Published online Sep 15, 1996. doi: 10.3748/wjg.v2.i3.125
Revised: August 14, 1996
Accepted: August 29, 1996
Published online: September 15, 1996
AIM: To evaluate the effects of chronic alcohol abuse on the mucosal permeability to lipopolysaccharide in the colon in rats.
METHODS: Escherichia coli lipopolysaccharide (LPS, 20 μg/mL) was injected into the colon of chronic alcoholic rats (n = 10) and the rats were supplied with Lieber diets every other day for 6 weeks. Before LPS injection and 5, 10, 20, 30 min after injection, blood samples from the portal vein were obtained and contents of LPS in the blood were measured. The distribution of LPS in the colon tissues was observed with a confocal laser scanning microscope by immunofluorescent technique using a monoclonal antibody specific to the lipid A region of LPS. Normal rats were used as controls (n = 6).
RESULTS: Before LPS injection into the colon, LPS levels in the blood of portal vein of chronic alcoholic rats were significantly higher than those of normal controls (3.56 ± 0.67 pg/mLaa, vs 2.45 ± 0.15 pg/mLaa, P < 0.01). At 5, 10, 20, 30 min after injection of LPS, LPS contents were significantly higher than those before LPS injection (173.56 ± 23.45 pg/mLaa, 154.78 ± 20.57 pg/mLaa, 43.89 pg/mLaa ± 8.67 pg/mLaa, 45.38 ± 7.89 pg/mLaa vs 3.56 ± 0.67 pg/mLaa, P < 0.01 respectively). Most mucosal cells showed strong positive reactions to LPS in the rats of chronic alcohol abuse, but no significant changes of LPS contents in blood from the portal vein and fluorescentreactions to LPS in mucosal cells of normal rats were found after LPS injection.
CONCLUSION: Chronic alcohol abuse resulted in a significant increase of permeability to LPS in colon mucosal cells in rats.
- Citation: Chen XM, Xu RL, Ma XH, Zhou YC, Han DW. Mucosal permeability to lipopolysaccharides in the colon in chronic alcoholic rats. World J Gastroenterol 1996; 2(3): 125-127
- URL: https://www.wjgnet.com/1007-9327/full/v2/i3/125.htm
- DOI: https://dx.doi.org/10.3748/wjg.v2.i3.125
Endotoxins are potent biological moieties from the outer membrane of Gram-negative bacteria and mainly composed of lipopolysaccharides (LPS) and proteins[1]. LPS causes all the toxic actions endotoxin inflicts[2]. Endotoxemia is a common complication in patients with alcoholic hepatitis, and the liver injury was associated with the development of endotoxemia. Normally, a lot of endotoxins present in the gut and nonpathogenic amounts of this toxic macromolecule material are absorbed through the intestinal wall, reaching the liver[3]. The sinusoidal cell is the key to the uptake and detoxication of endotoxins in the liver[4] Under pathological conditions, such as alcoholic hepatitis, endotoxins in the portal vein may enter the peripheral blood (known as endogenous endotoxemia) as a result of depression of Kupffer cells, or portal systemic circulation[5,6]. However, there is little information concerning the permeability mechanism of LPS in the gut under those pathological conditions. In the present study, by using a monoclonal antibody specific to the lipid A region of endotoxin, the distribution and localization of endotoxins in the colon in rats of chronic alcohol abuse was observed with confocal laser scanning microscopy. LPS concentrations in the portal vein were measured.
Thirty male Wistar rats were divided into two groups: chronic alcoholic group and control group. Rats were fed on a complete liquid diet with the Lieber protocol[7] in chronic alcoholic group. Diets were supplied every other day with homogenation and administered through Richter feeding tubes for 6 wk. Rats in control group (n = 6) were fed ad libitum with ordinary rat chow. Under the anaesthesia by intraperitoneal injection of sodium pentobarbital (50 mg/kg body weight), abdomens of rats of both groups were opened. The colons about 1.5 cm-2.0 cm long were ligated at two ends without interruption to their blood circulation. After being washed with sterile saline, the colon was injected Escherichia coli LPS. LPS was diluted with sterile saline to 20 μg/mL and the amount of the injected LPS corresponded to the pressure in the colon which was maintained to 10 cm/H2O. About 0.5 mL-1.0 mL of LPS was injected in to each rat. Before LPS injection and 5, 10, 20, 30 min after the injection of LPS into the colon, blood samples about 0.5 mL were obtained from the portal vein for the determination of LPS in the blood. At 30 min after LPS injection, the colon was taken and washed. Tissue specimens were fixed in buffered formalin, embedded in paraffin and 4 μm sections were cut. The sections were then performed for immunofluorescent staining.
As for location of LPS in tissues and cells, the indirect immunofluorescence staining technique was used. Briefly, sections (4 μm thick) were dewaxed with xylene and washed successively with 100%, 96%, 70% aqueous ethanol. After being washed with phosphate buffered saline (PBS), each section was treated with 1% normal goat serum (Vectastain, Vector Laboratories, Inc. Burlingame) for 30 min to cover possible nonspecific sites. Then, all the sections were washed and incubated with the monoclonal antibody against LPS (a gift from Dr. Noguchi) for 60 min at 37 °C. After washing with PBS for three times, fluorescein isocthiocyanate (FITC) conjugated goat anti mouse IgM (H&L) F(ab’)2 fragments (1:30, O.E.M. Concepts Inc.) was added for 60 min at 37 °C. Excess conjugate was removed by washing with PBS. Then the sections were mounted with VECTASHIEID mounting medium (Vector Laboratories, North Chicago) for fluorescence under coverslip and ready for observation with Confocal Laser Scanning Microscopy. For the sake of negative controls, sections were incubated with PBS omitting the first antibody or FITC conjugated secondary antibody.
Sections were observed within 2 h after immunofluorescence staining. With a confocal laser scanning microscope (LSM-GB200, Olympus, Japan), cellular localization and distribution of LPS in the mucosal cells of the colon were observed.
All data were expressed as x-± s, and were analyzed with the Stat View Statistical Program. Student’s t and Anoval F test were employed where appropriated.
In rat of chronic alcohol abuse for 6 weeks, LPS concentration in portal vein blood was significantly higher than that of control even before LPS injection into the colon. At 5 min after the injection of LPS, LPS level in the portal vein blood increased markedly and reached the peak point. At 10 min after LPS injection, LPS level in the blood decreased a little but even at 30 min after LPS injection, the LPS level was still significantly higher than that of the normal control. For the rats of normal control, LPS levels in the portal vein blood increased a little after LPS injection, but no significant difference was found in comparison with that before LPS injection (Table 1).
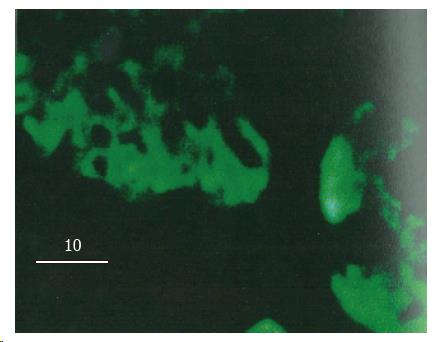
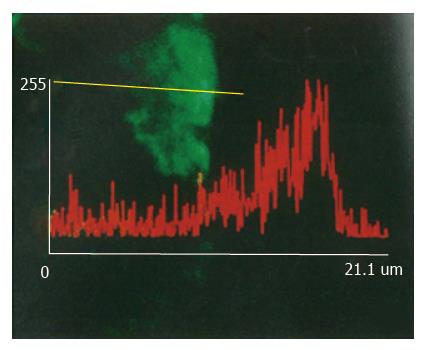
In normal control rats after LPS injection, colon mucosal cells showed no obvious FITC fluorescence reaction to LPS except membrane cells on the cavity side of the mucosa which showed slight fluorescence. In the rats of chronic alcoholic abuse, nearly all the mucosal cells showed strong FITC fluorescent reactions to LPS 30 min after LPS injection. The fluorescent reactions were found in the cytoplasm of mucosal cells, tissues under the basement membrane, but not found in the nuclei of the mucosal cells (Figure 1). Fluorescent reactions did not significantly increase between the membrane of the mucosal cells (Figure 2). The positive fluorescent reactions to LPS were evenly scattered, not in spot or particulate form. No fluorescence was detected in negative control tissues using only the monoclonal antibody or FITC labelled secondary antibody.
Confocal laser scanning microscopy can scan tissues or cells at thickness as thin as 1 μm, and can actually reflect the FITC fluorescent distribution in tissues and cells. For the location and distribution of LPS in tissues and cells, the monoclonal antibody specific to the lipid A region of LPS is more specific than antibody against the polysaccharide chain of LPS by the immunohistochemical technique, because the antigen is characterized by the fact that the polysaccharide chain of LPS might be changed or separated from lipid A part after LPS enter cells[8]. Most of the toxic effects of LPS, if not all, reside in the core lipid A part[9]. No fluorescence was detected in negative controls using only the monoclonal antibody or FITC labelled secondary antibody, and it was reasonable to estimate that the fluorescence reaction detected was specific for the location and distribution of LPS in tissues and cells.
Endotoxins are mainly composed of lipopolysaccharides and proteins[1]. There is good evidence that portal vein endotoxemia is in a normal state[3]. It is generally known that the liver, mainly by Kupffer cells and hepatocytes, is responsible for the clearance of endotoxins in the portal vein blood[4]. In patients with alcoholic hepatitis, endotoxemia is a common complication[5,6] and the endotoxins are affirmed primarily from the gut by the portal vei[10] and the lymphatic vessels[11] in intestine. The liver injury due to alcohol and its clinical manifestation were confirmed to be associated with the development of endotoxemia[12], suggesting that endotoxin might play a role in hepatic injury induced by alcohol. In the rats of chronic alcohol abuse with Lieber diets for 6 wk, we found that endotoxin concentration in the portal vein blood was markedly higher than that of the control, and the level increased progressively at various time point after LPS injection, suggesting that the permeability to LPS in the intestine increased after the stimulation by alcohol abuse, a phenomenon which might be critical to the development of endotoxemia in alcoholic hepatitis.
Two different mechanisms, namely specific and non-specific, are suggested to be involved in the initial interaction of LPS with cells[13]. Specific interactions result from the binding of LPS to a specific receptor on the plasma membrane, and, on the other hand, non-specific interactions result from the binding of LPS macromolecule to any membrane constituents other than the receptors. Both mechanisms are involved in the uptake procedure of LPS by cells[14,15]. A number of receptors specific to different regions of LPS were recently reported. By using a LPS derivative as a probe to define LPS specific binding structures, Lei MG et al[16] identified a 73 kDa membrane-localized protein which existed in almost all the mammalian cell subpopulations and was lipid A specific. LPS was found in the cytoplasm of macrophages in spot and particulate form after phagocytized by those cells[17]. In the present study, the positive reactions to LPS were found in nearly all the mucosal cells in chronic alcoholic rats, indicating that there was no cellular specificity to the permeability of LPS in the colon. There were no positive reactions to LPS among those mucosal cells membranes also suggested that the permeability to LPS in the colon may be through the cells, not through the injection between cells. The positive fluorescent reactions to LPS in the cytoplasm of mucosal cells were evenly distributed, not in spot or particulate form. This phenomenon suggested that the uptake mechanism of LPS by those mucosal cells might not be through phagocytic procedure.
Presented at the Symposium on Alcoholic Liver Diseases and Cirrhosis, Nanjing, 26-28 July 1995.
Original title:
S- Editor: Yang ZD L- Editor: Filipodia E- Editor: Li RF
| 2. | Morrison DC, Ulevitch RJ. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978;93:526-618. [PubMed] |
| 3. | Jacob AI, Goldberg PK, Bloom N, Degenshein GA, Kozinn PJ. Endotoxin and bacteria in portal blood. Gastroenterology. 1977;72:1268-1270. [PubMed] |
| 4. | Freudenberg N, Piotraschke J, Galanos C, Sorg C, Askaryar FA, Klosa B, Usener HU, Freudenberg MA. The role of macrophages in the uptake of endotoxin by the mouse liver. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;61:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Bradfield JW. Control of spillover. The importance of Kupffer-cell function in clinical medicine. Lancet. 1974;2:883-886. [PubMed] |
| 6. | Mathison JC, Ulevitch RJ. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol. 1979;123:2133-2143. [PubMed] |
| 7. | Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res. 1982;6:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 567] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 8. | Freudenberg M, Galanos C. Metabolic fate of endotoxin in rat. Adv Exp Med Biol. 1990;256:499-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Moldow CF, Bach RR, Staskus K, Rick PD. Induction of endothelial tissue factor by endotoxin and its precursors. Thromb Haemost. 1993;70:702-706. [PubMed] |
| 10. | Nolan JP, Hare DK, McDevitt JJ, Ali MV. In vitro studies of intestinal endotoxin absorption. I. Kinetics of absorption in the isolated everted gut sac. Gastroenterology. 1977;72:434-439. [PubMed] |
| 11. | Daniele R, Singh H, Appert HE, Pairent FW, Howard JM. Lymphatic absorption of intraperitoneal endotoxin in the dog. Surgery. 1970;67:484-487. [PubMed] |
| 12. | Bhagwandeen BS, Apte M, Manwarring L, Dickeson J. Endotoxin induced hepatic necrosis in rats on an alcohol diet. J Pathol. 1987;152:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 123] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Morrison DC. Nonspecific interactions of bacterial lipopolysaccharides with membranes and membrane components. Cellular biology of endotoxin. New York: Elsevier 1985; 25-30. |
| 14. | Kriegsmann J, Gay S, Bräuer R. Endocytosis of lipopolysaccharide in mouse macrophages. Cell Mol Biol (Noisy-le-grand). 1993;39:791-800. [PubMed] |
| 15. | Fox ES, Thomas P, Broitman SA. Comparative studies of endotoxin uptake by isolated rat Kupffer and peritoneal cells. Infect Immun. 1987;55:2962-2966. [PubMed] |
| 16. | Lei MG, Stimpson SA, Morrison DC. Specific endotoxic lipopolysaccharide-binding receptors on murine splenocytes. III. Binding specificity and characterization. J Immunol. 1991;147:1925-1932. [PubMed] |
| 17. | Kang YH, Dwivedi RS, Lee CH. Ultrastructural and immunocytochemical study of the uptake and distribution of bacterial lipopolysaccharide in human monocytes. J Leukoc Biol. 1990;48:316-332. [PubMed] |