Published online Dec 15, 1996. doi: 10.3748/wjg.v2.i4.236
Revised: August 21, 1996
Accepted: September 14, 1996
Published online: December 15, 1996
AIM: To investigate the expression of P53 oncoprotein in benign and malignant lesions of the large bowel as well as the relationship between p53 expression and clinicopathological factors.
METHODS: Immunohistochemistry was used to detect P53 protein in large bowel tissues of 146 cases with benign and malignant lesions.
RESULTS: All normal large bowel mucosae and non-neoplastic polyps were negative for P53 protein. However, the positive rates of P53 protein in adenomas, paracancerous mucosae and carcinomas were 18.18% (2/11), 13.21% (7/53) and 42.11% (32/76), respectively. The P53 expression in both adenomas and paracancerous mucosae presented only weak staining, whereas 75% of p53 positive cancers displayed very intense staining (++ or +++). The rates of P53 protein detection in poorly differentiated carcinoma and mucous carcinoma were 63.64% (7/11) and 62.5% (10/16), respectively, which were much higher than that of well/moderately differentiated carcinomas (30.16%, 15/40) (p < 0.05), and the carcinomas with marked positive p53 expression were more likely to penetrate the bowel wall and metastasize to lymph nodes (p < 0.05). However, no relationship between p53 expression and massive type, tumor size, location, Dukes stage or 3-year survival was found in this study.
CONCLUSION: P53 gene mutation and overexpression are common in colorectal cancers, and seem to be associated with histological type, progression and lymph node metastasis of colorectal cancer.
- Citation: Peng DF, Lin HM. Expression of P53 oncoprotein in benign and malignant lesions of large bowel. World J Gastroenterol 1996; 2(4): 236-237
- URL: https://www.wjgnet.com/1007-9327/full/v2/i4/236.htm
- DOI: https://dx.doi.org/10.3748/wjg.v2.i4.236
The p53 suppressor gene is the most frequently altered gene in solid human malignancies[1,2]. In colorectal neoplasia, p53 dysfunction is important in the progression from adenomas into invasive carcinomas[3,4], and mutation of p53 seems associated with tumor biology of colorectal and other neoplasias[5,6]. In this study, an immunohistochemical method was used to detect p53 protein in 76 cases of colorectal cancers and 70 cases of other large bowel tissues.
Specimens of 76 colorectal carcinomas, 11 adenomas, 6 non-neoplastic polyps, and 53 paracancerous mucosae were obtained from Yijishan Hospital. The tissues were fixed in 10% neutral buffered formalin. Paraffin sections (5 μm) were cut and used for HE staining and immunohistochemistry. Mouse antibody against P53 protein DO7 and S-P Kits were purchased from Maxim Co.
S-P immunohistochemistry was used in this study, and both negative and positive controls were set. The staining patterns observed were scored according to the relative intensity and extent of immunoperoxidase staining. The intensity of staining was scored as negative (0), weak-yellow (1), yellow-brown (2) and dark-brown (3). The extent of staining was scored as negative (0), < 30% cells stained (1), 30%-70% cells stained (2), and > 70% cells stained (3). Then the cases were divided into four stages according to the scores obtained by adding the two above-mentioned scores: those not stained at all being negative (-), those scoring 2 points grade (+), those scoring 3-4 points grade (++) and those scoring 5-6 points grade (+++).
P53 protein positive rates and intensities in colorectal cancers and other large bowel tissues are shown in Table 1.
| Group | n | - | + | ++ | +++ | Positive rate (%) |
| NMOLB | 13 | 13 | 0 | 0 | 0 | 0 |
| Non-neoplastic polyps | 6 | 6 | 0 | 0 | 0 | 0 |
| Paracancerous mucose | 53 | 46 | 3 | 0 | 0 | 13.21 |
| Adenomas | 11 | 9 | 2 | 0 | 0 | 18.18 |
| Carcinomas | 76 | 44 | 8 | 16 | 8 | 42.11b |
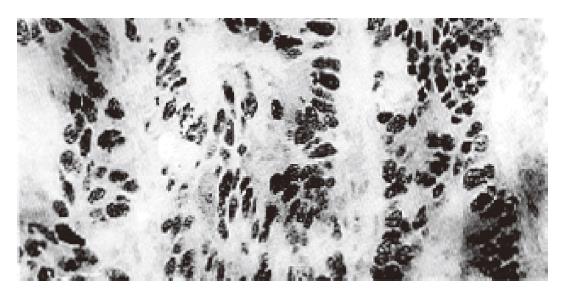
P53 protein in colorectal cancer cells was mainly located in nuclei (Figure 1) and the distribution of stained cells was very variable. In some tumors the majority of cells and sometimes all cells showed overexpression at a very high level, indicating very intense P53 staining. In some tumors there were only occasionally stained cells. But in the majority of p53 positive cases, the percentage of stained cells ranged from 30% to 70%. However, p53 expression in both adenomas and paracancerous mucosas presented occasional cell staining in some cases.
The correlations between p53 expression and clinicopathological parameters of colorectal carcinoma are shown in Tables 2 and 3. No significant relation was found between P53 expression and tumor size, location, massive type, Dukes stage or 3-year survival (P > 0.05). However, P53 expression in colorectal cancer was associated with tumor differentiation, histological type, serosal invasion and lymph node metastasis. Poorly differentiated and mucous carcinomas possessed much higher rates of P53 detection than well and moderately differentiated cancer, and the cancers with marked positive p53 expression were more likely to penetrate the bowel wall and metastasize to lymph nodes (P < 0.05).
Growing evidence suggests that the accumulation of multiple genetic events is responsible for the pathogenesis and/or progression of tumors. The abnormalities of the p53 gene were found to represent the most common molecular change in human cancer[1-4]. Such abnormalities can be detected in many ways. For example, mutations can be demonstrated by sequencing of the commonly mutated exons or inferred by SSCP analysis. The mRNA expression can be detected by hybridization and the expression of oncoprotein can be investigated by immunochemical methods, including immunohistochemistry. Of all the above-mentioned methods, immunohistochemistry is valuable in clinical practice. Recent studies have shown that there are several mechanisms to stabilize P53 protein[7]. In addition to p53 gene mutation, SV40 and other viral genomes encoded proteins can bind to, stabilize and inactivate P53 protein. It has been reported thatthe product of the mdm2 gene may directly interact with P53 and stabilize it[8]. However, Bass et al[9] used six kinds of antibodies for P53 (DO7, Pab1801, Pab240, Bp53-12, CM1, and Signet) on 19 archival colorectal neoplasms and compared the results with mutation status of the p53 gene and 17p allelic deletion status. They found that there was a very close correlation between overexpression of P53 and p53 genetic alterations, which produced the same results. They suggested that immunohistochemistry is valuable for assessing p53 gene mutation in colorectal neoplasms.
In our study, we used DO7 antibody for P53 protein and found that overexpression of P53 protein can be detected in colorectal cancers in 32 of 76 cases, and that most of them presented far intensive staining (++ or +++), but only 18.18% of adenomas and 13.21% of paracancerous mucosas presented occasionally weak staining. This agreed with the results of other studies[10].
We found that the positive rates of P53 in poorly differentiated and mucous carcinomas were much higher than those in well/moderately differentiated cancers, and that carcinomas with positive P53 protein were more likely to invade the serosa and metastasize to lymph nodes. Our results suggest that overexpression of P53 protein in colorectal cancers is associated with tumor histological differentiation, and may play an important role in tumor progression and biological behavior.
Original title:
S- Editor: Yang ZD L- Editor: Wang TQ E- Editor: Li RF
| 1. | Vogelstein B, Kinzler KW. p53 function and dysfunction. Cell. 1992;70:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1346] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 2. | Levine AJ. The p53 tumour suppressor gene and product. Cancer Surv. 1992;12:59-79. [PubMed] |
| 3. | Baker SJ, Preisinger AC, Jessup JM, Paraskeva C, Markowitz S, Willson JK, Hamilton S, Vogelstein B. p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990;50:7717-7722. [PubMed] |
| 4. | Kikuchi-Yanoshita R, Konishi M, Ito S, Seki M, Tanaka K, Maeda Y, Iino H, Fukayama M, Koike M, Mori T. Genetic changes of both p53 alleles associated with the conversion from colorectal adenoma to early carcinoma in familial adenomatous polyposis and non-familial adenomatous polyposis patients. Cancer Res. 1992;52:3965-3971. [PubMed] |
| 5. | Laurent-Puig P, Olschwang S, Delattre O, Remvikos Y, Asselain B, Melot T, Validire P, Muleris M, Girodet J, Salmon RJ. Survival and acquired genetic alterations in colorectal cancer. Gastroenterology. 1992;102:1136-1141. [PubMed] |
| 6. | Barnes DM, Dublin EA, Fisher CJ, Levison DA, Millis RR. Immunohistochemical detection of p53 protein in mammary carcinoma: an important new independent indicator of prognosis? Hum Pathol. 1993;24:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 165] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Hall PA, Lane DP. p53 in tumour pathology: can we trust immunohistochemistry--Revisited! J Pathol. 1994;172:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 425] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 8. | Wu X, Bayle JH, Olson D, Levine AJ. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993;7:1126-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1257] [Cited by in RCA: 1323] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 9. | Baas IO, Mulder JW, Offerhaus GJ, Vogelstein B, Hamilton SR. An evaluation of six antibodies for immunohistochemistry of mutant p53 gene product in archival colorectal neoplasms. J Pathol. 1994;172:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 389] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 10. | van den Berg FM, Tigges AJ, Schipper ME, den Hartog-Jager FC, Kroes WG, Walboomers JM. Expression of the nuclear oncogene p53 in colon tumours. J Pathol. 1989;157:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 133] [Article Influence: 3.7] [Reference Citation Analysis (0)] |