroenterol Hepatol. 2008;23:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 90. | Kawaguchi T, Nagao Y, Sata M. Independent factors associated with altered plasma active ghrelin levels in HCV-infected patients. Liver Int. 2013;33:1510-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 91. | Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 937] [Cited by in RCA: 981] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 92. | Alvisi G, Madan V, Bartenschlager R. Hepatitis C virus and host cell lipids: an intimate connection. RNA Biol. 2011;8:258-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 93. | André P, Komurian-Pradel F, Deforges S, Perret M, Berland JL, Sodoyer M, Pol S, Bréchot C, Paranhos-Baccalà G, Lotteau V. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol. 2002;76:6919-6928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 517] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 94. | Hwang SJ, Lee SD. Hepatic steatosis and hepatitis C: Still unhappy bedfellows? J Gastroenterol Hepatol. 2011;26 Suppl 1:96-101. [PubMed] [DOI] [Full Text] |
| 95. | Moriya K, Yotsuyanagi H, Shintani Y, Fujie H, Ishibashi K, Matsuura Y, Miyamura T, Koike K. Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J Gen Virol. 1997;78:1527-1531. [PubMed] |
| 96. | Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, Chrétien Y, Koike K, Pessayre D, Chapman J, Barba G. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 428] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 97. | Dharancy S, Malapel M, Perlemuter G, Roskams T, Cheng Y, Dubuquoy L, Podevin P, Conti F, Canva V, Philippe D. Impaired expression of the peroxisome proliferator-activated receptor alpha during hepatitis C virus infection. Gastroenterology. 2005;128:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 98. | Ferré P. The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes. 2004;53 Suppl 1:S43-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 556] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 99. | Kim KH, Hong SP, Kim K, Park MJ, Kim KJ, Cheong J. HCV core protein induces hepatic lipid accumulation by activating SREBP1 and PPARgamma. Biochem Biophys Res Commun. 2007;355:883-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 100. | Ueki K, Kondo T, Tseng YH, Kahn CR. Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc Natl Acad Sci USA. 2004;101:10422-10427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 292] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 101. | Moriishi K, Mochizuki R, Moriya K, Miyamoto H, Mori Y, Abe T, Murata S, Tanaka K, Miyamura T, Suzuki T. Critical role of PA28gamma in hepatitis C virus-associated steatogenesis and hepatocarcinogenesis. Proc Natl Acad Sci USA. 2007;104:1661-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 102. | Abid K, Pazienza V, de Gottardi A, Rubbia-Brandt L, Conne B, Pugnale P, Rossi C, Mangia A, Negro F. An in vitro model of hepatitis C virus genotype 3a-associated triglycerides accumulation. J Hepatol. 2005;42:744-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 103. | Jackel-Cram C, Babiuk LA, Liu Q. Up-regulation of fatty acid synthase promoter by hepatitis C virus core protein: genotype-3a core has a stronger effect than genotype-1b core. J Hepatol. 2007;46:999-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 104. | de Gottardi A, Pazienza V, Pugnale P, Bruttin F, Rubbia-Brandt L, Juge-Aubry CE, Meier CA, Hadengue A, Negro F. Peroxisome proliferator-activated receptor-alpha and -gamma mRNA levels are reduced in chronic hepatitis C with steatosis and genotype 3 infection. Aliment Pharmacol Ther. 2006;23:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 105. | Piodi A, Chouteau P, Lerat H, Hézode C, Pawlotsky JM. Morphological changes in intracellular lipid droplets induced by different hepatitis C virus genotype core sequences and relationship with steatosis. Hepatology. 2008;48:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 106. | Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 774] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 107. | Patton HM, Patel K, Behling C, Bylund D, Blatt LM, Vallée M, Heaton S, Conrad A, Pockros PJ, McHutchison JG. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol. 2004;40:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 272] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 108. | Rubbia-Brandt L, Quadri R, Abid K, Giostra E, Malé PJ, Mentha G, Spahr L, Zarski JP, Borisch B, Hadengue A. Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3. J Hepatol. 2000;33:106-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 401] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 109. | Kumar D, Farrell GC, Fung C, George J. Hepatitis C virus genotype 3 is cytopathic to hepatocytes: Reversal of hepatic steatosis after sustained therapeutic response. Hepatology. 2002;36:1266-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 256] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 110. | Poynard T, Ratziu V, McHutchison J, Manns M, Goodman Z, Zeuzem S, Younossi Z, Albrecht J. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 431] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 111. | Farinati F, Cardin R, De Maria N, Della Libera G, Marafin C, Lecis E, Burra P, Floreani A, Cecchetto A, Naccarato R. Iron storage, lipid peroxidation and glutathione turnover in chronic anti-HCV positive hepatitis. J Hepatol. 1995;22:449-456. [PubMed] |
| 112. | Shan Y, Lambrecht RW, Bonkovsky HL. Association of hepatitis C virus infection with serum iron status: analysis of data from the third National Health and Nutrition Examination Survey. Clin Infect Dis. 2005;40:834-841. [PubMed] |
| 113. | Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 680] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 114. | Takeo M, Kobayashi Y, Fujita N, Urawa N, Iwasa M, Horiike S, Tanaka H, Kaito M, Adachi Y. Upregulation of transferrin receptor 2 and ferroportin 1 mRNA in the liver of patients with chronic hepatitis C. J Gastroenterol Hepatol. 2005;20:562-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 115. | Fujita N, Sugimoto R, Takeo M, Urawa N, Mifuji R, Tanaka H, Kobayashi Y, Iwasa M, Watanabe S, Adachi Y. Hepcidin expression in the liver: relatively low level in patients with chronic hepatitis C. Mol Med. 2007;13:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 116. | Fenton HJH. Oxidation of tartaric acid in presence of iron. J Chem Soc. 1894;65:899-910. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2257] [Cited by in RCA: 2250] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 117. | Fujita N, Miyachi H, Tanaka H, Takeo M, Nakagawa N, Kobayashi Y, Iwasa M, Watanabe S, Takei Y. Iron overload is associated with hepatic oxidative damage to DNA in nonalcoholic steatohepatitis. Cancer Epidemiol Biomarkers Prev. 2009;18:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 118. | Bassett SE, Di Bisceglie AM, Bacon BR, Sharp RM, Govindarajan S, Hubbard GB, Brasky KM, Lanford RE. Effects of iron loading on pathogenicity in hepatitis C virus-infected chimpanzees. Hepatology. 1999;29:1884-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 119. | Furutani T, Hino K, Okuda M, Gondo T, Nishina S, Kitase A, Korenaga M, Xiao SY, Weinman SA, Lemon SM. Hepatic iron overload induces hepatocellular carcinoma in transgenic mice expressing the hepatitis C virus polyprotein. Gastroenterology. 2006;130:2087-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 120. | Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11:351-356. [PubMed] |
| 121. | Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267-2277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 922] [Cited by in RCA: 858] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 122. | Walsh MJ, Jonsson JR, Richardson MM, Lipka GM, Purdie DM, Clouston AD, Powell EE. Non-response to antiviral therapy is associated with obesity and increased hepatic expression of suppressor of cytokine signalling 3 (SOCS-3) in patients with chronic hepatitis C, viral genotype 1. Gut. 2006;55:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 123. | Gadina M, Hilton D, Johnston JA, Morinobu A, Lighvani A, Zhou YJ, Visconti R, O’Shea JJ. Signaling by type I and II cytokine receptors: ten years after. Curr Opin Immunol. 2001;13:363-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 150] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 124. | Duong FH, Filipowicz M, Tripodi M, La Monica N, Heim MH. Hepatitis C virus inhibits interferon signaling through up-regulation of protein phosphatase 2A. Gastroenterology. 2004;126:263-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 125. | Kobayashi Y, Kawaguchi Y, Mizuta T, Kuwashiro T, Oeda S, Oza N, Takahashi H, Iwane S, Eguchi Y, Anzai K. Metabolic factors are associated with serum alanine aminotransferase levels in patients with chronic hepatitis C. J Gastroenterol. 2011;46:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 126. | Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, Conti M, Huet S, Ba N, Buffet C. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 390] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 127. | Ohki T, Tateishi R, Sato T, Masuzaki R, Imamura J, Goto T, Yamashiki N, Yoshida H, Kanai F, Kato N. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clin Gastroenterol Hepatol. 2008;6:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 128. | Akiyama T, Mizuta T, Kawazoe S, Eguchi Y, Kawaguchi Y, Takahashi H, Ozaki I, Fujimoto K. Body mass index is associated with age-at-onset of HCV-infected hepatocellular carcinoma patients. World J Gastroenterol. 2011;17:914-921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 129. | Takahashi H, Mizuta T, Eguchi Y, Kawaguchi Y, Kuwashiro T, Oeda S, Isoda H, Oza N, Iwane S, Izumi K. Post-challenge hyperglycemia is a significant risk factor for the development of hepatocellular carcinoma in patients with chronic hepatitis C. J Gastroenterol. 2011;46:790-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 130. | Saito K, Inoue S, Saito T, Kiso S, Ito N, Tamura S, Watanabe H, Takeda H, Misawa H, Togashi H. Augmentation effect of postprandial hyperinsulinaemia on growth of human hepatocellular carcinoma. Gut. 2002;51:100-104. [PubMed] |
| 131. | Yamaoka K, Tango T. Effects of lifestyle modification on metabolic syndrome: a systematic review and meta-analysis. BMC Med. 2012;10:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 273] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 132. | Fujii N, Jessen N, Goodyear LJ. AMP-activated protein kinase and the regulation of glucose transport. Am J Physiol Endocrinol Metab. 2006;291:E867-E877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 133. | Di Bisceglie AM, Axiotis CA, Hoofnagle JH, Bacon BR. Measurements of iron status in patients with chronic hepatitis. Gastroenterology. 1992;102:2108-2113. [PubMed] |
| 134. | Haque S, Chandra B, Gerber MA, Lok AS. Iron overload in patients with chronic hepatitis C: a clinicopathologic study. Hum Pathol. 1996;27:1277-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 135. | Iwasa M, Iwata K, Kaito M, Ikoma J, Yamamoto M, Takeo M, Kuroda M, Fujita N, Kobayashi Y, Adachi Y. Efficacy of long-term dietary restriction of total calories, fat, iron, and protein in patients with chronic hepatitis C virus. Nutrition. 2004;20:368-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 136. | Hayashi H, Takikawa T, Nishimura N, Yano M, Isomura T, Sakamoto N. Improvement of serum aminotransferase levels after phlebotomy in patients with chronic active hepatitis C and excess hepatic iron. Am J Gastroenterol. 1994;89:986-988. [PubMed] |
| 137. | Yano M, Hayashi H, Yoshioka K, Kohgo Y, Saito H, Niitsu Y, Kato J, Iino S, Yotsuyanagi H, Kobayashi Y. A significant reduction in serum alanine aminotransferase levels after 3-month iron reduction therapy for chronic hepatitis C: a multicenter, prospective, randomized, controlled trial in Japan. J Gastroenterol. 2004;39:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 138. | Kato J, Kobune M, Nakamura T, Kuroiwa G, Takada K, Takimoto R, Sato Y, Fujikawa K, Takahashi M, Takayama T. Normalization of elevated hepatic 8-hydroxy-2’-deoxyguanosine levels in chronic hepatitis C patients by phlebotomy and low iron diet. Cancer Res. 2001;61:8697-8702. [PubMed] |
| 139. | Kato J, Miyanishi K, Kobune M, Nakamura T, Takada K, Takimoto R, Kawano Y, Takahashi S, Takahashi M, Sato Y. Long-term phlebotomy with low-iron diet therapy lowers risk of development of hepatocellular carcinoma from chronic hepatitis C. J Gastroenterol. 2007;42:830-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 140. | Nathanson MH, Muir A, McLaren GD. Iron absorption in normal and iron-deficient beagle dogs: mucosal iron kinetics. Am J Physiol. 1985;249:G439-G448. [PubMed] |
| 141. | McCartney EM, Beard MR. Impact of alcohol on hepatitis C virus replication and interferon signaling. World J Gastroenterol. 2010;16:1337-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 142. | Singal AK, Anand BS. Mechanisms of synergy between alcohol and hepatitis C virus. J Clin Gastroenterol. 2007;41:761-772. [PubMed] |
| 143. | Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, Maisonneuve P, Boyle P. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 590] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 144. | Tipoe GL, Leung TM, Hung MW, Fung ML. Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection. Cardiovasc Hematol Disord Drug Targets. 2007;7:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 145. | Wheeler DS, Catravas JD, Odoms K, Denenberg A, Malhotra V, Wong HR. Epigallocatechin-3-gallate, a green tea-derived polyphenol, inhibits IL-1 beta-dependent proinflammatory signal transduction in cultured respiratory epithelial cells. J Nutr. 2004;134:1039-1044. [PubMed] |
| 146. | Yang F, de Villiers WJ, McClain CJ, Varilek GW. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J Nutr. 1998;128:2334-2340. [PubMed] |
| 147. | Lin YT, Wu YH, Tseng CK, Lin CK, Chen WC, Hsu YC, Lee JC. Green tea phenolic epicatechins inhibit hepatitis C virus replication via cycloxygenase-2 and attenuate virus-induced inflammation. PLoS One. 2013;8:e54466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 148. | Baba Y, Sonoda JI, Hayashi S, Tosuji N, Sonoda S, Makisumi K, Nakajo M. Reduction of oxidative stress in liver cancer patients by oral green tea polyphenol tablets during hepatic arterial infusion chemotherapy. Exp Ther Med. 2012;4:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 149. | Liu K, Zhou R, Wang B, Chen K, Shi LY, Zhu JD, Mi MT. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr. 2013;98:340-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 150. | Shimizu M, Kubota M, Tanaka T, Moriwaki H. Nutraceutical approach for preventing obesity-related colorectal and liver carcinogenesis. Int J Mol Sci. 2012;13:579-595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 151. | Darvesh AS, Bishayee A. Chemopreventive and therapeutic potential of tea polyphenols in hepatocellular cancer. Nutr Cancer. 2013;65:329-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 152. | Li Y, Chang SC, Goldstein BY, Scheider WL, Cai L, You NC, Tarleton HP, Ding B, Zhao J, Wu M. Green tea consumption, inflammation and the risk of primary hepatocellular carcinoma in a Chinese population. Cancer Epidemiol. 2011;35:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 153. | Ruhl CE, Everhart JE. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2005;128:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 154. | Modi AA, Feld JJ, Park Y, Kleiner DE, Everhart JE, Liang TJ, Hoofnagle JH. Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology. 2010;51:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 215] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 155. | Gelatti U, Covolo L, Franceschini M, Pirali F, Tagger A, Ribero ML, Trevisi P, Martelli C, Nardi G, Donato F. Coffee consumption reduces the risk of hepatocellular carcinoma independently of its aetiology: a case-control study. J Hepatol. 2005;42:528-534. [PubMed] |
| 156. | Tunnicliffe JM, Shearer J. Coffee, glucose homeostasis, and insulin resistance: physiological mechanisms and mediators. Appl Physiol Nutr Metab. 2008;33:1290-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 157. | Bravi F, Bosetti C, Tavani A, Gallus S, La Vecchia C. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1413-1421.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 158. | Yu JW, Sun LJ, Zhao YH, Kang P, Yan BZ. The effect of metformin on the efficacy of antiviral therapy in patients with genotype 1 chronic hepatitis C and insulin resistance. Int J Infect Dis. 2012;16:e436-e441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 159. | Romero-Gómez M, Diago M, Andrade RJ, Calleja JL, Salmerón J, Fernández-Rodríguez CM, Solà R, García-Samaniego J, Herrerías JM, De la Mata M. Treatment of insulin resistance with metformin in naïve genotype 1 chronic hepatitis C patients receiving peginterferon alfa-2a plus ribavirin. Hepatology. 2009;50:1702-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 160. | Khattab M, Emad M, Abdelaleem A, Eslam M, Atef R, Shaker Y, Hamdy L. Pioglitazone improves virological response to peginterferon alpha-2b/ribavirin combination therapy in hepatitis C genotype 4 patients with insulin resistance. Liver Int. 2010;30:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 161. | Harrison SA, Hamzeh FM, Han J, Pandya PK, Sheikh MY, Vierling JM. Chronic hepatitis C genotype 1 patients with insulin resistance treated with pioglitazone and peginterferon alpha-2a plus ribavirin. Hepatology. 2012;56:464-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 162. | Kawaguchi T, Taniguchi E, Morita Y, Shirachi M, Tateishi I, Nagata E, Sata M. Association of exogenous insulin or sulphonylurea treatment with an increased incidence of hepatoma in patients with hepatitis C virus infection. Liver Int. 2010;30:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 163. | Nkontchou G, Cosson E, Aout M, Mahmoudi A, Bourcier V, Charif I, Ganne-Carrie N, Grando-Lemaire V, Vicaut E, Trinchet JC. Impact of metformin on the prognosis of cirrhosis induced by viral hepatitis C in diabetic patients. J Clin Endocrinol Metab. 2011;96:2601-2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 164. | Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:881-891; quiz 892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 235] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 165. | Zhu Q, Li N, Han Q, Zhang P, Yang C, Zeng X, Chen Y, Lv Y, Liu X, Liu Z. Statin therapy improves response to interferon alfa and ribavirin in chronic hepatitis C: a systematic review and meta-analysis. Antiviral Res. 2013;98:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 166. | Sheridan DA, Neely RD, Bassendine MF. Hepatitis C virus and lipids in the era of direct acting antivirals (DAAs). Clin Res Hepatol Gastroenterol. 2013;37:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 167. | Chauvin B, Drouot S, Barrail-Tran A, Taburet AM. Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin Pharmacokinet. 2013;52:815-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 168. | Tsan YT, Lee CH, Ho WC, Lin MH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol. 2013;31:1514-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 169. | Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 352] [Article Influence: 29.3] [Reference Citation Analysis (0)] |