Published online Jan 7, 2014. doi: 10.3748/wjg.v20.i1.296
Revised: November 2, 2013
Accepted: November 18, 2013
Published online: January 7, 2014
Processing time: 146 Days and 19.2 Hours
AIM: To investigate the clinical features, diagnosis, treatment and prognosis of intestinal T-cell lymphomas (ITCL) by retrospective analysis.
METHODS: Sixty-eight patients who were diagnosed with ITCL in case reports in the Chinese literature were compiled and reviewed. Age, gender, CD56 expression, surgical management, multifocal nature, perforation and cyclophosphamide chemotherapy were analyzed as the prognostic factors. The Kaplan-Meier method was adopted for the univariate analysis and the cumulative survival curve analysis.
RESULTS: The male-to-female ratio was 1.52 to 1. The median age was 41.7 years. Twenty-seven patients had symptoms of abdominal pain or diarrhea. Thirty-six of 60 patients with temperature records had high fevers at the onset of the illness. Twenty-six of 34 patients who underwent fiberoptic colonoscopy were misdiagnosed with Crohn’s disease, intestinal tuberculosis or cancer. Sixty-one patients underwent surgery. Twelve of 61 surgical patients required a second operation for anastomotic leakage or secondary perforation. The sites of lesion involvement were the jejunum (8.82%), ileum (29.41%), ileum and colon (4.41%), colon (55.88%) and appendix (1.47%). The median cumulative survival rate was 3 mo (3.00 ± 0.48).
CONCLUSION: Efforts should be made to correctly diagnose ITCL and select the proper operative approach that may reduce serious complications and create opportunities for further treatment.
Core tip: Intestinal T-cell lymphoma (ITCL) is a rare non-Hodgkin lymphoma of T-cell origin. ITCL is difficult to diagnose because its unique characteristics are clinically rare. The disease characteristics differ between the Western world and Asia. Most of these patients are misdiagnosed and suffer serious complications due to improper operative approaches. The prognosis of ITCL is poor. Large studies on this cancer remain scarce. We performed a statistical analysis of 68 cases collected from the Chinese literature to enhance our understanding of the histological definition, epidemiology, etiology, clinical features, surgical treatment and prognosis of ITCL.
- Citation: Sun ZH, Zhou HM, Song GX, Zhou ZX, Bai L. Intestinal T-cell lymphomas: A retrospective analysis of 68 cases in China. World J Gastroenterol 2014; 20(1): 296-302
- URL: https://www.wjgnet.com/1007-9327/full/v20/i1/296.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i1.296
Intestinal T-cell lymphoma (ITCL) is a rare non-Hodgkin lymphoma of T-cell origin. ITCL is a heterogeneous lymphoid neoplastic entity with variable clinical and pathological features[1]. ITCL has similar clinicopathological features and is only distinguished by immunohistochemical staining. The term ITCL should be used only in the setting of incomplete information regarding the precise diagnosis. According to the current World Health Organization classification system (2008)[2], two diseases associated with ITCL are intestinal NK/T-cell lymphoma and enteropathy-associated T-cell lymphoma (EATL). Primary gastrointestinal lymphomas constitute 5% to 10% of all gastrointestinal tumors[3]. Approximately 9% of gastrointestinal lymphomas are intestinal T-cell lymphomas[4]. In a Chinese report[5], ITCL constituted 9.8% of all primary intestinal non-Hodgkin lymphomas. The disease characteristics are distinct between Western countries and the Orient. EATL as defined by the WHO classification is rare in oriental countries[6,7]. Patients with ITCL in these countries do not always have a history of celiac disease (CD). In China, there have recently been increasing numbers of case reports of ITCL. However, large analyses are still scarce, particularly regarding the clinical and surgical management of the disease. ITCL is difficult to diagnose clinically. The improper selection of an operative approach may lead to serious complications due to misjudgment. To enhance our understanding of ITCL, we performed a statistical analysis of 68 case reports from the Chinese literature.
In total, 68 patients who were diagnosed with intestinal NK/T-cell lymphoma or T-cell lymphoma were compiled from case reports in the Chinese literature between January 2001 and December 2012. All patients were histologically confirmed to have intestinal NK/T-cell lymphoma or T-cell lymphoma based on the following criteria: expression of CD3ε+, CD3+, CD45RO+, CD56+, Granzyme B+ and TIA-1+[8,9].
The following clinical data were collected from the case reports: patient age, gender, body temperature, presence of bloody stools, CD, WBC count, fiberoptic colonoscopy, emergency surgery, perforation, hematochezia, sites of involvement, multifocal nature, second operation, expression of CD56, expression of EBV-encoded mRNA (EBER) and cyclophosphamide (CHOP) chemotherapy. The overall survival time was measured from the date of diagnosis to the date of death or the last follow-up.
Age, gender, expression of CD56, surgery, multifocal nature, perforation and CHOP chemotherapy were analyzed as the prognostics factors. The Kaplan-Meier method was adopted for the univariate analysis and the cumulative survival curve analysis. The log-rank test was used to analyze the factors of survival time for any significant differences. P-values less than 0.05 were considered significant. All statistical analyses were performed using the Statistical Software Package for the Social Sciences version 19.
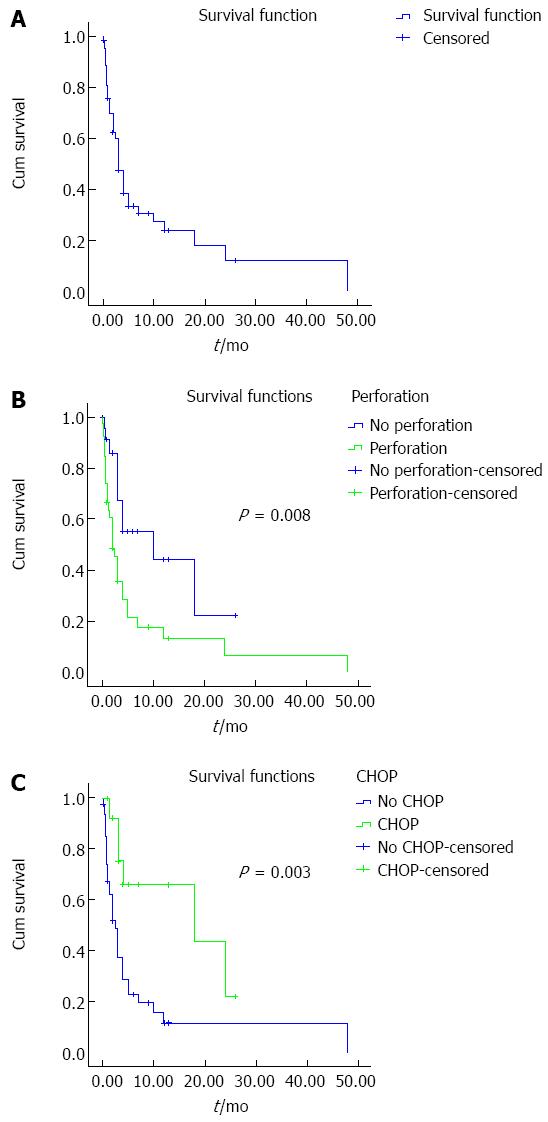
Sixty-eight ITCL patients were reported in the literature within a 12-year period. The pertinent clinical characteristics are listed in Table 1. There was a male preponderance with a male-to-female ratio of 1.52:1. The median age at diagnosis was 41.7 years (range, 14-81 years). Approximately 72.06% of the patients were ≤ 50 years. One patient (1.47%) had a history of CD. Twenty-seven (39.71%) patients had symptoms of abdominal pain or diarrhea. Twenty-six (38.24%) patients had a history of bloody stools. Thirty-six (60.00%) of 60 patients with temperature records had high fevers (more than 39.0 degrees centigrade) at the onset of the illness. Twenty-six (76.47%) of 34 patients with fiberoptic colonoscopy records were misdiagnosed as having Crohn’s disease, intestinal tuberculosis, or cancer, whereas 8 (23.53%) patients were definitely diagnosed with ITCL. Sixty-one (89.70%) patients underwent surgery and were diagnosed with ITCL on surgical histopathology. Forty-four (72.13%) of 61 surgical patients underwent emergency operations due to acute perforation or massive hematochezia. The remaining patients (27.87%) underwent elective surgery. Twelve (19.67%) of 61 surgical patients required a second operation for anastomotic leakage or secondary perforation. The sites of lesion involvement were the jejunum (6 cases, 8.82%), ileum (20 cases, 29.41%), ileum and colon (3 cases, 4.41%), colon (38 cases, 55.88%) and appendix (1 case, 1.47%). Forty-two (61.76%) patients had multifocal lesions. Forty-two (72.41%) of 58 patients with CD56 staining results were CD56-positive. Ten (62.5%) of 16 patients with EBER staining results via the in situ hybridization technique were positive. Ten (100%) patients with EBER+ expression simultaneously were CD56+ or granzyme B+ and were able to be precisely diagnosed with intestinal NK/T-cell lymphoma. Five (83.33%) of 6 patients with EBER-negative expression simultaneously were CD56+ and most likely were diagnosed with NK-like T-cell lymphoma. One patient with associated CD was CD56+. Sixteen patients completed CHOP chemotherapy after diagnosis. Sixty-three (92.65%) patients had follow-up data. The remaining 5 cases lacked detailed follow-up data. A cumulative survival curve is shown in Figure 1A (n = 63). The average cumulative survival was 10.6 mo (10.59 ± 2.67, 95%CI: 5.36-15.8). The median cumulative survival rate was 3 mo (3.00 ± 0.48, 95%CI: 2.07-3.94). The univariate analysis of prognostic factors using the Kaplan-Meier method showed the relationship of gender (P = 0.636), age over 50 years (P = 0.724), multifocal nature (P = 0.297), surgical management (P = 0.729), CD56 positivity (P = 0.449), perforation (7.12 ± 2.46 vs 12.00 ± 2.67, P = 0.008) and CHOP chemotherapy (8.32 ± 2.65 vs 15.97 ± 3.04, P = 0.003). The Kaplan-Meier survival curves and log-rank test for cumulative survival of the perforated group and the non-perforated group are shown in Figure 1B. The cumulative survival of the perforated group (n = 39) was significantly worse than that of the non-perforated group (n = 24). The median cumulative survival was 2 mo (2.00 ± 0.54, 95%CI: 0.95-3.05) in the perforated group. The Kaplan-Meier survival curves and log-rank test for cumulative survival of the CHOP group and the no CHOP group are shown in Figure 1C. The cumulative survival of the no CHOP group (n = 47) was significantly worse than that of the CHOP group (n = 16). The median cumulative survival was 18 mo (18 ± 12.87, 95%CI: 0.00-43.22) in the CHOP group.
| Characteristics | No./total (%) |
| Median age (yr), range | 41.7 (14-81) |
| ≤ 50 yr | 49 (70.06) |
| > 50 yr | 19 (27.94) |
| Gender (M:F) | 41:27 (1.52:1) |
| Pain or diarrhea | 27/68 (39.71) |
| Bloody stool | 26/68 (38.24) |
| Celiac disease | 1/68 (1.47) |
| Multifocal | 42/68 (61.76) |
| High fever | 36/60 (60.00) |
| Jejunum | 6/68 (8.82) |
| Ileum | 20/68 (29.41) |
| Colon | 38/68 (55.88) |
| Ileum and colon | 3/68 (4.41) |
| Appendix | 1/68 (1.47) |
| Diagnosed by fiberoptic colonoscopy | 8/34 (23.53) |
| Acute perforation | 42/68 (61.76) |
| Large hematochezia | 2/68 (2.94) |
| Emergency surgery | 44/61 (72.13) |
| Elective operation | 17/61 (27.87) |
| No surgery | 7/68 (10.29) |
| Secondary operation | 12/61 (19.67) |
| CD56+ | 42/58 (72.41) |
| EBER+ | 10/16 (62.5) |
| CD56+ or granzyme B+ and EBER+ | 10/10 (100) |
| CD56+ and EBER- | 5/6 (83.33) |
Extranodal NK/T-cell lymphoma (ENKTL) and EATL are the most characteristic subtypes of ITCL according to the current World Health Organization classification system (2008). NK/T is designated to instead of “NK”, because whereas most cases appear to be genuine NK-cell neoplasms, some manifest a cytotoxic T-cell phenotype. ENKTL is subcategorized into nasal and nasal-type NK/T-cell lymphomas according to the major sites of anatomic involvement. The nasal NK/T-cell lymphoma commonly presents with midline facial destructive disease, has a strong association with Epstein-Barr virus (EBV) and occurs prototypically within the nasal cavity. The nasal-type NK/T-cell lymphoma occurs in extranasal sites and shares a similar immunophenotypical profile with nasal NK/T-cell lymphoma. The preferential sites of extranasal involvement include the skin, soft tissue, gastrointestinal tract and testis[2]. EATL is divided into two types[10,11]. Type I is associated with CD and accounts for the majority of cases in Western countries. EATL type I tumor cells are CD3+, CD5-, CD7+, CD8+, CD4 -, CD56 - and CD103+. Type II is not associated with CD and is the most prevalent in Asian countries. EATL type II tumor cells are CD3+, CD4 -, CD8+ and CD56+[12]. NK/T-cell lymphoma is divided into two types. The first is a true NK/T-cell lymphoma that is CD3ε+, CD56+, granzyme B+, TIA-1+, sCD3-, CD45RO+ and EBV+. However, a few NK/T-cell lymphomas are negative for CD56 whereas nearly all are positive for EBER by in situ hybridization (ISH)[13]. The second type is an NK-like T-cell lymphoma that is CD56+, sCD3+, TIA-1+, CD45RO+ and EBV-. EATL is CD56- or CD56+, CD3+, CD45RO+, EBV+ or EBV-, and some may belong to NK-like T-cell lymphoma. ITCL has similar clinicopathological features. True NK-cell lymphomas do not have rearrangement of the T-cell receptor gene. Therefore, EATL can be distinguished from NK/T-cell lymphoma by reliable clonality testing of T-cell receptor genes[11]. In fact, because of the lack of detailed immunohistochemical profiles, the term ITCL should be used only in the setting of incomplete information regarding the precise diagnosis.
ENKTL is much more common in persons residing in Southeast Asia, Mesoamerica and Southern America and is rarely observed in the United States and Europe. Individuals with ENKTL reported in California and Texas, in fact, have usually been of Asian or Hispanic descent. In Southeast Asia, the ENKTL prevalence appears to be strongly associated with different ethnic backgrounds. In Malaysia, for example, the disease occurs more commonly in those of Chinese descent (22.51%-79%) followed by those of Malay descent (5%-16.37%) and lastly Indian descent (2.5%-16%). A male predominance of 2-3.6:1 has been reported[14].
EATL type I is frequently seen in those areas with a high prevalence of CD, in particular Northern Europe[2,7]. Previous reports indicate that type I EATL has a high frequency in Western countries, comprising approximately 80% of cases[15]. In a recent international series, type I was the most frequent subtype (66%)[10]. Type I in Western countries occurs with equal frequency in men and women aged on average from 56 to 60.1 years. Approximately 49% of the patients have a history of CD, which is localized mostly in the jejunum. Only 10% to 20% of cases are type II[16,17]. In our data, only one patient was diagnosed with type I EATL. Type II EATL appears to have a broader geographic distribution and is encountered in Asia and other regions where CD is rare. Type II EATL has been reported to be the predominant subtype in Asia, representing 90% of cases[7,18,19]. However, data on the occurrence of EATL in Asians are highly conflicting. A Korean series described 11 cases of EATL; all type II[19]. A study of 5 cases of type II EATL from Japan reported a total absence of histologic evidence of “enteropathy”[18]. In contrast, another study from Japan reported enteropathy-like changes in 14 of 18 cases (78%) of type II EATL and 2 of 3 cases of classical EATL[20]. However, among 38 cases of primary intestinal T-cell and NK-cell lymphomas recently reported from China, there were only 7 cases of EATL, all belonging to type II and all showing enteropathy-like changes. Only 1 patient with clinically suspected CD was considered type I EATL[11].
Little is known about the etiology of ENKTL. However, the very strong association of ENKTL with EBV, irrespective of the ethnic origin of the patients, suggests a probable pathogenic role of the virus[2,7,14]. In China, the positive frequency of EBER in ENKTL of nasal type is 83.78% to 93% and 85.71% in ITCL[16]. In our data, the EBER positive ratio is 62.5%. Ten (62.5%) of 16 patients with EBER staining were simultaneously EBER+ and CD56+ or granzyme B+, which can precisely diagnose intestinal NK/T-cell lymphoma. Five (83.33%) of 6 patients with EBER- also manifested CD56+ tumor cells, which may lead to a diagnosis of intestinal NK-like T-cell lymphoma. This result confirmed that ITCL is a heterogeneous lymphoid neoplastic group, and a small proportion of primary ITCLs in China and extranodal NK/T-cell lymphomas of the nasal type belong to the same spectrum[16].
Type I EATL is strongly associated with CD, which is an autoimmune disease that affects genetically susceptible individuals and is triggered by the ingestion of gluten[12,21]. Type II EATL usually shows a CD3+, CD4-, CD8+ and CD56+ immunophenotype and is characterized by monomorphic cytology with frequent expression of CD56, in addition to a lack of association with EBV and a weak or no association with CD[6,10,18]. In Western countries, 80% to 90% of EATL cases are type I with a CD56-positive rate of 10%. Approximately 10% to 20% of EATL cases are type II with a CD56-positive rate of 90%[2]. In our data, 83.3% of EBER negative patients are CD56 positive. There are some distinct differences in the disease characteristics between the West and East. Thus, the etiological agents may vary. It is currently unclear whether type II EATL represents a variant of classical EATL or a separate lymphoma entity[17,22]. In China, the intestinal NK/T-cell and EATL (Type II) are the main types[11,16,17].
In China, ITCL is commonly seen in young men. The lesions are mostly localized to the ileocecum and colon. The tumor has multifocal features[17]. In our data, the average age was 41 years. The male-to-female ratio was 1.52 to 1. The sites of lesion involvement were the jejunum (8.82%), ileum (29.41%), colon (55.88%), ileum and colon (4.41%) and appendix (1.47%). Only 1 (1.47%) patient had a history of CD. Approximately 61.76% of 68 patients had multifocal lesion. These results differ from those in the Western literature. A multicenter study from the Asia Lymphoma Study Group identified 38 EATL patients within a 19-year period[22]. All cases were type II EATL. Men were affected twice as often as women, at a median age of 59. None had a history of CD. The sites of involvement were the small bowel and stomach (5%), the small bowel (63%), the small and large bowel (16%) and the large bowel (18%). Common presenting features included bowel perforation (34%).
Abdominal pain or diarrhea is a common presenting symptom, with an occurrence rate of 39.7%. Approximately 38.24% of 68 patients had a history of bloody stools. A high fever may be a valuable characteristic for diagnosis. Sixty percent of 60 patients had a high fever, usually above 39 °C and even as high as 40 °C, but the white blood cell counts were normal or somewhat increased (generally ≤ 12 × 109). A few patients manifested hemophagocytic syndrome (HPS)[14]. Two patients with HPS were recorded in our data. Acute perforation is a common complication. The perforation rate was 61.76%, and massive hematochezia occurred in 2.94%. In total, 89.71% of 68 patients underwent surgery, and 75% of the patients had an emergency operation for an acute perforation or massive bleeding.
ITCL is difficult to diagnose because the unique characteristics are clinically rare. Spontaneous intestinal perforation accompanied by high fever was common in this group of patients. A few patients presented to the hospital with an abdominal mass. Most of these patients presented due to abdominal pain or diarrhea and bloody stools. Fiberoptic colonoscopy is the most commonly used method of examination, but the results are not satisfactory. Only 21% of intestinal non-Hodgkin lymphomas were diagnosed endoscopically[23]. In our data, approximately 76.47% of the 34 patients with reports of fiberoptic colonoscopy were misdiagnosed with Crohn’s disease, intestinal tuberculosis, or cancer. Only 23.53% of the patients who underwent frequent fiberoptic colonoscopy were definitely diagnosed with intestinal lymphoma. The reasons may include the following: (1) The biopsy specimens from the fiberoptic colonoscopy were not sufficiently large, deep and numerous to diagnose correctly. Histopathologically, ITCL is known to be more aggressive to blood vessels, and the lesion is primarily located in the submucosa and smooth muscle where there are abundant capillaries. Overall, 89.29% of the patients were diagnosed with ITCL by immunophenotypical analysis of the surgical resection specimens postoperatively; (2) Pathological diagnosis is difficult because the tumor cell is variable; and (3) The disease is ignored because of its rarity. Hence, more attention should be paid to those patients with a persistently high fever, hematochezia and intestinal spontaneous perforation, especially with normal or slightly elevated WBC counts. Video capsule enteroscopy and double-balloon enteroscopy can be used as diagnostic modalities. A study has demonstrated that the pretreatment plasma EBV-DNA copy number is a good indicator for both the treatment response and overall survival in extranodal NKTCL. The EBV-DNA level is a good indicator of the response and overall survival[24].
Surgery alone is not therapeutic in the management of non-Hodgkin lymphoma; its use delays the initiation of chemotherapy, and the underlying undernutrition exposes the patient to problems of healing and infection. Surgery is usually performed on an emergency basis for diagnostic purposes when endoscopy is not possible and/or to treat one or several complications (45% to 72% of surgical interventions, depending on the series)[5,6]. Over 50% of patients are diagnosed based on a surgical complication that reveals the disease[23]. Hence, the surgical purposes are the treatment of acute perforation and hematochezia complications; diagnosis when endoscopy is not possible; and to permit further treatment by proper surgical treatment. When deciding how to proceed during the operation, the nature of the disease should be considered first. Ileotyphus with perforation is the disease that we first think of that has the same signs and symptoms as ITCL when the patient has a high fever. There are some differences between the two entities. ITCL lesions occur mostly in the colon and ileocecum and are multifocal, which differs from ileotyphus with a single focus in the ileum. The pictures from the case reports in our study and our own experience demonstrated the long axis of the ellipse of the ITCL perforation to be perpendicular to the intestinal longitudinal axis, which differs from the parallel to the intestinal longitudinal axis observed with ileotyphus. Perhaps the pathologic vascular invasion feature of ITCL is consistent with the anatomy of the intestinal marginal artery. This phenomenon differs from ileotyphus in which the pathologic feature of the typhoid is lymphoid infiltrates in the intestinal wall.
Enterectomy and enteroanastomosis are usually performed to treat the perforation. However, primary anastomosis is not ideal. In our data, 12 (19.67%) of the 61 surgical patients went to the operating room a second or even third time due to anastomotic leakage or secondary perforation. The reasons for this finding may be as follows: (1) The histopathological feature of ITCL is that the lymphomatous infiltrate is diffuse in the wall of the intestine and multifocal. The edge of the surgical specimen may be not clean and may cause anastomotic leakage. Multifocal lesions may cause later perforation. The multifocal rate was 61.8% in our data and 54% in data from a German study group[23]; (2) Most patients were treated with steroids, and some were even treated with CHOP chemotherapy; and (3) High fever and malnutrition influence the anastomotic healing. Thus, the proper operative management requires careful planning. We believe that a temporary enterostomy may be a better choice for those patients with a persistently high fever, unexplained intestinal spontaneous perforation and a history of steroid medication use. The time of the enteroenterostomy should be determined based on the histopathological findings of the operation.
Chemotherapy is often used after precise diagnosis of ITCL. A variety of regimens have been used. The treatment strategy for peripheral T-cell lymphomas (PTCLs) is still based on anthracycline-containing chemotherapy such as CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) or CHOP-like regimens despite the fact that their outcome was not satisfactory in the majority of PTCLs[25,26]. A report by Bortezomib administering CHOP as first-line treatment shows the complete response of only 30% for ENKTL[27]. In fact, 30% of the patients never received the chemotherapy due to their poor general health status and early death from complications (initial 3 mo). In addition, among patients who are able to receive the initial chemotherapy, an average of 50% is unable to finish their therapeutic program[6,23]. The data in the literature are inconsistent[6,12,22]. In our data, the use of CHOP chemotherapy was a statistically significant difference. The cumulative survival of the no CHOP group was worse than that of the CHOP group. The median cumulative survival rate was 18 mo in the CHOP group.
The prognosis of ITCL is very poor. The 1- and 5-year survival rates of EATL were 31%-39% and 8%-20%, respectively[12]. The median OS was 3 to 7 mo[16,22]. In our data, the average cumulative survival was 10.6 mo. The median cumulative survival was 3 mo. The 6-mo, 1-year and 5-year survival rates were 33.4%, 23.8% and 11.9%, respectively. The gender, age, surgery and multifocal nature were not associated with a poor prognosis in the univariate analysis.
Perforation is a poor predictive factor. The median cumulative survival was 2 mo in the perforated group and 10 mo in the non-perforated group, which was a statistically significant difference.
In conclusion, the epidemiological, pathological features and clinical characteristics of ITCL in Chinese populations are very different from those in the West, suggesting that a small proportion of primary ITCLs in China and ENKTL of the nasal type may belong to the same spectrum. ITCL is difficult to diagnose because of the rare clinical characteristics and unique pathological features. We should be vigilant with regard to spontaneous intestinal perforation accompanied by a high fever, which may portend this disease. The outcome of colonoscopy for ITCL is not satisfactory because ITCL is more aggressively localized to blood vessels and the tumor cells are variable. Although surgery is not a radical therapy in the management of ITCL, most of these patients will undergo surgical treatment and reach a final diagnosis. The key point is that efforts should be made to diagnose the disease correctly during the operation and to select the proper operative technique that may reduce serious complications and create opportunities for further treatment. The prognosis of ITCL is poor. Although CHOP chemotherapy may prolong survival, the survival rate is not satisfactory.
Intestinal T-cell lymphoma (ITCL) is a rare non-Hodgkin lymphoma of T-cell origin and a heterogeneous lymphoid neoplastic entity with variable clinical and pathological features. Different ITCL entities have similar clinicopathological features and are only distinguished by immunohistochemical staining. The disease characteristics are distinct between Western and Eastern countries. In China, there have recently been increasing numbers of case reports. However, large studies are still scarce, especially regarding clinical and surgical management. ITCL is difficult to diagnose clinically. Although surgery is not radically therapeutic in the disease’s management, the improper selection of the operative management may lead to serious complications due to misjudgment.
To enhance our understanding of ITCL, we collected 68 cases reported in the Chinese literature and performed a statistical analysis to investigate the disease’s features in terms of the histological definition, epidemiology, etiology, clinical diagnosis, surgical treatment and prognosis.
The research results reveal that the epidemiology and etiology of ITCL in China are different from those in the West, providing insight for the clinical diagnosis and surgical treatment via statistical analysis. Univariate analysis revealed that the prognosis is related to perforation and cyclophosphamide chemotherapy. The cumulative survival curve was plotted.
The results of this study would be useful for physicians to diagnose and treat ITCL, especially for surgeons selecting the proper operative method to avoid or reduce serious complications due to misjudgment. Although surgery is not a radical therapy in the management of ITCL, most patients will undergo surgical treatment to reach a final diagnosis. Proper surgical treatment may create the opportunity for further treatment. The cumulative survival curve will direct physicians to communicate with families of patients efficiently.
ITCL is a rare non-Hodgkin lymphoma of T-cell origin. ITCL is a heterogeneous lymphoid neoplastic entity with variable clinical and pathological features and subtypes that are only able to be distinguished by immunohistochemical staining. The term ITCL should be used only in the setting of incomplete information regarding the precise diagnosis.
This is an interesting study. Although intestinal T-cell lymphoma is rare clinically, there is a clinical relevance. In a well-conducted study, the authors analyzed a representative population of Chinese ITCL cases, providing a complete compilation of epidemiological, histological and clinical findings that are useful to clarify the state of the art in this rare pathology.
P- Reviewers: De Silva AP, Martinez-Lostao L, Su C S- Editor: Cui XM L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Domizio P, Owen RA, Shepherd NA, Talbot IC, Norton AJ. Primary lymphoma of the small intestine. A clinicopathological study of 119 cases. Am J Surg Pathol. 1993;17:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 146] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid tissues. 4th ed. Lyon: IARC press 2008; 12-15. |
| 3. | Radaszkiewicz T, Dragosics B, Bauer P. Gastrointestinal malignant lymphomas of the mucosa-associated lymphoid tissue: factors relevant to prognosis. Gastroenterology. 1992;102:1628-1638. [PubMed] |
| 4. | Franssila KO, Jaser N, Sivula A. Gastrointestinal non-Hodgkin’s lymphoma. A population-based clinicopathological study of 111 adult cases with a follow-up of 10-15 years. APMIS. 1993;101:631-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Wang GB, Xu GL, Luo GY, Shan HB, Li Y, Gao XY, Li JJ, Zhang R. Primary intestinal non-Hodgkin’s lymphoma: a clinicopathologic analysis of 81 patients. World J Gastroenterol. 2011;17:4625-4631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Chandesris MO, Malamut G, Verkarre V, Meresse B, Macintyre E, Delarue R, Rubio MT, Suarez F, Deau-Fischer B, Cerf-Bensussan N. Enteropathy-associated T-cell lymphoma: a review on clinical presentation, diagnosis, therapeutic strategies and perspectives. Gastroenterol Clin Biol. 2010;34:590-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Chuang SS, Chang ST, Chuang WY, Huang WT, Hsieh PP, Tsou MH, Liao YL, Lin SH, Hsieh YC, Lu CL. NK-cell lineage predicts poor survival in primary intestinal NK-cell and T-cell lymphomas. Am J Surg Pathol. 2009;33:1230-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Suzuki R. NK/T-cell lymphomas: pathobiology, prognosis and treatment paradigm. Curr Oncol Rep. 2012;14:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Ishida F, Kwong YL. Diagnosis and management of natural killer-cell malignancies. Expert Rev Hematol. 2010;3:593-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Delabie J, Holte H, Vose JM, Ullrich F, Jaffe ES, Savage KJ, Connors JM, Rimsza L, Harris NL, Müller-Hermelink K. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the international peripheral T-cell lymphoma project. Blood. 2011;118:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 11. | Sun J, Lu Z, Yang D, Chen J. Primary intestinal T-cell and NK-cell lymphomas: a clinicopathological and molecular study from China focused on type II enteropathy-associated T-cell lymphoma and primary intestinal NK-cell lymphoma. Mod Pathol. 2011;24:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | van de Water JM, Cillessen SA, Visser OJ, Verbeek WH, Meijer CJ, Mulder CJ. Enteropathy associated T-cell lymphoma and its precursor lesions. Best Pract Res Clin Gastroenterol. 2010;24:43-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Lu D, Lin CN, Chuang SS, Hwang WS, Huang WT. T-cell and NK/T-cell lymphomas in southern Taiwan: a study of 72 cases in a single institute. Leuk Lymphoma. 2004;45:923-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Al-Hakeem DA, Fedele S, Carlos R, Porter S. Extranodal NK/T-cell lymphoma, nasal type. Oral Oncol. 2007;43:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Chott A, Haedicke W, Mosberger I, Födinger M, Winkler K, Mannhalter C, Müller-Hermelink HK. Most CD56+ intestinal lymphomas are CD8+CD5-T-cell lymphomas of monomorphic small to medium size histology. Am J Pathol. 1998;153:1483-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 143] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Zhang WY, Li GD, Liu WP, Ouyang Q, Ren XC, Li FY, Xu H. Features of intestinal T-cell lymphomas in Chinese population without evidence of celiac disease and their close association with Epstein-Barr virus infection. Chin Med J (Engl). 2005;118:1542-1548. [PubMed] |
| 17. | Chan JK, Chan AC, Cheuk W, Wan SK, Lee WK, Lui YH, Chan WK. Type II enteropathy-associated T-cell lymphoma: a distinct aggressive lymphoma with frequent γδ T-cell receptor expression. Am J Surg Pathol. 2011;35:1557-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Akiyama T, Okino T, Konishi H, Wani Y, Notohara K, Tsukayama C, Tsunoda T, Tasaka T, Masaki Y, Sugihara T. CD8+, CD56+ (natural killer-like) T-cell lymphoma involving the small intestine with no evidence of enteropathy: clinicopathology and molecular study of five Japanese patients. Pathol Int. 2008;58:626-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Ko YH, Karnan S, Kim KM, Park CK, Kang ES, Kim YH, Kang WK, Kim SJ, Kim WS, Lee WY. Enteropathy-associated T-cell lymphoma--a clinicopathologic and array comparative genomic hybridization study. Hum Pathol. 2010;41:1231-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Takeshita M, Nakamura S, Kikuma K, Nakayama Y, Nimura S, Yao T, Urabe S, Ogawara S, Yonemasu H, Matsushita Y. Pathological and immunohistological findings and genetic aberrations of intestinal enteropathy-associated T cell lymphoma in Japan. Histopathology. 2011;58:395-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 1272] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 22. | Tse E, Gill H, Loong F, Kim SJ, Ng SB, Tang T, Ko YH, Chng WJ, Lim ST, Kim WS. Type II enteropathy-associated T-cell lymphoma: a multicenter analysis from the Asia Lymphoma Study Group. Am J Hematol. 2012;87:663-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Daum S, Ullrich R, Heise W, Dederke B, Foss HD, Stein H, Thiel E, Zeitz M, Riecken EO. Intestinal non-Hodgkin‘s lymphoma: a multicenter prospective clinical study from the German Study Group on Intestinal non-Hodgkin‘s Lymphoma. J Clin Oncol. 2003;21:2740-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 162] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Suzuki R, Yamaguchi M, Izutsu K, Yamamoto G, Takada K, Harabuchi Y, Isobe Y, Gomyo H, Koike T, Okamoto M. Prospective measurement of Epstein-Barr virus-DNA in plasma and peripheral blood mononuclear cells of extranodal NK/T-cell lymphoma, nasal type. Blood. 2011;118:6018-6022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 25. | Gallamini A, Stelitano C, Calvi R, Bellei M, Mattei D, Vitolo U, Morabito F, Martelli M, Brusamolino E, Iannitto E. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103:2474-2479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 529] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 26. | Abouyabis AN, Shenoy PJ, Lechowicz MJ, Flowers CR. Incidence and outcomes of the peripheral T-cell lymphoma subtypes in the United States. Leuk Lymphoma. 2008;49:2099-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Kim SJ, Yoon DH, Kang HJ, Kim JS, Park SK, Kim HJ, Lee J, Ryoo BY, Ko YH, Huh J. Bortezomib in combination with CHOP as first-line treatment for patients with stage III/IV peripheral T-cell lymphomas: a multicentre, single-arm, phase 2 trial. Eur J Cancer. 2012;48:3223-3231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |