Copyright
©2014 Baishideng Publishing Group Co.
World J Gastroenterol. Mar 14, 2014; 20(10): 2515-2532
Published online Mar 14, 2014. doi: 10.3748/wjg.v20.i10.2515
Published online Mar 14, 2014. doi: 10.3748/wjg.v20.i10.2515
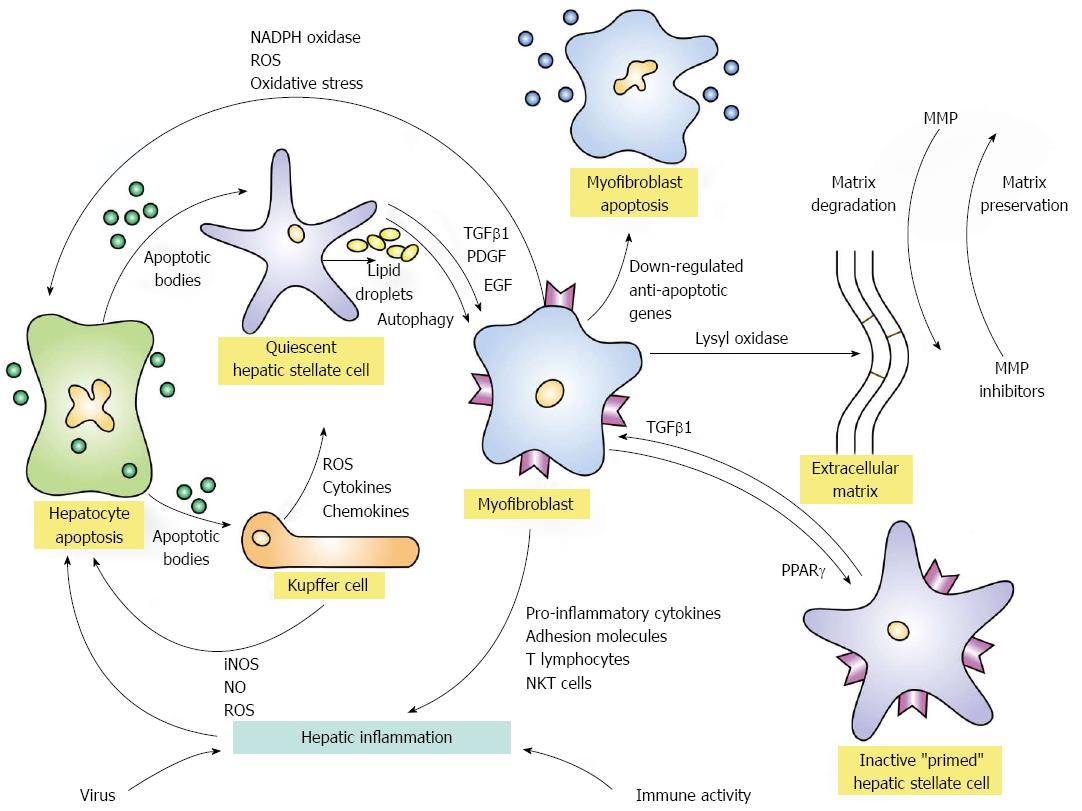
Figure 1 Activation and de-activation pathways of hepatic fibrosis.
Hepatic inflammation triggered by virus infection or immune-mediated mechanisms can initiate fibrogenesis by inducing apoptosis of hepatocytes. The released apoptotic bodies can activate quiescent hepatic stellate cells and transform them into myofibroblasts under the mediation of transforming growth factor beta 1 (TGFβ1), platelet-derived growth factor (PDGF), and endothelial growth factor (EGF). The activation process is fueled by the release of lipid droplets (autophagy). The apoptotic bodies from the damaged hepatocytes can also stimulate Kupffer cells to generate reactive oxygen species (ROS) and nitric oxide (NO) by inducible nitric oxide synthase (iNOS). The ROS in turn can enhance hepatocyte apoptosis and continue the activation of hepatic stellate cells. Kupffer cells can also release ROS, cytokines and chemokines that contribute to the transformation of quiescent hepatic stellate cells to myofibroblasts. The myofibroblasts can also generate ROS and increase oxidative stress on hepatocytes by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase pathway and promote hepatic inflammation by enhancing the expression of pro-inflammatory cytokines, adhesion molecules, activated T lymphocytes, and natural killer T (NKT) cells. The myofibroblasts generate the extracellular matrix which can be cross-linked by lysyl oxidases and rendered resistant to degradation by metalloproteinases (MMP). The reversibility of the extracellular matrix depends in part on the cross-linkage of collagen fibrils and the counterbalance of activities between MMP and MMP inhibitors. Myofibroblast activity can be attenuated by the down-regulation of anti-apoptotic genes which then favor myofibroblast apoptosis and the expression of the peroxisome proliferator-activated receptor-gamma (PPARγ) gene which may contribute to hepatic stellate cell inactivation. Inactivated stellate cells are different from quiescent hepatic stellate cells in that they are “primed” to have a low threshold for reactivation by TGFβ1.
- Citation: Czaja AJ. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol 2014; 20(10): 2515-2532
- URL: https://www.wjgnet.com/1007-9327/full/v20/i10/2515.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i10.2515