INTRODUCTION
Ulcerative colitis (UC) is one of the main inflammatory bowel diseases (IBD) which affect primarily the colonic segment of the gut. Inflammation is continuous and limited to the mucosa, accompanied by ulceration, pseudopolyps, oedema, crypt abscesses and spontaneous haemorrhage. Patients present urgent diarrhoea with blood and mucus, abdominal pain, fever, continual tiredness, anaemia, weight loss and potentially serious complications, such as megacolon toxicum. Symptoms often require steroid therapy and are characterised by alternating acute and remission periods. Although its etiology is unknown, its development and acute exacerbation have been related to both physical and mental stress[1-9].
It is well established that corticotropin-releasing factor (CRF) and its homologue peptides urocortin 1, 2 and 3, are the key mediators of the endocrine, behavioural, autonomic and visceral responses to stress[10-12]. These neuropeptides exert their multiple actions through activation of two distinct receptor types, CRF1 and CRF2, both belonging to the class B G-protein coupled receptor superfamily[13,14]. CRF1 is expressed primarily in the brain and the pituitary[15-17] whereas expression of CRF2 has been reported in the central nervous system but also in various peripheral tissues such as heart, skeletal muscle, gut and testis[18-22]. CRF and urocortin 1 display equal affinity for the CRF1 receptor while urocortin 1 is 40 times more potent than CRF in binding CRF2[23]. In contrast, urocortins 2 and 3 bind selectively to CRF2 and have been established as the endogenous ligands for this receptor subtype[13].
The gastrointestinal tract is one of the primary peripheral systems affected by stress and there is accumulating evidence that the CRF signalling pathways are responsible for mediating these effects via both central and peripheral routes[24-26]. Previous reports indicate that CRF, urocortin 1 and urocortin 2 are elevated in the colonic mucosal of patients with active UC[27,28] and are involved in the pathogenesis of this disease, participating in the regulation of motility and/or inflammatory process via autocrine/paracrine actions[29-31]. Local expression of CRF receptors is a prerequisite for mediation of these effects and understanding their histological distribution would provide anatomical support to the physiological and pathophysiological condition/phenomena. Furthermore, they could provide targets for new strategies of pharmacotherapy[32-34].
We have recently reported that CRF1 is up-regulated in the colonic mucosa of UC patients, particularly in macrophages supporting its involvement in the immune/inflammatory process[35]. However, recent studies in a rat model of chemically induced colitis showed that CRF2 rather than CRF1 is pivotal for macroscopic spread of colitis and resolution of edema[36]. Here, we investigated the expression pattern of CRF2 in the distal/sigmoid colonic mucosal biopsies of healthy human subjects and compared it to inflamed colonic tissues from patients with UC or with human immunodeficiency virus (HIV) infection and functional bowel diseases (FBD), without signs of inflammation.
MATERIALS AND METHODS
Tissues
All sigmoid colonic mucosal biopsy specimens were obtained from the Mucosal Immunology Core (UCLA AIDS Institute Center for AIDS Research). Approval to conduct the study was obtained from the UCLA Human Subjects Protection Committee. All participants provided written consent at the time they presented for scheduled screening endoscopy. Sigmoid colonic biopsies were collected by flexible sigmoidoscopy, 10-20 cm from the anal verge, from 6 healthy controls (33-65 years; 1 male, 5 females) and 10 UC patients (32-83 years; 6 males and 4 females). Patients had UC for > 10 years, based on history, endoscopic and pathology reports over the years. Four patients had active clinical disease presentation and met the criteria for mild-moderately active disease (grade as 2-3) and 6 UC patients met the criteria for minimal to no active inflammation with markers of quiescent disease (grade as 1-2). Grades were based on Matts UC classifications as used in other studies[35]. No participants were taking steroids and one was taking a low dose of the immunosuppressive agent, 6-mercaptopurine (25 mg/d). All were treated with 5-aminosalicylate except one patient who was taking only omega-3 fatty acid gel caps. The mucosal biopsies of patients with HIV (n = 4) or with IBD (n = 7) revealed no inflammation at pathological examination. All biopsies were fixed in formalin, embedded in paraffin and stored at room temperature until further use.
Cell line
The human colorectal adenocarcinoma cell line Caco2 was obtained by the American Type Culture Collection (ATCC HTB-37, Manassas, VA, United States). Cells were maintained in ATCC-formulated Eagle’s Minimum Essential Medium (Invitrogen, Grand Island, NY, United States), supplemented with 1% antibiotic-antimycotic solution and 20% fetal bovine serum (Invitrogen) in tissue culture flasks (Nunclon, Rochester, NY) at 37 °C in 5% CO2. Cell culture medium was replaced every 2-3 d and cells were passaged when subconfluent.
Reverse transcription polymerase chain reaction
Universal reference total RNAs from normal human colon and from whole human brain were purchased from Clontech (Mountain View, CA, United States). RNA from Caco2 cells was extracted using Trizol Reagent, according to the manufacturer’s instructions. Reverse transcription (RT) was performed using the SuperScript Preamplification System (Invitrogen, Paisley, United Kingdom) and random hexamers in a total volume of 20 μL. Two microliter of the RT product was used as a template, amplified by polymerase chain reaction (PCR) using 2 mol/L MgCl2, PCR buffer, 0.2 mol/L of sense and antisense primers, 0.2 mol/L dNTPs and 2.5 U Taq polymerase (Invitrogen) in a final reaction volume of 50 μL. PCR was performed in a Perkin-Elmer DNA Thermal Cycler with the following cycling parameters: a pre-amplification cycle (denaturation for 5 min at 96 °C), 35 cycles of amplification (denaturation for 30 s at 96 °C, annealing for 40 s at 56 °C and extension for 50 s at 72 °C) and a final extension step for 7 min at 72 °C. The primers were designed to amplify specifically the human CRF2 (sense AAGCTTGCCATGGACGCGGCACTGCTC antisense AAGGGCGATGCGGTAGTGC, in the area of the gene encoding the transmembrane part of the receptor and thus targeting all splice variants) according to the GenBank published sequences. The size of the amplified products was expected to be 308 bp. Negative control samples where no RT enzyme was added in a total brain RNA sample (no RT) or without DNA template (no DNA) were included in every assay in order to exclude the possibility of genomic or other DNA contamination. RT-PCR for β-actin, with expected PCR product size of 175 bp, was also performed for every sample in order to assure RNA quality. The amplified PCR products were fractionated by 1.5% agarose gel electrophoresis and detected by ethidium bromide staining under UV.
Indirect immunofluorescence
Immunofluorescence was conducted as previously described[37]. Briefly, four-micron distal/sigmoid colonic tissue sections (4 μm) were cut, deparaffinized, and rehydrated by standard procedures. Sections were then incubated with Antigen Retrieval Solution (Dako, Glostrup, Denmark) for 15 min, pre-blocked in 1% normal goat serum (Vector Laboratories, Burlingame, CA, United States) in phosphate buffered saline (PBS). They were then incubated with the primary antisera 4392a-CRF1and2 (1:2000) (raised against a synthetic peptide corresponding to amino acids 381-415 of the human/rat CRF1/2 C-terminus) and 2064a-CRF2 (1:500) (raised against a synthetic peptide corresponding to amino acids 404 to 438 of the human/rat CRF2 C-terminus)[38,39], diluted in 1% normal goat serum in PBS for 45 min at room temperature in a humid chamber, in parallel with negative controls using normal rabbit IgG instead of the primary antiserum. Following washing in PBS, secondary antibody conjugated to a fluorescent dye, goat anti-rabbit AlexaFluor594 (Molecular Probes, Eugene, OR, United States; 1:500 or 4 μg/mL) was added for 30 min at room temperature. Slides were subsequently mounted in antifade mounting media (Molecular Probes) and visualized by standard fluorescence microscopy. Replacement of the antisera by non-specific rabbit IgG (negative) was used as negative control. The number of immunoreactive cells were counted and quantified in an average number of 5 fields (340 μm × 260 μm/field) from each specimen in a blinded fashion such that the information on the clinical, endoscopic and pathological findings were unknown until all counting was completed.
Statistical analysis
Statistical significance was assessed by Fisher’s exact method for small samples using the SPSS 17.0 statistical software (SPSS Inc. Chicago, Illinois, United States). Significance was set at a P value < 0.05.
RESULTS
CRF2 receptor gene expression in the distal/sigmoid colon of healthy human subjects and cell lines
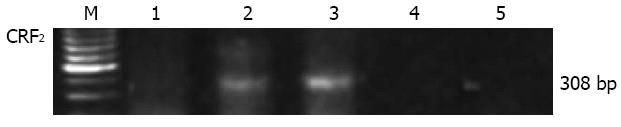
The expression of CRF2 receptor gene was investigated by RT-PCR in total RNA preparations from normal human distal/sigmoid colon tissue or whole cellular extract isolated from the human colon cancer cell line Caco2. A unique RT-PCR product was amplified in RNA preparations from human distal/sigmoid colon tissue but not from the Caco2 cells (Figure 1). The size of the product was as the expected size for CRF2 transcripts and was identical to that amplified from human brain mRNA extracts. No PCR product was amplified in the human mRNA sample when reverse transcriptase enzyme was not added in the RT reaction, or when PCR was performed in the absence of DNA template, excluding the possibility of genomic or other DNA contamination.
Figure 1 Reverse transcription polymerase chain reaction for corticotropin-releasing factor 2 receptor gene expression in total RNA isolated from the human colon cancer cell line Caco2 (1), human colon (2), and human brain (3) from healthy subjects.
The predicted size products (308 bp) were amplified in the human colon and brain samples. Negative controls in the absence of reverse transcription enzyme (4) or DNA template (5) are also presented. CRF: Corticotropin-releasing factor; M: Marker.
CRF2 receptor protein expression in the distal/sigmoid colon biopsies of healthy human subjects
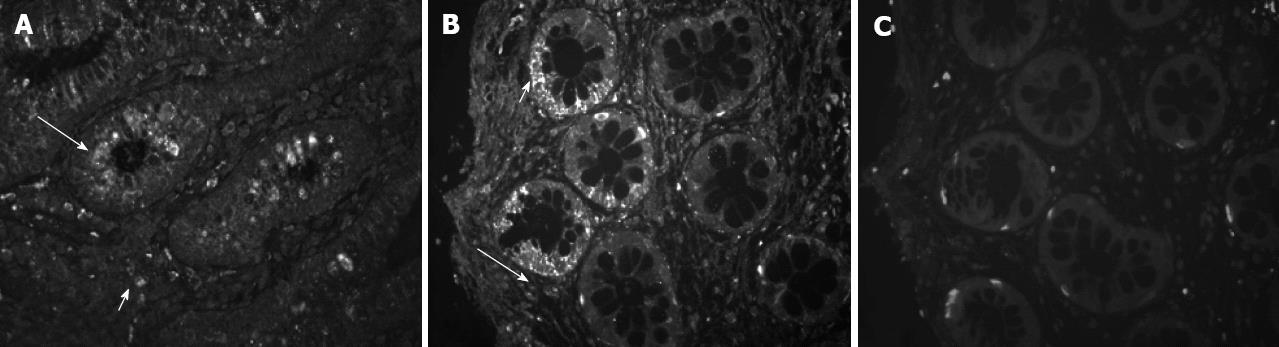
Serial tissue sections from 10 human colonic biopsies from healthy subjects were stained by immunofluorescence for CRF2 receptor protein, using two specific polyclonal antisera (Figure 2), one of them selective for the CRF2 receptor type. Both antibodies revealed membranic staining and similar patterns. CRF2 positive cells were localized in the lamina propria of the colonic mucosal and in the epithelial cells of the intestinal crypts. Replacement of the antisera by non-specific rabbit IgG abolished all specific staining (although there is still some background due to non-specific absorption of secondary antibody). There were no significant differences on the pattern of distribution of receptor expression in the serial sections of the colon biopsy samples examined.
Figure 2 Mapping of corticotropin-releasing factor 2 receptor protein expression in distal/sigmoid colon biopsies of healthy human subjects.
Tissue sections from normal mucosal biopsies were stained by immunofluorescence using specific antisera against corticotropin-releasing factor 2 (CRF2) (A, 2064a-CRF2) and both CRF1 and CRF2 (B, 4392a-CRF1 and 2). Immunoreactivity was localized in isolated structures of the lamina propria (long arrows) and in the epithelial cells of the intestinal crypts (short arrows). Replacement of the antiserum by non-specific rabbit IgG (C, negative) abolished all specific staining. Original magnification 100×.
Intestinal CRF2 receptor protein expression in distal/sigmoid colonic biopsies of patients with ulcerative colitis, HIV and FBD
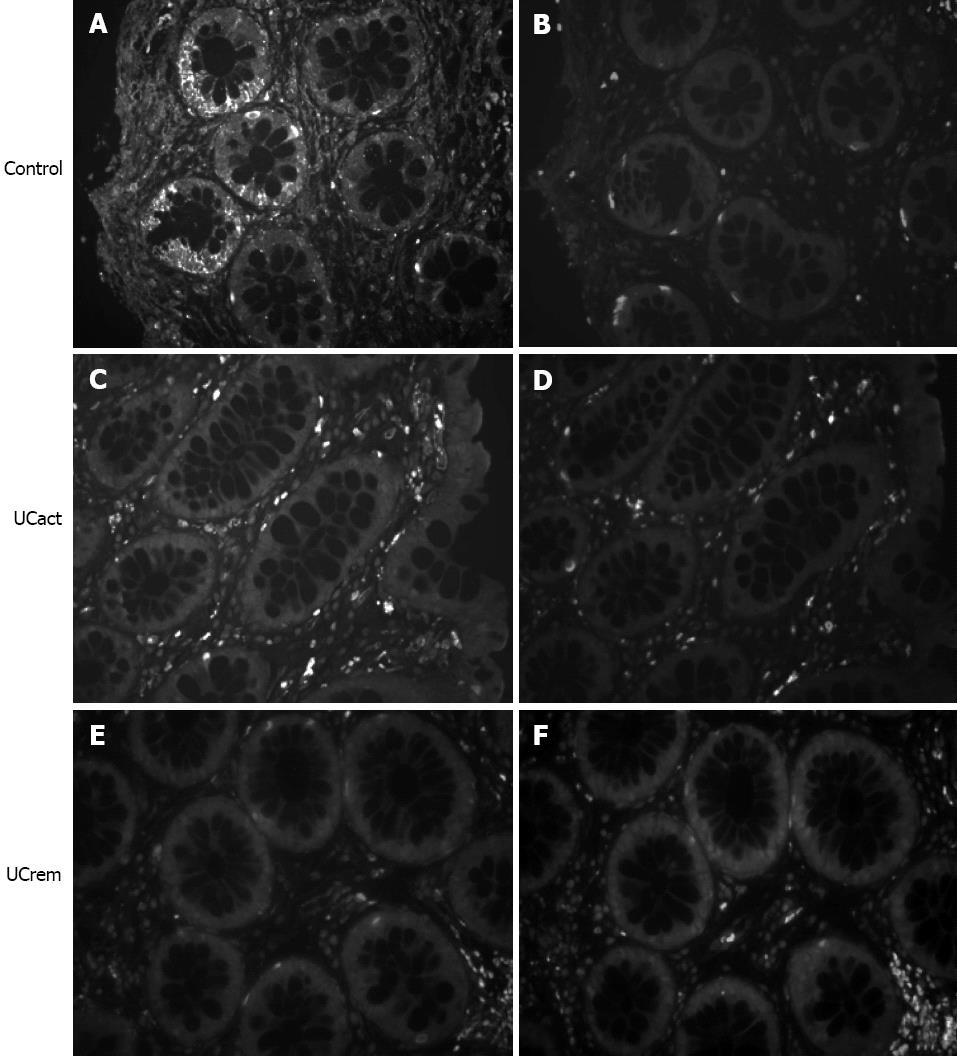
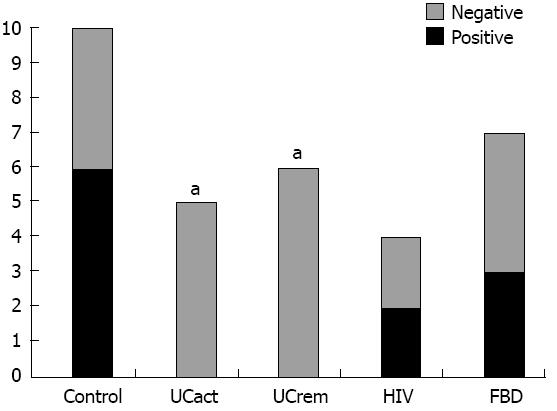
CRF2 receptor protein expression was compared in tissue sections from five groups: A: healthy subjects (control, n = 10), B: UCact (n = 4), C: UCrem (n = 6), D: HIV (n = 4), and E: FBD (n = 4). The results are presented in Figures 3 and 4. CRF2 expression in the colonic epithelial cells was down regulated in both UC patient groups, those with moderately active UC and those with disease in remission. In particular, CRF2 immunoreactivity was detected in the epithelial cells of the crypts in 6/10 (60%) of healthy subjects, 2/4 (50%) in the HIV group and 3/4 (75%) in the FBD group, but in none of the UCact (0/5, 0%) and UCrem group (0/6, 0%) and these differences where statistically significant (P < 0.05). Positive CRF2 staining in the lamina propria was seen in all the tissues examined.
Figure 3 Comparison of corticotropin-releasing factor 2 receptor immunoreactivity in distal/sigmoid biopsies from healthy human subjects and patients with moderate ulcerative colitis and patients in remission.
Tissue sections from mucosal biopsies were stained by immunofluorescence using specific antisera 2064a- corticotropin-releasing factor 2 (CRF2) (A, C, E). Immunoreactivity for CRF2 observed in the epithelial cells of the intestinal crypts in the control group, was not found in the UCact and UCrem groups. Replacement of the antiserum by non-specific rabbit IgG (B, D, F) abolished all specific staining. Original magnification 100×.
Figure 4 Corticotropin-releasing factor 2 receptor protein expression in epithelial cells in distal/sigmoid biopsies from five groups: Healthy subjects, patients with moderately active ulcerative colitis, patients with ulcerative colitis in remission, patients with human immunodeficiency virus and functional bowel disease.
UCact: Active ulcerative colitis; UCrem: Ulcerative colitis in remission; HIV: Human immunodeficiency virus; FBD: Functional bowel disease. aP < 0.05 vs control group.
DISCUSSION
In the present study, we describe the expression pattern of CRF2 in the distal/sigmoid colonic mucosa of patients with colonic inflammation (UC) or disease without inflammation (HIV infection and FBD) and compare it to healthy control subjects. Receptor protein was prominent in both the lamina propria and in epithelial cells of the intestinal crypts in tissues from healthy subjects. Interestingly, in patients with UC either moderately active or with disease in remission, expression was limited only to the lamina propria of the mucosa, whereas in the intestinal crypts it was found to be down regulated. No such difference was observed in tissues from the HIV and FBD patients.
Gene expression was confirmed by RT-PCR in total RNA preparations from full thickness intestinal tissues in healthy human subjects. Previous studies also showed CRF2 gene expression in isolated lamina propria mononuclear cells and lower levels in the epithelial cells fraction isolated macroscopically from normal colorectal biopsies of patients undergoing surgery for non-obstructive colorectal cancer[28,40]. More recently Wallon et al[41] also found CRF2 in subepithelial cells of human sigmoid biopsies, proven to be mast cells by colocalization with tryptase. In experimental studies, CRF2 receptors have also been localized in the colonic enteric plexus[38,42,43], the endothelium and vascular smooth muscle, and closely resemble human colonic expression patterns of urocortin 1 and urocrotin 3[44]. These results complement our present findings on the receptor protein histological localization, showing the respective/predicted expression pattern. By contrast, we found that the human colorectal adenocarcinoma cell line Caco2 did not express any receptor gene. These data seem specific to this cell line since previous studies showed that adenocaricnoma HT-29 cells expressed mRNA for CRF2α[45] and the non-transformed human, NCM460 colonocyte[46] also express CRF2α although at a low level[45].
Saruta et al[28] showed CRF2 mRNA expression in the lamina propria macrophages of UC patients without glucocorticoid treatment, along with an upregulation of urocortin 1 within the same cells in proportion to the severity of inflammation. We could also confirm these findings, observing an increase in urocortin 1 immunoreactivity in the colonic mucosa of UC patients (moderately active or in remission) although not a thorough quantification was performed (results not shown). Another report indicates that there is an increased gene expression of both urocortin 2 and CRF2 in biopsies from UC patients with moderately active disease undergoing colonoscopy[30]. The differential up-regulation of CRF2 mRNA levels previously reported in UC and the decreased CRF2 immunoreactivity in colonic biopsies of UC patients (present study) is not clear at the present time and may reflect alterations in CRF2 transcription under UC conditions. However experimental studies in a rat model of chemically-induced colitis showed increased urocortin 2 accompanied by down-regulation of CRF2 expression. CRF was also enhanced in the colonic mucosa of UC patients, in both inflammatory (namely macrophages and eosinophils) and epithelial cells[27,47]. It is possible that the increase of peptide ligands related to the local inflammatory process, accounts for the down regulation of CRF2 receptor protein expression reported here (being either a cause or a resultant effect). Regulation of G-protein receptor expression by its ligands is a frequent homeostatic mechanism observed in many endocrine/paracrine pathways[48].
The down regulation of CRF2 protein expression in the sigmoid observed in the UC patients seems to be specific to UC. This notion is enforced by our findings from a small number of two more patient groups, with HIV infection or FBD, where CRF2 protein expression in colonic mucosa was not down-regulated. Thus, we could conclude that CRF2 down-regulation is specific to acute or chronic inflammation of UC patients with moderately active disease and persist in colitis in remission (also inflamed) in patients receiving conventional, non-steroid therapy.
Data from experimental animal models point to a prominent role for CRF2 in colitis-related inflammation. In a 2,4,6-trinitrobenzenesulfonic acid-induced colitis model in rats, CRF2 expression, present on myenteric neurons and macrophages, decreased in the early phase (day 1-3) of inflammation[49]. However a study by Moss et al[30] performed on a graft of human fetal small intestinal and colonic tissues in immunodeficient mice and in which inflammation is induced by C. difficile toxin A 12 wk later, demonstrated increased mucosal urocortin 2 mRNA expression and up-regulation of mucosal CRF2 expression (after 6 h), as was also the case in the murine toxin A model[50]. Taken together, while the experimental and clinical studies provide convergent data showing an up regulation of CRF ligands including CRF, urocortin 1 and urocortin 2 under conditions of colonic inflammation or UC, this can be associated with either up or down regulation of CRF2 receptor.
A number of studies involve the expression of the CRF system (ligands and receptors) in the regulation of local inflammation in many different tissues. In particular, local expression of urocortin 1 has been reported to act as a proinflammatory factor in rheumatoid arthritis[29,51] or having anti-inflammatory actions in Helicobacter pylori-related gastritis[52]. It seems likely that the two receptor types CRF1 and CRF2, being distributed in different cellular types, could mediate distinct, even opposite effects in the process of local inflammatory phenomena. Opposite signalling of the two receptor types and CRF neuropeptides has been previously reported[53-55]. This is further supported by our data showing increased CRF1 positive macrophages in the colonic mucosa of UC patients.
In conclusion, we report the expression of CRF2 in the mucosal epithelium of normal human colon at gene and protein levels, and its histological mapping in the colonic mucosal and lamina propria cells. Moreover, we show down regulation of the CRF2 protein receptor in tissues from UC patients either with moderately active disease or in remission that were not treated with glucocorticoids. These findings along with our recent studies showing the upregulation of CRF1 receptor protein expression in macrophages of the lamina propria from sigmoid biopsies in UC patients suggest the involvement of CRF receptors in the modulation of colonic mucosa inflammation which needs further investigation. These results along with existing evidence[32] point to the potential therapeutic use of drugs targeting CRF signaling systems to interfere with UC pathophysiology.
COMMENTS
Background
An established etiological factor for the development of ulcerative colitis (UC) is stress acting via initial nervous disturbance and subsequent immune dysfunction through brain-gut interactions. Activation of corticotropin-releasing factor (CRF) receptors is the principal mediator of the neuroendocrine stress responses.
Research frontiers
Previous reports indicate that CRF neuropeptides are elevated in the colonic mucosal of patients with active UC and are involved in the pathogenesis of this disease. Local expression of CRF receptors is a prerequisite for mediation of these effects and understanding their histological distribution would provide anatomical support to the physiological and pathophysiological condition/phenomena. Furthermore, they could provide targets for new strategies of pharmacotherapy.
Innovations and breakthroughs
The authors have recently reported that CRF1 is up-regulated in the colonic mucosa of UC patients, particularly in macrophages supporting its involvement in the immune-inflammatory process. However, recent studies in a rat model of chemically induced colitis showed that CRF2 rather than CRF1 is pivotal for macroscopic spread of colitis and resolution of edema. In order to elucidate the molecular mechanism underlying the stress-related activation of UC symptomatology, here, we investigated the expression pattern of CRF2 in the distal/sigmoid colonic mucosal biopsies of healthy human subjects and compared it to inflamed colonic tissues from patients with UC or with human immunodeficiency virus infection and functional bowel diseases, without signs of inflammation.
Applications
The results suggest an involvement of CRF2 receptor in the process of inflammation in the colon and that alterations in CRF receptor expression might participate in the pathophysiology of UC, by arresting direct stress effects on the colonic tissue. These findings could be exploited for the development of effective drugs against colitis.
Terminology
UC is one of the main inflammatory bowel diseases which affect primarily the colonic segment of the gut. Although its aetiology is unknown, its development and acute exacerbation have been related to both physical and mental stress. The hypothalamic neuropeptide CRF and its homologues urocortin 1, 2 and 3, are the key mediators of the endocrine, behavioural, autonomic and visceral responses to stress, acting via 2 G-protein coupled receptors, CRF1 and CRF2.
Peer review
The work is well written. Authors should better specify the expression of CRF2 in response to therapy and how it changes in activity or remission state of disease.