Published online Mar 14, 2014. doi: 10.3748/wjg.v20.i10.2695
Revised: December 22, 2013
Accepted: January 8, 2014
Published online: March 14, 2014
Processing time: 194 Days and 4.2 Hours
AIM: To evaluate the impact of antiviral treatment on cytomegalovirus (CMV)-positive ulcerative colitis patients.
METHODS: We performed a systematic review and meta-analysis (MA) of comparative cohort and case-control studies published between January 1966 and March 2013. Studies focusing on colectomy series and studies including only less than 3 patients in the treated or non-treated arm were excluded. The primary outcome was colectomy within 30 d of diagnosis. Secondary outcomes included colectomy during the follow-up period Subgroup analyses by method of detection of CMV, study design, risk of bias and country of origin were performed. Quality of studies was evaluated according to modified New-Castle Ottawa Scale.
RESULTS: After full-text review, nine studies with a total of 176 patients were included in our MA. All the included studies were of low to moderate quality. Patients who have received antiviral treatment had a higher risk of 30-d colectomy (OR = 2.40; 95%CI: 1.05-5.50; I2 = 37.2%). A subgroup analysis including only patients in whom CMV diagnosis was based did not demonstrate a significant difference between the groups (OR = 3.41; 95%CI: 0.39-29.83; I2 = 56.9%). Analysis of long-term colectomy rates was possible for 6 studies including 110 patients. No statistically significant difference was found between the treated and untreated groups (OR = 1.71; 95%CI: 0.71-4.13; 6 studies, I2 = 0%). Analysis of mortality rate was not possible due to a very limited number of cases. Stratification of the outcomes by disease severity was not possible.
CONCLUSION: No positive association between antiviral treatment and a favorable outcome was demonstrated. These findings should be interpreted cautiously due to primary studies’ quality and potential biases.
Core tip: We have undertaken a meta-analysis of the existing literature in order to evaluate the impact of antiviral therapy on the outcome (colectomy rate) of ulcerative colitis patients with documented presence of cytomegalovirus. Nine studies of low to moderate quality with significant heterogeneity were included. Patients treated with antivirals did not have a better outcome in comparison to those who were not. These results should be interpreted with caution in view of low quality of the included studies and several potential biases. Additional high-quality studies are required to define the optimal diagnostic and therapeutic strategy for these patients.
- Citation: Kopylov U, Eliakim-Raz N, Szilagy A, Seidman E, Ben-Horin S, Katz L. Antiviral therapy in cytomegalovirus-positive ulcerative colitis: A systematic review and meta-analysis. World J Gastroenterol 2014; 20(10): 2695-2703
- URL: https://www.wjgnet.com/1007-9327/full/v20/i10/2695.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i10.2695
Cytomegalovirus (CMV) infections are very common in the general population[1]. The infection is usually clinically mild and often confused with other minor viral infections. Reported rates of CMV-IgG positivity, signifying past exposure to the virus, are as high as 100%, depending on the age and geographic location of the population studied[2]. Even though systemic CMV disease in the immunocompetent patients is extremely rare, primary CMV infection or reactivation may lead to disseminated disease or end-organ involvement in immunocompromised patients (post-solid organ transplantation, chemotherapy-treated, HIV, recipients of immunosuppressive drugs, etc.)[3]. CMV is the most common virus causing disease and death in solid organ transplant recipients (kidney, heart, liver, lung and pancreas)[4].
The role of CMV infection in patients with inflammatory bowel disease (IBD) is controversial. Although CMV has been specifically associated with refractory disease, the strength and nature of this association has been a subject of debate[5]. The detection of CMV in the colon is frequent in patients with acute severe ulcerative colitis (UC). CMV has been reported to be present in colonic tissue of 21%-34% of all UC patients and in 33%-36% of steroid refractory patients[6].
The clinical significance of detecting CMV in UC remains debatable[7]. It has been suggested that intestinal CMV detection may be a marker of severe disease that is more likely to be refractory to corticosteroid and immunosuppressive therapy. Alternatively, CMV might only be an “innocent bystander”, reflecting a remote infection of the involved mucosa, without significantly impacting on outcomes[2]. Conversely, CMV is often considered an undisputable infection with potentially grave outcome. As such, the standard therapy for CMV infection in UC patients includes intravenous gancyclovir, with a possible switch to oral valgancyclovir upon improvement. Intravenous foscarnet is usually reserved for patients who do not tolerate or respond to gancyclovir[2]. However, prospective controlled trials to validate the clinical benefit of such treatment in IBD patients are still largely unavailable.
The aim of this study is to evaluate the impact of antiviral therapy on the outcome of CMV-positive UC patients using a systematic review and meta-analysis (MA) of the published studies pertaining to the subject.
We searched Pubmed, Embase and World of Science databases for articles published between 1966 and March 2013. In addition, we manually scanned the abstracts presented at the following medical conferences: DDW, UEGW, ECCO for the years 2004-2012. In our analysis, we have included studies published as full papers or conference abstracts.
The search strategy included the following search terms: “ulcerative colitis” or “inflammatory bowel disease” and “cytomegalovirus” or “CMV”. Their MESH terms were crossed. We manually scanned references of all included studies to identify additional relevant publications.
We included prospective and retrospective cohort studies comparing outcomes of treated and untreated CMV- positive UC patients.
The following types of studies were excluded: (1) Studies including less than 4 patients in one or more of the arms (treated and non treated patients); (2) Studies pertaining exclusively to CD patients. For studies involving mixed cohorts (CD and UC), we analyzed only the UC data. If this was not possible, we contacted the first and last author of the study and requested that they provide the relevant data. If no response was received, the study was excluded from analysis; (3) Studies describing antiviral treatment with an antiviral agent other than gancyclovir, valgancyclovir or foscarnet were also excluded; and (4) Series exclusively reporting colectomy data, i.e. only patients who reached the outcome of colectomy, were included. Patients were excluded from the analysis if they underwent colectomy before gancyclovir treatment was considered or the results of CMV assessment were available.
The primary outcome assessed was the rate of colectomy during the hospitalization period or within 30 d of diagnosis. Secondary outcomes was colectomy rate for the available follow-up duration (3 mo since the index hospitalization).
Subgroup analysis was done according to the method of CMV diagnosis, study design, study location and quality of studies, based on Newcastle Ottawa Scale (see below).
Data extraction and quality of data assessment: Two reviewers (UK, NE) independently applied inclusion criteria, selected the studies, and extracted data, outcomes and quality. In cases with disagreement between the two reviewers, the issues were resolved by discussion. Authors of studies were contacted if clarification was needed. The following data were collected: period and location of the studies, year of publication, inclusion criteria for participants in each study, demographic and disease characteristics of the included patients, method of diagnosis of CMV colitis, details of anti-inflammatory treatment preceding the diagnosis of CMV, type, duration and dose of the antiviral treatment, and outcome measures as mentioned above.
Quality of studies was assessed using the Newcastle Ottawa Scale (NOS), modified for this review. The quality of the included studies was evaluated based on questions regarding the selection and comparability of the cohort (treated and non-treated CMV-infected UC patients) and the outcome of the study. A higher score indicates higher methodological quality. We defined high-quality studies as mean total points ≥ 5; low quality as < 4, and moderate, between those values.
Study results were expressed as odds ratio (OR) with 95%CI. We used a fixed-effect model to pool results. We assessed heterogeneity using the χ2-test of heterogeneity and the I2 measure of inconsistency. If significant heterogeneity had a χ2-test P value < 0.1 or an I2 measure > 50% we conducted a random effects meta-analysis. Sensitivity analysis was done without the less qualified studies or the study which showed the most significant difference, with the same statistical methods. Analyses were performed using RevMan 4.2 (The Nordic Cochrane Centre, The Cochrane Collaboration Copenhagen, 2003).
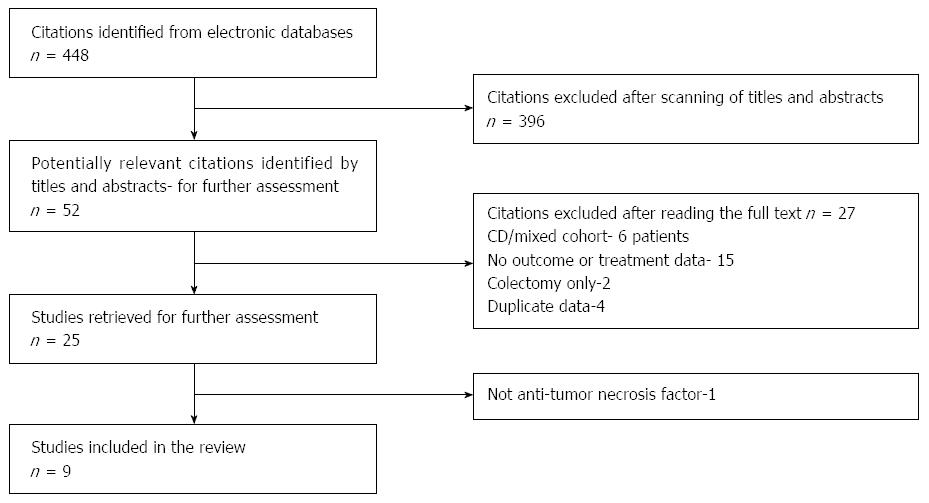
Our search yielded 448 studies. After title and abstract scanning 397 of them were excluded. An additional 42 studies were excluded after full-text review (Figure 1). A total of 9 studies[8-16] were included in our systematic review and MA, with data available for 176 patients. Table 1 shows the characteristics of the included studies.
| Ref. | Year of publication | Type | Design | n | Included patients | Severity criteria | Method of diagnosis | Anti-viral tx | Follow-up (mo) | Short-term colectomy rate | Long-term colectomy rate | |||
| TX | C | TX | C | TX | C | |||||||||
| Omiya et al[8] | 2010 | Full | P1 | 10 | 10 | Moderate to severe UC | Seo’s score | Tissue PCR (IHC/HE -) | GCV | 12 | 0/10 | 0/10 | 3/10 | 0/10 |
| Zeki et al[9]1 | 2010 | Abstract | R2 | 7 | 10 | UC (no severity data) | NA | HE/IHC+ | GCV-5, | 12 | 4/7 | 7/10 | 4/7 | 7/10 |
| VGCV-2 | ||||||||||||||
| Maconi et al[10]3 | 2011 | Abstract | R2 | 6 | 14 | Moderate to severe UC | Mayo/Baron score | HE/IHC+ | GCV | 12 | 0/6 | 0/14 | 0/6 | 2/13 |
| Criscuoli et al[17] | 2011 | Full | P1 | 7 | 21 | Moderate to severe UC | Truelove/Witts | IHC +, pp65+ | GCV-5 FC-2 | 12 | 2/7 | 4/21 | 2/7 | 4/18 |
| Kim et al[12] | 2012 | Full | P1 | 14 | 17 | Moderate to severe UC | Mayo/Baron score | HE/IHC/PCR+ | GCV | hospitalization | 3/14 | 0/17 | NA | NA |
| Roblin et al[13]3 | 2011 | Full | P1 | 8 | 8 | Moderate to severe UC, failure of CS +rescue therapy (IFX/CSA) | Mayo | qPCR, IHC/HE- | GCV | ≥ 10 | 1/8 | 2/8 | 3/8 | 2/8 |
| Al-Zafiri et al[14]3 | 2012 | Full | R2 | 7 | 8 | Hospitalized UC | NA | IHC/HE+ | GCV | hospitalization | 3/7 | 0/8 | NA | NA |
| Maruyama et al[15] | 2012 | Abstract | R2 | 4 | 12 | Moderate to severe UC | NA | IHC/HE+ | GCV | Hospitalization | 3/4 | 0/12 | NA | NA |
| Kopylov et al[16] | 2013 | Full | R2 | 7 | 6 | UC | NA | IHC/HE+ | GCV-6 VGCV-1 | 13 | 1/7 | 0/6 | 3/7 | 0/6 |
All studies collected data on patients admitted between 1990-2011 and published between 2010 and 2013. Four studies were conducted in Europe[9,10,13,17], two in Japan[8,15] and the others in Canada[14], South Korea[12] and Israel[16]. Six studies were published as ull text articles[8,12-14,16,17] and three[9,10,15] as conference abstracts. Duration of follow-up ranged between 1 to 40 mo. Four studies[8,12,13,17] were prospective and five[9,10,14-16] retrospective. In four studies, the primary objective was to compare the outcome of patients treated with antivirals to the outcome of patients who did not receive antiviral therapy[9,12,15,16]. The quality of the included studies was determined according to the modified Newcastle Ottawa Scale (Table 2). The overall quality of the studies was moderate-low; no study met the criteria for high quality.
| Total | Outcome | Comparability | Selection | Ref. |
| 1 | * | Al-Zafiri et al[14], 2012 | ||
| 3 | * | ** | Criscuoli et al[17], 2011 | |
| 4 | * | * | ** | Kim et al[12], 2012 |
| 1 | * | Kopylov et al[16], 2013 | ||
| 3 | * | ** | Maconi et al[10], 2011 | |
| 3 | * | ** | Maruyama et al[15], 2012 | |
| 4 | * | * | ** | Omiya et al[8], 2010 |
| 4 | * | *** | Roblin et al[13], 2011 | |
| 1 | * | Zeki et al[9], 2010 | ||
| 24/54 (44.4%) | 4/9 (44.4%) | 4/18 (22.2%) | 16/27 (59.3%) | Total |
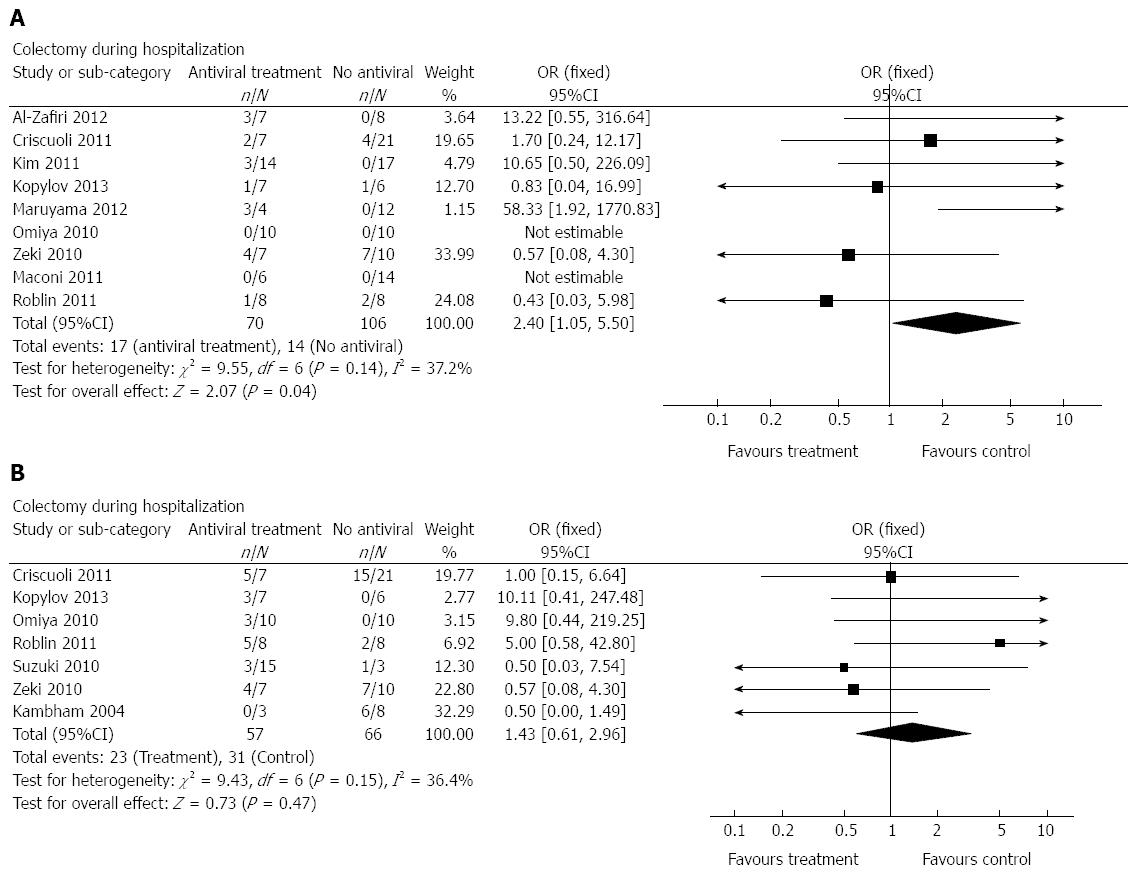
Analysis of colectomy rates during hospitalization was possible for all included studies[8-16] (Figure 2) and for all patients (70 treated, 106 controls). Patients who had received antiviral treatment had a higher risk of requiring a subsequent colectomy (OR = 2.40; 95%CI: 1.05-5.50; I2 = 37.2%). Subgroup analysis including only patients in whom CMV diagnosis was based on immunohistochemistry (IHC) staining showed the same trend, with much wider CI (OR = 3.41; 95%CI: 0. 39-29.83; 5 studies, Random effect, I2 = 56.9%). Subgroup analyses using only the prospective studies (n = 4) or studies with moderate (n = 3) vs high risk of bias (n = 6) showed the same trend, but without reaching statistical significance because of small group size and wide CI. We performed an additional subgroup analysis, comparing studies conducted in Europe[9,10,13,17] to those taken place in Asia[8,12,15] (omitting the Canadian and Israeli studies). No case of colectomy during hospitalization was reported among the non treated group in the Asian studies, therefore, patients who received antivirals underwent more colectomies than the non treated patients in the Asian studies. In the European studies no difference was recorded between the two groups (OR = 19.85; 95%CI: 1.94-203.61; and OR = 0.81; 95%CI: 0.24-2.79 for studies taken in Asia and in Europe, respectively).
In this systematic review and MA, we have attempted to compare the outcome of CMV-positive UC patients who were treated with antivirals to that of untreated patients. We did not observe a favorable effect of antiviral therapy for either the primary (short-term colectomy rate) or the secondary (long-term colectomy rate) outcomes. In fact, the patients who had not been treated had a significantly lower risk of a short-term colectomy, and a trend towards improved long-term outcome. However, these results should be interpreted cautiously in view of important confounders and biases that are discussed in detail below.
Although CMV infection in IBD patients is frequently described, the vast majority of the studies pertaining to the outcome of this condition are case-reports and case series[18]. Very few prospective studies evaluating the outcomes of such cases have been published, and none employed a randomized blinded design. The true pathological and clinical consequences of the presence of CMV in the colonic tissue in patients with ulcerative colitis have been debated for many years, since the initial report by Powell et al[19]. Although evidence of CMV infection in the inflamed colonic mucosa of IBD patients is quite common, particularly in steroid-resistant patients[20,21], the actual clinical significance of this finding remains unclear. CMV is trophic for inflamed and replicating tissue, and commonly affects immunosuppressed patients[6,7]. Evidence of viral shedding and replication is often found in IBD patients, almost exclusively in the inflamed mucosa[2]. However, the virus has been shown to disappear from the colonic tissue of UC patients without the administration of antiviral therapy[22]. We will try to address some of the more important controversies on the subject of CMV colitis that are the focus of the present study.
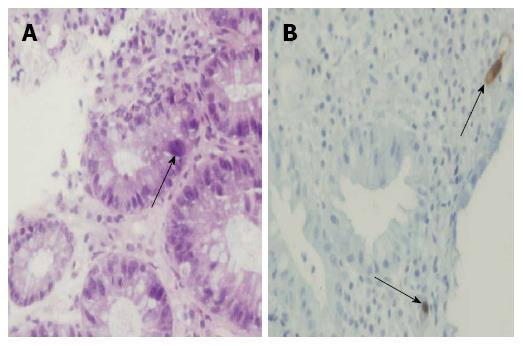
The first issue is the method of diagnosis of CMV infection. Several diagnostic techniques have been described for UC patients. In the past, viral culture was considered a “gold standard” technique for detection of CMV. However, this technique is not sufficiently sensitive and is cumbersome. CMV serology is usually uninformative, as positive CMV IGG is very common. However, a positive CMV IgM is indicative of acute infection[1], and the risk of CMV infection in patients negative for both IgG and IgM is extremely low[23]. None of the studies included in this MA used positive serology as a sole criterion for definition of CMV infection or colitis. Viral particles (pp65 antigen) can be detected in fluid specimens. However, this technique is susceptible to subjective interpretation and can be positive without evidence of colitis[6,24]. Only one of the studies included in this systematic review employed pp65 antigenemia as an indicator of a need for antiviral therapy, along with positive immunohistochemistry[11]. CMV antigenemia testing has generally been replaced by viral DNA detection. CMV DNA can be identified by PCR with a sensitivity of 65%-100% and specificity of 40%-92%[2]. PCR can be positive in patients without colonic involvement, and a correlation with histologic CMV disease has not been universally reported[6,22,25].The presence of CMV in colonic tissue can also be detected by histological methods [hematoxylin-eosin staining (HE), IHC] (Figure 3), as well as PCR. Earlier reports have included steroid-resistant patients with evidence of CMV-induced cytopathic damage on HE staining (“inclusion bodies”)[20,26,27]. These patients usually had a severe disease and high rates of colectomy (up to 67%)[21,26,28]. The detection of inclusion bodies on HE staining is clinically relevant[6] and implies ongoing destruction of the colonic epithelial cells by the virus. Unfortunately, this technique has low sensitivity (10%-87%)[2]. Immunohistochemistry with a monoclonal antibody targeting the early CMV antigen has been reported to improve the diagnostic sensitivity to 78%-93%[2,29]. Current ECCO guidelines recommend the combination of HE staining and IHC for detection of CMV infection in patients with a flare-up of UC[30]. Detection of CMV DNA in the colonic tissue by PCR is highly sensitive, but in the absence of histological evidence of tissue damage possibly represents a remote or latent infection or a low-key viral replication of unclear significance[2]. Recently, reports have been published[13,25] utilizing a quantitative cut-off for a number of CMV particles detected by real-time PCR.
Despite the available evidence, the question as to what test truly defines CMV disease in the colon and thus signifies the need for treatment remains unanswered. There was significant heterogeneity in the definition of CMV among the studies included in this MA. Five[9,10,14-16] defined CMV colitis by presence of a positive IHC staining for CMV. Two studies[8,13] included IHC/HE-negative patients who were positive for CMV in colonic tissue using PCR. Two additional studies[11,12] employed a combination of several techniques to define CMV positivity. A subgroup analysis of studies including only IHC-positive patients did not demonstrate a significant difference in the outcome between the groups (OR = 3.41; 95%CI: 0. 39-29.83, 5 studies).
The standard treatment recommended for CMV colitis employs intravenous gancyclovir (5 mg/kg intravenously every 12 h for 2-3 wk) with a possible switch to oral gancyclovir (1 g/8 h) after clinical improvement for the reminder of the course[2]. These recommendations are not based on experience in IBD, but are derived from data in organ transplant patients[31]. The vast majority of patients in the included studies were initially treated intravenously, usually with gancyclovir, or with foscarnet in gancyclovir-resistant cases. The duration of the intravenous treatment and whether the patients were eventually switched to oral valgancyclovir were not available for majority of the studies. Importantly, administration of both gancyclovir and foscarnet is associated with significant adverse effects (for gancyclovir- bone marrow depression, headaches, somnolence, psychosis, abnormal liver function, fever, and rash; for foscarnet-nephrotoxicity and severe electrolyte abnormalities[2]. These adverse effects were not clearly reported in the majority of the included studies. Two of the studies[9,16] included a total of 3 patients initially treated with oral valgancyclovir. An additional important question that we could not address due to very limited data was whether anti-inflammatory treatment should be stopped when antiviral treatment is instituted.
The included studies were heterogeneous with regards to the severity of disease, as well as the clinical severity score employed (Table 1). Most of the studies included patients with at least moderate colitis, although the scoring system employed was not uniform. In addition, it was not possible to extract data pertaining to individual patient severity categories in order to determine whether severity of underlying disease affected the outcome, as might be expected. The same is true with regards to anti-inflammatory medications used by the patients and the proportion of steroid resistant patients, which could have served as a surrogate marker of severity and the degree of immune suppression. One study[13] included only steroid resistant patients who had failed a rescue medication (infliximab or cyclosporine), while other studies included patients with a wide variety of anti-inflammatory medications. Most of the included studies did not describe a clear strategy supporting the decision to institute or withhold antiviral therapy. Omiya et al[8] administered antiviral treatment only to patients with ulcers > 10 mm on colonoscopy. Kim et al[12] treated only steroid resistant patients, with steroid-responsive patients having excellent outcome without antiviral treatment. Criscuoli et al[17] based the decision to treat on a combination of positive IHC and pp65 antigenemia. Roblin et al[13] treated only steroid-resistant patients who were not improved after rescue infliximab or cyclosporine therapy. The rest of the studies treated all CMV- positive patients; however, it appears that patients who quickly responded to anti-inflammatory treatment after hospitalization were less likely to be treated with antivirals and have a favorable outcome without antivirals. On the contrary, patients with a very severe clinical presentation were frequently operated early in the course of the hospitalization, in many cases before their CMV status was established.
There are several important drawbacks to our study. The main weakness was the quality of the included studies. None of the included studies was randomized controlled trial. Only 4 of the studies had a prospective design. The total number of patients included in the analysis is small, reflecting a lack of well-designed large studies. The most significant drawback stems from inability to stratify the patients by disease severity, along with an inherent selection bias that resulted in administration of the antiviral therapy to the sicker patients in the majority of the studies. In addition, the definition of CMV infection differed significantly. We have attempted to overcome this heterogeneity in diagnostic criteria by performing a subgroup analysis of studies with a histological definition of CMV colitis. Mortality (2 patients overall) was reported in only 2 of the included studies, precluding any analysis of the impact of antiviral treatment on mortality.
Our MA has several strengths. We employed a stringent inclusion strategy aimed at minimizing selection and publication bias. Primarily, we excluded exclusive colectomy series, as they naturally included only patients who had reached the primary outcome (colectomy). In addition, we excluded the patients in whom the diagnosis of CMV was only available after colectomy, as these patients had never had a chance to receive the treatment. In order to minimize reporting bias, we excluded studies that did not compare patients with and without antiviral therapy, or included 3 or less patients in each arm, as these studies were likely to be biased towards one of the strategies.
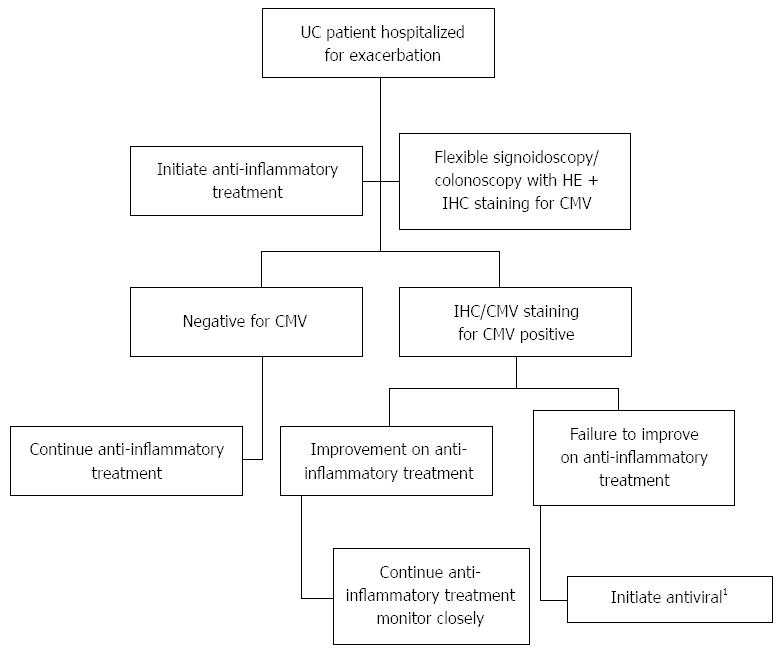
In summary, our MA did not demonstrate a benefit of antiviral therapy in CMV-positive patients with UC. The results were not changed if the analysis was restricted to studies using histological (IHC/HE) criteria for diagnosis of CMV. Based on the available literature, we are suggesting an algorithm for management of CMV-positive (as demonstrated by HE/IHC staining) patients hospitalized for UC exacerbation, stratified by clinical response to initial anti-inflammatory treatment (Figure 4). To the best of our knowledge, this is the first attempt to perform a systematic analysis of the multiple studies published on the subject. While the results are hampered by the weakness of the included studies, they do indicate the heterogeneity of this challenging patient cohort, showing that at least some patients with CMV probably do not require antiviral therapy. Thus, these findings underscore the dire need for prospective studies employing stringent clinical, endoscopic and virologic measures to identify the subgroups of patients who are likely to benefit from antiviral therapy, vs those who recuperate without this intervention. Such a study should also aim to establish the optimal dose and duration of treatment and the clinical benefit of withholding anti-inflammatory agents.
Cytomegalovirus (CMV) is a very common infection endemic almost ubiquitously. In immunocompromised patients CMV infection can be associated with severe end-organ and systemic infection. CMV presence is frequently detected in the mucosa of patients investigated for exacerbation of ulcerative colitis (UC). However, the impact of CMV infection on the prognosis of UC patients is unclear, and the impact of antiviral therapy on the outcome of these patients has not been well established.
Multiple studies describing the outcome of CMV-positive UC patients had been published in the last twenty years. However, a vast majority of these publications are case series or small case-controlled studies with heterogenous patients cohorts. In this study, we aimed to evaluate the impact of antiviral therapy on the outcome of CMV-positive UC patients using a meta-analysis of the currently available literature.
The authors did not demonstrate a positive impact of antiviral therapy with gancyclovir on either a short-term (colectomy within 30 d) or long-term (colectomy for the duration of follow-up) outcomes. These results should be addressed with caution due to a low quality of the included studies and important potential biases. The results underline a significant heterogenity of these population, that potentially includes both patie ts with mild disease who do not necessarily require antiviral therapy along with severy ill patients refractory to several lines of anti-inflammatory treatment. This study was underpowered to detect the impact of the disease severity on the outcome of these patient.
The results point out that not all of these patients benefit from antiviral therapy, and it is quite possible that patients with good initial response to conventional antiviral treatment may do well on this treatment alone. Well designed randomized controlled studies with stringent disease and outcome definitions are required in order to delineate the optimal treatment strategy for CMV-positive UC patients, and to define the patient subgroups that benefit from such treatment.
Cytomegalovirus - a very common virus that is rarely associated with significant morbidity in healthy individuals, but may be associated with severe complications in immunocompromised patients. It is commonly treated with intravenous gancyclovir
This is an interesting paper on one of the controversial issues in inflammatory bowel disease (IBD) literature on whether to treat or not and under which conditions CMV infection in patients with IBD. The methodology and analysis is technically solid, however a cautious interpretation due to a heavy selection bias is required.
P- Reviewers: Abraham P, Ali T, Fazel M, Lakatos PL, Taxonera C S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | de la Hoz RE, Stephens G, Sherlock C. Diagnosis and treatment approaches of CMV infections in adult patients. J Clin Virol. 2002;25 Suppl 2:S1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Lawlor G, Moss AC. Cytomegalovirus in inflammatory bowel disease: pathogen or innocent bystander? Inflamm Bowel Dis. 2010;16:1620-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 3. | Manuel O, Perrottet N, Pascual M. Valganciclovir to prevent or treat cytomegalovirus disease in organ transplantation. Expert Rev Anti Infect Ther. 2011;9:955-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Hodson EM, Ladhani M, Webster AC, Strippoli GF, Craig JC. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2013;2:CD003774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Maher MM, Nassar MI. Acute cytomegalovirus infection is a risk factor in refractory and complicated inflammatory bowel disease. Dig Dis Sci. 2009;54:2456-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol. 2006;101:2857-2865. [PubMed] |
| 7. | Hommes DW, Sterringa G, van Deventer SJ, Tytgat GN, Weel J. The pathogenicity of cytomegalovirus in inflammatory bowel disease: a systematic review and evidence-based recommendations for future research. Inflamm Bowel Dis. 2004;10:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Omiya M, Matsushita M, Tanaka T, Kawamata S, Okazaki K. The absence of large ulcer predicts latent cytomegalovirus infection in ulcerative colitis with positive mucosal viral assay. Intern Med. 2010;49:2277-2282. [PubMed] |
| 9. | Zeki SS, Kodati S, Jordan A, Hansi N, Thomas-Gibson S, Nightingale JM. T1322 Colectomy Rates for Patients Treated for CMV Disease in the Context of Ulcerative Colitis do Not Differ From Those Who are Not Treated for CMV Disease. Gastroenterology. 2010;138:S-537. [DOI] [Full Text] |
| 10. | Maconi G, Lombardini M, Ardizzone S, Cassinotti A, de Franchis R. Outcome of Cytomegalovirus (CMV) Colitis in Inflammatory Bowel Disease (IBD). A Retrospective Study. Gastroenterology. 2011;140:S-589. |
| 11. | Minami M, Ohta M, Ohkura T, Ando T, Ohmiya N, Niwa Y, Goto H. Cytomegalovirus infection in severe ulcerative colitis patients undergoing continuous intravenous cyclosporine treatment in Japan. World J Gastroenterol. 2007;13:754-760. [PubMed] |
| 12. | Kim YS, Kim YH, Kim JS, Cheon JH, Ye BD, Jung SA, Park YS, Choi CH, Jang BI, Han DS. The prevalence and efficacy of ganciclovir on steroid-refractory ulcerative colitis with cytomegalovirus infection: a prospective multicenter study. J Clin Gastroenterol. 2012;46:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Roblin X, Pillet S, Oussalah A, Berthelot P, Del Tedesco E, Phelip JM, Chambonnière ML, Garraud O, Peyrin-Biroulet L, Pozzetto B. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am J Gastroenterol. 2011;106:2001-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Al-Zafiri R, Gologan A, Galiatsatos P, Szilagyi A. Cytomegalovirus complicating inflammatory bowel disease: a 10-year experience in a community-based, university-affiliated hospital. Gastroenterol Hepatol (N Y). 2012;8:230-239. [PubMed] |
| 15. | Maruyama Y, Matsuoka K, Iwao Y, Yajima T, Inoue N, Hisamatsu T, Sujino T, Takabayashi K, Yoneno K, Mikami Y. Sa1936 Anti-Viral Therapy is Not Necessarily Indicated in Ulcerative Colitis Patients With Cytomegalovirus Infection Detected by Immunohistochemistry. Gastroenterology. 2012;142:S-363. |
| 16. | Kopylov U, Sasson G, Geyshis B, Oikawa MT, Barshack I, Eliakim R, Ben-Horin S. Cytomegalovirus positive ulcerative colitis: A single center experience and literature review. World J Gastrointest Pathophysiol. 2013;4:18-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Criscuoli V, Rizzuto MR, Montalbano L, Gallo E, Cottone M. Natural history of cytomegalovirus infection in a series of patients diagnosed with moderate-severe ulcerative colitis. World J Gastroenterol. 2011;17:633-638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Drouin E, Seidman E, Russo P, Deslandres C. Gastrointestinal cytomegalovirus infection complicating Crohn’s disease in an adolescent without AIDS. J Pediatr Gastroenterol Nutr. 1997;25:210-213. [PubMed] |
| 19. | Powell RD, Warner NE, Levine RS, Kirsner JB. Cytomegalic inclusion disease and ulcerative colitis; report of a case in a young adult. Am J Med. 1961;30:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Berk T, Gordon SJ, Choi HY, Cooper HS. Cytomegalovirus infection of the colon: a possible role in exacerbations of inflammatory bowel disease. Am J Gastroenterol. 1985;80:355-360. [PubMed] |
| 21. | Cottone M, Pietrosi G, Martorana G, Casà A, Pecoraro G, Oliva L, Orlando A, Rosselli M, Rizzo A, Pagliaro L. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn’s colitis. Am J Gastroenterol. 2001;96:773-775. [PubMed] |
| 22. | Matsuoka K, Iwao Y, Mori T, Sakuraba A, Yajima T, Hisamatsu T, Okamoto S, Morohoshi Y, Izumiya M, Ichikawa H. Cytomegalovirus is frequently reactivated and disappears without antiviral agents in ulcerative colitis patients. Am J Gastroenterol. 2007;102:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Osaki R, Andoh A, Tsujikawa T, Ogawa A, Koizumi Y, Nakahara T, Hata K, Sasaki M, Saito Y, Fujiyama Y. Acute cytomegalovirus infection superimposed on corticosteroid-naïve ulcerative colitis. Intern Med. 2008;47:1341-1344. [PubMed] |
| 24. | Yoda Y, Hanaoka R, Ide H, Isozaki T, Matsunawa M, Yajima N, Shiozawa F, Miwa Y, Negishi M, Kasama T. Clinical evaluation of patients with inflammatory connective tissue diseases complicated by cytomegalovirus antigenemia. Mod Rheumatol. 2006;16:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Yoshino T, Nakase H, Ueno S, Uza N, Inoue S, Mikami S, Matsuura M, Ohmori K, Sakurai T, Nagayama S. Usefulness of quantitative real-time PCR assay for early detection of cytomegalovirus infection in patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm Bowel Dis. 2007;13:1516-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Begos DG, Rappaport R, Jain D. Cytomegalovirus infection masquerading as an ulcerative colitis flare-up: case report and review of the literature. Yale J Biol Med. 1996;69:323-328. [PubMed] |
| 27. | Loftus EV, Alexander GL, Carpenter HA. Cytomegalovirus as an exacerbating factor in ulcerative colitis. J Clin Gastroenterol. 1994;19:306-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Papadakis KA, Tung JK, Binder SW, Kam LY, Abreu MT, Targan SR, Vasiliauskas EA. Outcome of cytomegalovirus infections in patients with inflammatory bowel disease. Am J Gastroenterol. 2001;96:2137-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 201] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 29. | Kambham N, Vij R, Cartwright CA, Longacre T. Cytomegalovirus infection in steroid-refractory ulcerative colitis: a case-control study. Am J Surg Pathol. 2004;28:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 151] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Rahier JF, Ben-Horin S, Chowers Y, Conlon C, De Munter P, D’Haens G, Domènech E, Eliakim R, Eser A, Frater J. European evidence-based Consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2009;3:47-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 372] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 31. | Rowshani AT, Bemelman FJ, van Leeuwen EM, van Lier RA, ten Berge IJ. Clinical and immunologic aspects of cytomegalovirus infection in solid organ transplant recipients. Transplantation. 2005;79:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |