Published online Dec 15, 1996. doi: 10.3748/wjg.v2.i4.194
Revised: September 15, 1996
Accepted: November 10, 1996
Published online: December 15, 1996
AIM: To purify human fetal hepatic stimulating substance (hHSS) and to determine its activity.
METHODS: hHSS was purified by ion exchange chromatography, nondenaturing polyacrylamide gel electrophoresis (PAGE), and ISCO (In situ chemical oxidation) concentrator electrophoresis. The molecular weight was determined by sodium dodecyl sulfate (SDS)-PAGE and the activity of hHSS was examined by both 3H-thymidine incorporation assay and enzyme linked immunosorbent assay (ELISA).
RESULTS: The purity of hHSS was increased along with the purification process. After many steps, the major band of nondenaturing PAGE showed a high activity and purity, and two bands were revealed by SDS-PAGE.
CONCLUSION: The molecular weight of hHSS is about 70 kDa, and it consists of two subunits with molecular weights of 18 kDa and 53 kDa, respectively. The activity of hHSS was assayed by both 3H-TdR incorporation assay and ELISA, and good consistence was seen.
- Citation: Hou WJ, Liu SY, Xing W, Pan ZC, Liu JL, Wang DH. Purification and activity of human fetal hepatic stimulating substance. World J Gastroenterol 1996; 2(4): 194-196
- URL: https://www.wjgnet.com/1007-9327/full/v2/i4/194.htm
- DOI: https://dx.doi.org/10.3748/wjg.v2.i4.194
Hepatic stimulating substance (HSS) stimulates specifically DNA synthesis in liver cells both in vivo and in vitro[1]. It has organ specificity but no species specificity. Although HSS purification has been studied using rat livers, only crude human fetal hepatic stimulating substance (hHSS) was used for clinical treatment and research. The purification of human HSS remains to be further developed. In this study, hHSS was purified to near homogeneity by ion exchange chromatography, nondenaturing polyacrylamide gel electrophoresis (PAGE) and ISCO (In situ chemical oxidation) concentrator electrophoresis.
Kunming mice were purchased from the Department of Experimental Animal of China Medical University.
RC5C centrifuge was purchased from Dupont Company, United States; 2023 MinicoldLab from LKB Co., Sweden; and UV-260 spectrophotometer from Shimadzu Co., Japan. Liquid Scintillation system model 1801 was obtained from Beckman Co., United States; EL-309 Microplate Reader from BIO-TEK Co., United States; and 3H-thymidine (3H-TdR) (20 Ci/mmol) from the Institute of Atomic Energy of China. Antibody of hHSS was from the Department of Immunology, General Hospital of Chinese PLA (Nanjing, China). Cell culture reagents and Williams′ Medium E were purchased from Sigma Co., United States
A fresh fetal liver (from a mouse aged 5 mo) was excised and immediately homogenized in ice-cold 0.9% NaCl containing EDTA (Ethylenediaminetetraacetic acid) 10 mmol/L and PMSF 0.09 mmol/L with a polytron. Then it was centrifuged at 400 r/min for 20 min. The supernatant was heated at 95 °C for 15 min and centrifuged at 45000 g for 20 min, then 40% cold ethanol was added to the supernatant and stirred at 4 °C for 2 h[1]. After centrifugation, the precipitate was dissolved in 0.02 mol/L Tris-HCl buffer, and centrifuged at 12000 g for 20 min.
The supernatant was purified by chromatography on a DEAE-Sephadex A50 ion exchange column in Tris-HCl (0.02 mol/L, pH 7.8) buffer and eluted with a continuous gradient of 0-0.6 mol/L NaCl. The active peak of DEAE-Sephadex A50 was further purified by nondenaturing gradient PAGE. Following the electrophoresis, the major protein band was sliced and homogenized. Elution and concentration were done by the ISCO concentrator. The recovered protein was tested for hHSS activity using both enzyme linked immunosorbent assay (ELISA) and 3H-TdR incorporation assay. The molecular weight was determined by sodium dodecyl sulfate-PAGE (SDS-PAGE).
3H-TdR incorporation assay: This assay was performed according to the methods described by LaBrecque[5] and Schwarz[6]. An index of stimulation (IS) was then determined as: IS = Count per minute (cpm) for experimental hepatocytes/cpm for control hepatocytes.
hHSS- antibody diluted to 1:1000 with coating buffer was added to a 96-well microplate (0.2 mL/well) and kept at 4 °C overnight. After absorption, blocking solution was added to the wells at 0.4 mL/well and set at room temperature for 4 h. After the blocking solution was removed, 0.1 mL of sample was added to the coated wells and allowed to react at 37 °C for 1 h. After washing 5 times, 0.1 mL of peroxidase-conjugated anti-rabbit IgG secondary antibody was added to the testing wells and incubated at 37 °C for 1 h. After washing, the wells were subjected to chromogenic reaction.
A combination of the above steps produced an almost 200-fold purification of hHSS. The results of purification are shown in Table 1 (by 3H-TdR incorporation method) and Table 2 (by ELISA method).
| Sample | Sample volume (μL) | Concentration of protein (mg/mL) | cpm (mean ± SD) | IS | Specific activity (IS/mg protein) | Fold of purification |
| Control (0.9% NaCl) | 25 | - | 1275 ± 203 | - | - | - |
| Homogenization | 25 | 52.600 | 3162 ± 329 | 2.48 | 1.89 | 1.00 |
| ETOH-ppt hHSS | 25 | 8.280 | 3683 ± 401 | 3.03 | 14.64 | 7.74 |
| DEAE A50 peak V | 25 | 3.470 | 2920 ± 251 | 2.29 | 26.40 | 13.97 |
| Nondenaturing PAGE major band | 25 | 0.169 | 2039 ± 114 | 1.60 | 326.89 | 172.76 |
| Sample | Abs at 630 nm | Abs at 280 nm | Abs at 630 nm/Abs at 280 nm | Fold of purification |
| Control (0.9% NaCl) | 0.077 | 0 | - | - |
| Homogenization | 0.141 | 2.790 | 0.051 | 1.00 |
| ETOH-ppt hHSS | 0.159 | 0.964 | 0.165 | 3.24 |
| DEAE A50 peak V | 0.284 | 0.236 | 1.200 | 23.52 |
| Nondenaturing PAGE major band | 0.197 | 0.019 | 10.368 | 203.29 |
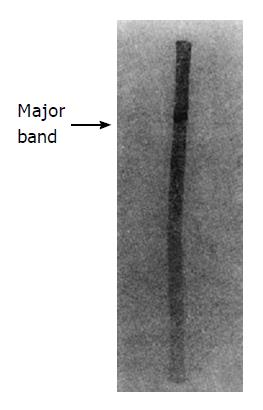
Peak V of DEAE-Sephadex A50 was loaded on a nondenaturing gradient polyacrylamide tube gel (Figure 1). After electrophoresis, three bands were shown on the tube gel. Assay of hHSS activity showed that the major band had a very high specific activity.
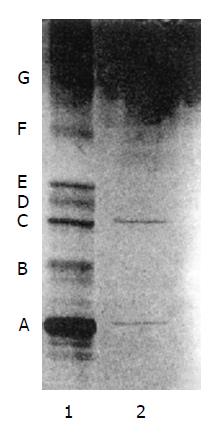
The major band of nondenaturing PAGE was subjected to SDS-PAGE, and the molecular weight of hHSS was shown as two bands with molecular weights of 18 kDa and 53 kDa, respectively (Figure 2).
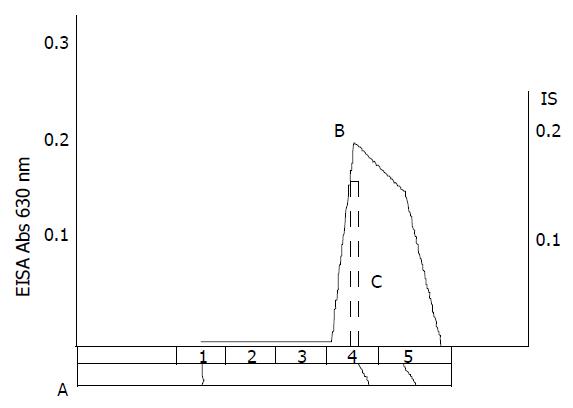
hHSS is able to initiate and/or stimulate hepatic cell growth and regeneration. Many techniques of purification and assay were studied in many laboratories[1-7], but few were concerned with the purification of hHSS. From the process of isolation, it was shown that large amounts of contaminated protein was removed after heating treatment and ISCO concentrator electrophoresis (Figure 3). SDS-PAGE showed that only two bands with molecule weights of 18 kDa and 53 kDa were found. So the molecular weight of hHSS may be 70 kDa, and it consists of two subunits with molecular weights of 18 kDa and 53 kDa, respectively.
3H-TdR incorporation assay is a standard method for the assay of HSS activity, but it is difficult to control when a large number of samples are assayed simultaneouly. In 1991, Tsubouchi used ELISA for measuring HSS in blood[7]. In the present study, we utilized ELISA for measuring the hHSS content in every step of the purification, and compared the measured values with those by 3H-TdR incorporation method. The values measured by both methods were basically parallel, and the ELISA method is easier to perform and is suitable for measuring a large number of samples simultaneously.
Original title: China National Journal of New Gastroenterology (1995-1997) renamed World Journal of Gastroenterology (1998-).
S- Editor: Tao T L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | LaBrecque DR, Steele G, Fogerty S, Wilson M, Barton J. Purification and physical-chemical characterization of hepatic stimulator substance. Hepatology. 1987;7:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Antonio F. The effect of hepatic stimulatory substance, isolated from regenerating hepatic cytosol, and 50000 and 300000 subfraction in enhancing survival in experimental acute hepatic failure in rats treated with D-galactosamine. Hepatology. 1986;6:1346-1352. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Díaz-Gil JJ, Escartín P, García-Cañero R, Trilla C, Veloso JJ, Sánchez G, Moreno-Caparrós A, Enrique de Salamanca C, Lozano R, Gavilanes JG. Purification of a liver DNA-synthesis promoter from plasma of partially hepatectomized rats. Biochem J. 1986;235:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Xie M. The study on human fetal hepatocyte regeneration factor. Zhonghua Neike Zazhi. 1990;29:602-606. |
| 5. | LaBrecque DR, Pesch LA. Preparation and partial characterization of hepatic regenerative stimulator substance (SS) from rat liver. J Physiol. 1975;248:273-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 162] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Schwarz LC, Makowka L, Falk JA, Falk R. The characterization and partial purification of hepatocyte proliferation factor. Ann Surg. 1985;202:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Tsubouchi H. Significance of hepatocyte proliferation factor in liver regeneration. Igako no ayumi. 1991;158:337-341. |