Published online Dec 15, 1996. doi: 10.3748/wjg.v2.i4.238
Revised: September 18, 1996
Accepted: October 9, 1996
Published online: December 15, 1996
AIM: To study the three-dimensional structure and distribution of the lymphatics in the rabbit appendix, and to reveal the correlation between the perifollicular lymphatic sinus (PLS) and lymphatics.
METHODS: Freeze-fractured tissues and lymphatic corrosion cast with the Mercox were used for scanning electron microscopy (SEM), and histologic and semithin sections were used for light microscopy. The Mercox was diluted and injected intraparenchymally into the appendix wall. The injected appendixes were cut and put in a concentrated NaOH solution until the tissues were corroded away.
RESULTS: The lymphatic capillary networks were found in the superficial layer of the mucosa and the lymphatic capillary plexuses were observed in the deep layer of the mucosa. From the plexuses, the short grove-like lymphatic capillaries were connected with the PLS. The luminal side of the sinus looked like a flower basket. Short lymphatic capillaries arising from the bottom of the PLS were continuous with the lymphatics of the submucosa. The lymphatics of the submucosa were connected with the lymphatics running in the muscular layer, then they were led into the serosal lymphatics and drained into the lymphatics in the mesoappendix.
CONCLUSION: The PLS and rich lymphatics in the rabbit appendix may play an important role in the drainage of lymph and the immune function.
- Citation: Tang FC, Zhang YF, Xu YD, Zhong SQ, Wang XP, Wang YX. Scanning electron microscopic study of lymphatic corrosion casts in the rabbit appendix. World J Gastroenterol 1996; 2(4): 238-240
- URL: https://www.wjgnet.com/1007-9327/full/v2/i4/238.htm
- DOI: https://dx.doi.org/10.3748/wjg.v2.i4.238
The appendix is one of the gut-associated lymphoid organs. The lymphatics and perifollicular lymphatic sinus (PLS) in the appendix have been studied by many authors[1-4]. However, using SEM technique and the lymphatic corrosion cast with Mercox to study the three-dimensional structure of lymphatics in the appendix and to reveal the relationship between the PLS and the lymphatics has not been reported in China. In our study, we used the lymphatic corrosion cast with Mercox, fracture specimens and semithin section methods to investigate the intramural lymphatics in the rabbit appendix and their three-dimensional structure.
Fifteen healthy rabbits of either sex, weighing 2.5-4.0 kg, were used: one for light microscopy with histologic sections, two for scanning electron microscopy (SEM) with fractured tissues, two for semithin sections, and ten for lymphatic corrosion cast.
About 2-3 mL Mercox (CL-2B-5, Velene Hospital, Tokyo, Japan) was diluted to 30%-40% (V/V) solution, then it was injected intraparenchymally into the lower part of the mucosa of the appendix wall. After the injected medium had filled the lymphatics of the rabbit appendix, the injected appendix was cut and put into 5%-20% NaOH solution until tissue elements were corroded away. Thus the corrosion casts were obtained by washing in water. The casts were cut into blocks of appropriate size, then they were dried, coated with gold and observed by SEM[5].
The rabbits were anesthetized with pentobarbital sodium and perfused with 0.85% NaCl and 2.5% PBS through the superior mesenteric artery. The appendixes were cut into small pieces, and freeze-fractured specimens were obtained by conducting electronic staining, dehydrating in a graded series of ethanol, immersing in isoamyl acetate, fracturing with the solution of liquid nitrogen and drying at critical point with liquid CO2. The dried specimens were mounted on a specimen holder, coated with gold and observed by SEM with increasing voltage of 10-20 kV. Semithin section samples were completed by using usual TEM technique, and were observed with a light microscope.
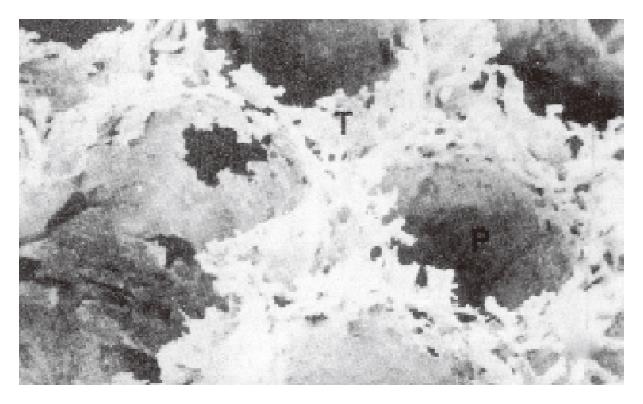
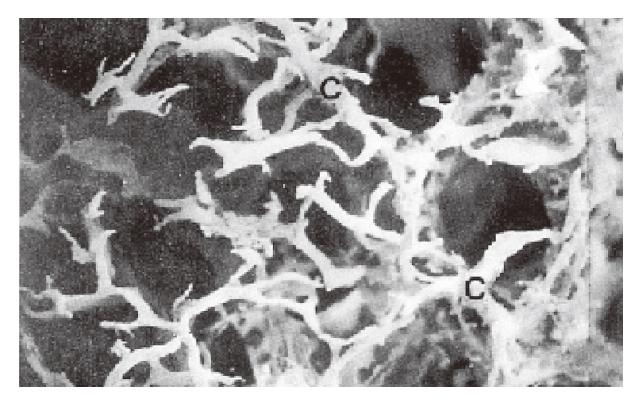
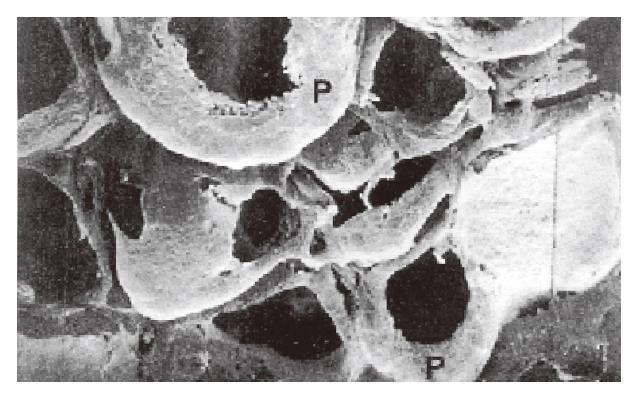
SEM of lymphatic casts showed that the PLSs were not continuous along the entire wall of each follicle but surrounded the lateral surface of the follicle (Figure 1). The upper end of each sinus possessed dense short grove-like lymphatic capillaries that projected toward the thymus-dependent areas (TDA). In the TDA, they formed dense lymphatic capillary plexuses (Figure 1). The straight lymphatics arose from the upper ends of the sinuses, and they were thicker than the lymphatic capillaries in the TDA. The straight lymphatics were continuous with the lymphatic capillaries in the mucosa. The latter interconnected and formed a well-developed network of lymphatic capillaries in the superficial layer of the mucosa (Figure 2). The bottoms of the PLS gave the appearance of incomplete sleeve. In many cases, the sleeves were round in shape (Figure 3). The fine lymphatics arose from the bottom of the sinuses and were continuous with the submucosa lymphatics. The submucosa lymphatics were connected with the lymphatics of the muscular layer, then they were led into the subserosa lymphatics and drained into the lymphatics in the mesoappendix. The fusiform impression of the endothelial nuclei was found on the casts. The casts of the thicker lymphatics showed notches corresponding to the bicuspid valves of the lymphatics.
The PLS and lymphatics in rabbit appendix demonstrated by SEM of fractured appendix and the semithin section observations by light microscopy were also similar to the scanning electron microscopic images of the lymphatic corrosion cast.
In general, the lymphatics do not exist in the superficial layer of the mucosa, but between the bottom of the large intestine glands and the mucosa muscle, there were the lymphatic capillaries[6,7]. In our study, a large number of lymphatic capillaries in the superficial layer of the mucosa in the rabbit appendix were observed. They connected with each other in circle, surrounded the mucosa glands and projected to the luminal side. The results are similar to those reported by Hirashima et al[8]. These lymphatic capillaries drain the lymph and may transfer some immune substances from the TDA to the lumen, which plays an important role in immune function. Ohtani[4] observed mucosa lymphatics (140 μm in diameter), but we only found the lymphatic capillaries (20-40 μm in diameter) circling the top of the PLS in the deep layer of the mucosa, and many lymphocytes in the lumen were identified. Our results may support the Waksman′s[3] opinion that the TDA was the place of the lymphocyte recirculation. We suggest that the lymphatic capillaries in TDA could transfer the lymphocytes not only to the PLS, but also to the lymphatic capillary networks in the superficial layer of the mucosa, then to the lumen of the intestine.
As we have described, each follicle was surrounded by a well-developed PLS as reported by Ohtani in 1984. Ohtani considered that two adjacent follicles frequently shared one PLS. But we found that each follicle had one PLS; rarely did two adjacent PLS fuse to form a large sinus.
According to our observation, we consider that PLS may have extensive drainage. They communicate with (1) the adjacent one by anastomotic channels; (2) the network of lymphatic capillaries in the mucosa by straight channels; (3) the plexuses of the lymphatic capillaries in TDA by short anastomotic channels; and (4) submucosal lymph channels by short lymphatics. It is suggested that the PLS may transport immune substances and lymphocytes to the network of the superficial layer in the mucosa by straight lymphatics. The immune substances may be supplied by TDA, transferred to the local lymph nodes, then to the lymph circulation. In general, the sinuses may act as a reservoir.
Baba[9] has reported that few lymphatics go to the lymphatic follicle. We have not observed intrafollicle lymphatics in the rabbit appendix.
Our observation is the same as that described by великоречин. In the submucosal layer, the lymphatic capillaries and lymphatics were situated at the same level. In the muscular layer, the lymphatics and lymphatic capillaries were also found. Baba[9] described that there were only lymphatics in the muscular layer. Sui Guang-Zhi reported that the lymphatic capillaries located in the superficial layer of the subserosa, and lymphatics in the subserosal deep layer. We have not found the lymphatics and lymphatic capillaries in the superficial layer of the subserosa.
Original title:
S- Editor: Cao LB L- Editor: Wang TQ E- Editor: Li RF
| 1. | Wang YX. The practical anatomy of lymphatic system. Beijing: The People s Health Pablication 1984; 215-238. |
| 2. | Bockman DE. Functional histology of appendix. Arch Histol Jpn. 1983;46:271-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Waksman BH, Ozer H, Blythman HE. Appendix and M-antibody formation. VI. The functional anatomy of the rabbit appendix. Lab Invest. 1973;28:614-626. [PubMed] |
| 4. | Ohtani O. Lymphatics of the rabbit small intestine and appendix. A study with scanning electron microscopy of corrosion casts (In Japanese). Biomed SEM. 1984;13:66-72. |
| 5. | Tang FC, Wang YX, Han MD. Observation of the corrosion casts of stomach lymphatics of the rabbit. Acta anat Sinaca. 1992;23:343-346. |
| 6. | Kamei Y. The distribution and relative location of the lymphatic and blood vessels in the mucosa of the rabbit colon. Nagoya Med J. 1969;15:223-238. [PubMed] |
| 8. | Hirashima T. The fine distribution of lymphatics in large intestine wall. Lymphatology. 1983;6:227-232. |
| 9. | Baba H. The fine distribution of lymphatics in the ileum, caecum and ileocecum of dog. J Tokyo Univ. 1976;34:441-446. |