Published online Dec 28, 2013. doi: 10.3748/wjg.v19.i48.9447
Revised: November 20, 2013
Accepted: December 5, 2013
Published online: December 28, 2013
Processing time: 92 Days and 22.9 Hours
AIM: To evaluate the efficacy and toxicity of nedaplatin (NDP) concurrent with radiotherapy in the treatment of locally advanced esophageal carcinoma.
METHODS: Sixty-eight patients with locally advanced esophageal carcinoma were randomized into either a NDP group (n = 34) or a cisplatin (DDP) group (n = 34). The NDP group received NDP 80-100 mg/m2iv on day 1 + leucovorin (CF) 100 mg/m2iv on days 1-5 + 5-fluorouracil (5-FU) 500 mg/m2iv on days 1-5. The DDP group received DDP 30 mg/m2iv on days 1-3 + CF 100 mg/m2 on days 1-5 + 5-FU 500 mg/m2iv on days 1-5. The treatment was repeated every 4 wk in both groups. Concurrent radiotherapy [60-66 Gy/(30-33 f)/(6-7 wk)] was given during chemotherapy.
RESULTS: There was no significant difference in the short-term response rate between the NDP group and DDP group (90.9% vs 81.3%, P = 0.528). Although the 1- and 2-year survival rates were higher in the NDP group than in the DDP group (75.8% vs 68.8%, 57.6% vs 50.0%), the difference in the overall survival rate was not statistically significant between the two groups (P = 0.540). The incidences of nausea, vomiting and nephrotoxicity were significantly lower in the NDP group than in the DDP group (17.6% vs 50.0%, P = 0.031; 11.8% vs 47.1%, P = 0.016; 8.8% vs 38.2%, P = 0.039). There was no significant difference in the incidence of myelosuppression, radiation-induced esophagitis or radiation-induced pneumonia between the two groups.
CONCLUSION: NDP-based concurrent chemoradiotherapy is effective and well-tolerated in patients with locally advanced esophageal carcinoma. NDP-based regimen has comparable efficacy to DDP-based regimen but is associated with lower incidences of gastrointestinal and renal toxicity.
Core tip: This paper describes patients with locally advanced esophageal carcinoma who underwent nedaplatin (NDP) concurrent with radiotherapy. The survival and local control as well as the side effects during follow-up were analyzed by comparing with cisplatin (DDP). We found that NDP-based concurrent chemoradiotherapy is effective and well-tolerated. Compared with DDP, NDP-based concurrent chemoradiotherapy exhibits favorable efficacy with lower toxicity.
- Citation: Shen ZT, Wu XH, Li B, Shen JS, Wang Z, Li J, Zhu XX. Nedaplatin concurrent with three-dimensional conformal radiotherapy for treatment of locally advanced esophageal carcinoma. World J Gastroenterol 2013; 19(48): 9447-9452
- URL: https://www.wjgnet.com/1007-9327/full/v19/i48/9447.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i48.9447
Radiotherapy is one of the main treatments for esophageal carcinoma, especially for patients with locally advanced esophageal cancer who have no indications for surgery. However, the 5-year survival rate for patients with non-early esophageal carcinoma after radiotherapy alone is only 8% to 17%[1]. Approximately 70%-80% of cases of radiotherapy failure are due to uncontrolled or recurrent localized disease. Chemotherapy given concurrently with radiotherapy can improve the efficacy of radiotherapy in esophageal carcinoma. Concurrent chemoradiotherapy has been recommended as the standard treatment for locally advanced esophageal carcinoma in some countries, and conventional fractionated radiotherapy plus cisplatin (DDP) and 5-fluorouracil (5-FU) has been advocated as a standard regimen for this malignancy[2,3]. However, the risk of gastrointestinal and renal toxicity associated with DDP-based PF regimen (DDP + 5-FU) limits its use. In the present study, we designed a randomized controlled phase II trial to compare the efficacy, acute adverse reactions and late toxicity of three-dimensional conformal radiotherapy plus nedaplatin (NDP) and 5-FU versus plus the PF regimen in the treatment of locally advanced esophageal carcinoma, with an aim to find a regimen that has fewer adverse reactions and better efficacy than the PF regimen.
NDP injection (trade name, Jiebaishu, 10 mL) was provided by Simcere Pharmaceutical (Nanjing, China).
Sixty-eight patients who were pathologically proven to have locally advanced esophageal squamous cell carcinoma by gastroesophagoscopy from March 2007 to September 2009 in Department of Radiation Oncology of the Nanjing General Hospital of Nanjing Military Region and had evaluable tumor lesions were included in the study. There were 38 males and 30 females, and their median age was 54 years (range, 26 to 72 years). According to the 1997 Union for International Cancer Control esophageal cancer staging system, 29 patients had stage II disease and 39 had stage III disease. The patients were randomly divided into either a NDP group (n = 34) or a DDP group (n = 34) to receive NDP + leucovorin (CF) + 5-FU and DDP + CF + 5-FU, respectively. In the NDP group, 14 patients had stage II disease and 20 had stage III disease. In the DDP group, 15 patients had stage II disease and 19 had stage III disease. The average age of patients in the NDP and DDP groups was 55 and 53 years old, and the median age was 54 and 53 years old, respectively. Clinical data for patients in both groups are shown in Table 1.
| NDP group | DDP group | χ2 | P value | |
| Case | 34 | 34 | ||
| Gender | ||||
| Male | 18 | 20 | 0.239 | 0.625 |
| Female | 16 | 14 | ||
| Age | ||||
| Range | 27-72 | 26-70 | ||
| Median | 54 | 53 | ||
| Clinical stage (Union for International Cancer Control ) | ||||
| IIa | 4 | 6 | 0.478 | 0.787 |
| IIb | 10 | 9 | ||
| III | 20 | 19 | ||
| Tumor length | ||||
| < 5 cm | 14 | 17 | 0.534 | 0.465 |
| ≥ 5 cm | 20 | 17 | ||
| Cervical | 5 | 3 | ||
| Location in the esophagus | ||||
| Upper | 12 | 15 | 1.130 | 0.770 |
| Middle | 14 | 12 | ||
| Lower | 3 | 4 | ||
| Medullary | 20 | 22 | ||
| Fungoid | 6 | 5 | ||
| Pathology | ||||
| Ulcer type | 5 | 5 | 0.386 | 0.943 |
| Sclerotic type | 3 | 2 | ||
| General status (Eastern Cooperative Oncology Group score) | ||||
| 0-1 | 24 | 21 | 0.591 | 0.442 |
| 2 | 10 | 13 | ||
Inclusion criteria are (1) previously untreated, histologically or pathologically proven locally advanced esophageal carcinoma, with at least one measurable lesion (≥ 2 cm); (2) Eastern Cooperative Oncology Group performance status score ≤ 2; (3) expected survival for three months or more; (4) age between 26 and 72 years; (5) basically normal heart, lung, liver, kidney functions; (6) no previous thoracic radiotherapy or chemotherapy, and no significant chemotherapy contraindications; (7) no other malignancy; or (8) willing to provide signed informed consent.
Exclusion criteria included (1) participation in other drug trial or receiving anti-tumor therapy within 4 wk; (2) other serious complications that made the patient not to fit to the study; (3) pregnant or lactating women; and (5) allergy to the test drug.
Withdrawal criteria included (1) serious adverse reactions during treatment, such as life-threatening bleeding due to thrombocytopenia, life-threatening infections for leukopenia, and grade III or more liver and kidney adverse reactions; (2) not being able to complete the treatment; (3) not willing to continue the trial; or (4) disease progression during treatment.
The NDP group received NDP 80-100 mg/m2iv on day 1 + CF 100 mg/m2iv on days 1-5 + 5-FU 500 mg/m2iv on days 1-5. The DDP group received DDP 30 mg/m2iv on days 1-3 + CF 100 mg/m2 on days 1-5 + 5-FU 500 mg/m2iv on days 1-5. The treatment was repeated every 4 wk in both groups. Before chemotherapy, prophylactic antiemetic therapy with 5-HT3 receptor antagonist was given. Granulocyte colony-stimulating factor (G-CSF) was administered when grade 3/4 neutropenia occurred. When anemia and grade 3/4 thrombocytopenia occurred, erythropoietin (EPO) and recombinant human interleukin-11 (IL-11) or therapeutic plateletpheresis were given, and the dose of main chemotherapy drugs was reduced by 25% in the next cycle or the interval between two cycles was extended. Concurrent radiotherapy was given during chemotherapy in both groups.
Three-dimensional conformal radiotherapy (3D-CRT) was adopted, with high-energy X-ray beams (6 MV) produced by a linear accelerator. Gross tumor volume (GTV) boundaries were determined by esophageal X-ray, barium meal, CT, and esophagoscopy. The upper and lower boundaries for clinical target volume (CTV) were defined as upper and lower boundaries for GTV plus 3 cm. The lateral boundaries for CTV were defined as the lateral boundaries for tumors plus 0.8 cm. Planning target volume (PTV) was defined as CTV plus 0.5 cm.
Efficacy was evaluated using the 2000 RECIST criteria based on physical examination and imaging data (X-ray, barium meal, chest CT). Imaging data were assessed independently by two professional radiologists. Patients were rated as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD). The response rate (RR) was defined as (number of cases with CR + number of cases with PR)/total number of cases (n). Acute radiation injury was assessed using the Radiation Therapy Oncology Group (RTOG) acute radiation morbidity criteria (grades 0 to 4). Chemotherapy-associated adverse reactions were assessed using the U.S. National Cancer Institute common toxicity criteria (NCI-CTC), version 3.0 (grades 0-4).
Statistical analyses were performed using SPSS 13.0 software. Rates or percentages between two groups were compared using the chi-square test and Fisher exact test. Survival was analyzed using the Kaplan-Meier method. Survival curves were compared using the Log-rank significance test. Survival was defined as the period from the date of diagnosis to death. Two-tailed P-values <0.05 were considered statistically significant.
In the NDP group, 33 of 34 patients completed two or more cycles of treatment and were evaluable for efficacy and toxicity. In the DDP group, 32 patients completed two or more cycles of chemotherapy and can be evaluated for efficacy and toxicity, and the remaining two cases discontinued the treatment after one cycle of chemotherapy (one for intolerable side effects and the other for poor incompliance) but can be evaluated for toxicity. Short-term responses in the two groups are shown in Table 2.
| Group | n | CR | PR | SD | PD | RR | χ2 | P value |
| NDP group | 33 | 6 (18.2) | 24 (72.7) | 3 (9.1) | 0 (0) | 90.9% | 1.276 | 0.528 |
| DDP group | 32 | 5 (15.6) | 21 (65.6) | 6 (18.8) | 0 (0) | 81.3% |
The 1-year overall survival rate was 75.8% (25/33) for the NDP group and 68.8% (22/32) for the DDP group, and the 2-year overall survival rate was 57.6% (19/33) and 50.0% (16/32), respectively. Although the overall survival rate was higher in the NDP group than in the DDP group, the difference was not statistically significant (χ2 = 0.375, P = 0.504).
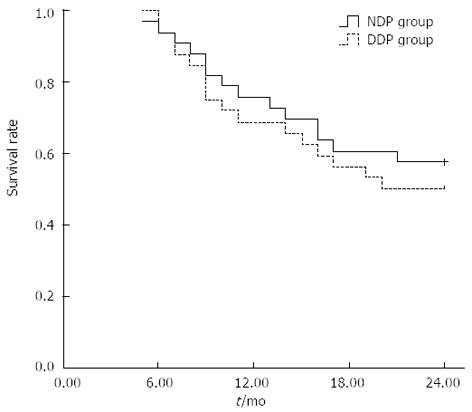
During the follow-up period, 19 patients survived and 14 died in the NDP group. Of 14 dead patients, 9 died of distant metastasis, 3 of local control failure, and 2 of distant metastasis plus local control failure. Of 16 dead patients in the DDP group, 4 died of local control failure, 7 of local control failure plus distant metastasis, and 5 of distant metastasis. These findings suggest that distant metastasis was the main cause of death in both groups. The percentage of patients who died of distant metastasis showed no significant difference between the NDP group and DDP group (78.6% vs 75.0%, χ2 = 0.053, P = 0.818) (Figure 1).
Toxicity could be evaluated in all cases. In the NDP group, grades I-IV decreased hemoglobin developed in 20 patients (58.8%), grades I-IV leukopenia in 21 patients (61.8%), and grades I-IV thrombocytopenia in 19 patients (55.9%); the corresponding figures in the DDP group were 18 (52.9%), 19 (55.9%) and 14 cases (41.2%). The incidences of decreased hemoglobin, leukopenia and thrombocytopenia showed no significant differences between the two groups (P = 0.990, 0.805, 0.540). Although the incidence of hepatic dysfunction did not differ significantly between the two groups (P = 0.565), the incidence of renal toxicity was significantly higher in the DDP group (38.2% vs 8.8%, P = 0.039). The incidences of nausea and vomiting were significantly lower in the NDP group than in the DDP group (17.6% vs 50.0%, 11.8% vs 47.1%, P = 0.031, 0.016) (Table 3).
| Acute adverse reactions | NDP group (n = 34) | DDP group (n = 34) | χ2 | P value | ||||||||||
| 0 | I | II | III | IV | Incidence | 0 | I | II | III | IV | Incidence | |||
| Hemoglobin | 14 | 9 | 5 | 5 | 1 | 58.80% | 16 | 8 | 5 | 4 | 1 | 52.90% | 0.303 | 0.990 |
| Leukopenia | 13 | 8 | 7 | 6 | 0 | 61.80% | 15 | 8 | 6 | 4 | 1 | 55.90% | 1.62 | 0.805 |
| Platelet | 15 | 7 | 6 | 5 | 1 | 55.90% | 20 | 4 | 7 | 3 | 0 | 41.20% | 3.109 | 0.540 |
| Bilirubin | 29 | 4 | 1 | 0 | 0 | 14.70% | 30 | 3 | 1 | 0 | 0 | 11.80% | 0.16 | 0.923 |
| Transaminase | 25 | 8 | 1 | 0 | 0 | 26.50% | 27 | 7 | 0 | 0 | 0 | 20.60% | 1.144 | 0.565 |
| Urea nitrogen | 30 | 4 | 0 | 0 | 0 | 11.80% | 29 | 3 | 2 | 0 | 0 | 14.70% | 2.16 | 0.340 |
| Creatinine | 31 | 2 | 1 | 0 | 0 | 8.80% | 21 | 9 | 3 | 1 | 0 | 38.20% | 8.378 | 0.039 |
| Nausea | 28 | 4 | 1 | 1 | 0 | 17.60% | 17 | 7 | 6 | 4 | 0 | 50.00% | 8.878 | 0.031 |
| Vomiting | 30 | 2 | 1 | 1 | 0 | 11.80% | 18 | 6 | 4 | 6 | 0 | 47.10% | 10.371 | 0.016 |
| Esophagitis | 8 | 18 | 7 | 1 | 0 | 76.50% | 4 | 19 | 9 | 1 | 1 | 88.20% | 2.61 | 0.625 |
| Pneumonia | 18 | 14 | 2 | 0 | 0 | 47.10% | 12 | 17 | 4 | 1 | 0 | 64.70% | 3.157 | 0.368 |
Late grade 4 esophageal toxicity was noted in one patient in the DDP group, but no patient developed late grade 3 or more esophageal toxicity in the NDP group. The incidences of late esophageal and lung toxicities showed no significant difference between the two groups (P > 0.05 for both). Serious late radiation toxicities such as radiation-induced myelitis and pericarditis were not observed (Table 4).
| Late adverse event | NDP group | DDP group | χ2 | P value |
| Late esophageal injury | ||||
| 0 | 18 (52.9) | 13 (38.2) | ||
| I | 10 (29.4) | 12 (35.3) | ||
| II | 4 (11.8) | 5 (14.7) | 2.299 | 0.681 |
| III | 2 (5.9) | 3 (8.8) | ||
| IV | 0 (0) | 1 (2.9) | ||
| Late lung injury | ||||
| 0 | 24 (70.6) | 20 (58.8) | ||
| I | 7 (20.6) | 8 (23.5) | ||
| II | 2 (5.9) | 4 (11.8) | 1.43 | 0.698 |
| III | 1 (2.9) | 2 (5.9) | ||
| IV | 0 (0) | 0 (0) | ||
Esophageal carcinoma is one of the most common malignancies in China. Surgical excision is the standard treatment for esophageal carcinoma. Because most patients with esophageal carcinoma are diagnosed at the advanced stage, most of them have missed the chance of radical surgery. For patients without indications for surgery or those with localized disease after surgical resection, radiotherapy is another possible cure. However, both surgery alone and radiotherapy alone can not significantly improve the five-year survival rate in patients with esophageal carcinoma. To overcome this problem, worldwide scholars have tried a variety of comprehensive treatment from the 1970s to improve the therapeutic effect against this malignancy[4-8]. Chemotherapy combined with radiotherapy has yielded encouraging results. Particularly, the RTOG8501 trial conducted by Cooper et al[5] has provided conniving evidence to support the effectiveness of concurrent chemoradiotherapy in the management of esophageal carcinoma.
Compared to radiotherapy alone, concurrent chemoradiotherapy will further increase the incidence of side effects. Main side effects include radiation-induced esophagitis, pneumonia, bone marrow suppression, nausea, and vomiting. Seung et al[9] reported that the incidences of grade 2 and 3 esophagitis were 89% and 39%, respectively. Severe radiation-induced esophagitis is difficult to manage and often affects the implementation of treatment regimens or extends the total treatment time, thereby affecting therapeutic effects. Many studies have shown that the most commonly used PF regimen plus concurrent radiotherapy is associated with a high incidence of esophagitis. DDP is the main factor causing toxicity and is intolerable in some patients. Therefore, researchers have been seeking more efficient drugs or regimens with lower toxicity.
NDP (cis-diam-mincgly-colatoplatinum; formula, C2H8N2O3Pt; molecular mass, 303.18 kD) is a second-generation anti-cancer platinum derivative developed by Japanese pharmaceutical company Shionogi and approved for marketing in Japan in June 1995. Clinical studies have demonstrated that NDP is effective in esophageal carcinoma, head and neck cancer, lung cancer, cervical cancer, ovarian cancer, bladder cancer, testicular cancer and other solid tumors. It can be used alone or in combination with other chemotherapeutic drugs or radiotherapy to improve efficacy and reduce side effects. The mechanism of action of NDP is the same as that of DDP; they bind to DNA by forming platinum-nucleoside complexes and inhibit DNA replication[10]. The solubility of NDP is about 10 times that of DDP, and there exists certain cross-resistance between DDP and NDP[11]. NDP does not require hydration, has low renal and gastrointestinal toxicity, and shows a good synergistic effect when being used with other chemotherapy drugs. There is no complete cross-resistance between CDDP and NDP[12]. Although NDP has a high therapeutic index, its side effects are low. The dose-limiting toxicity of NDP is myelosuppression-induced thrombocytopenia, and its renal and gastrointestinal toxicity is low[13]. In recent years, many foreign clinical studies have demonstrated that the response rate of NDP-based regimens is above 50% in patients with advanced esophageal carcinoma, which is higher than or similar to those of conventional DDP-based regimens, but adverse reactions could be expected and well tolerated[14]. A similar study has also been reported in China[15]. Watanabe et al[16] reported the use of NDP and 5-FU with concurrent radiotherapy for advanced esophageal carcinoma. Kato et al[14] reported that NDP and 5-FU combined with radiotherapy achieved an overall response rate of 77%, a 1-year survival rate of 30.7%, a 2-year survival rate of 10.2%, and the median survival time of 10.1 mo in patients with unresectable advanced esophageal squamous cell carcinoma.
The present study showed that the short-term response rate and the 1- and 2-year survival rates were higher in the NDP group than in the DDP group (90.9% vs 81.3%, 75.8% vs 68.8%, 57.6% vs 50.0%), although the differences were not statistically significant. These findings suggest that NDP-based regimen has a trend to improve the short- and long-term response rates in locally advanced esophageal carcinoma, and that the efficacy of NDP-based concurrent chemoradiotherapy regimen is not lower, or slightly higher than that of traditional CDDP-based concurrent chemoradiotherapy regimen. With regard to adverse effects, the incidences of nausea and vomiting were significantly lower in the NDP group than in the DDP group (17.6% vs 50.0%, 11.8% vs 47.1%, P < 0.05 for both). The majority of cases of nausea and vomiting in the NDP group were grades I-II and could be easily managed using antiemetic therapy with 5-HT3 receptor antagonist, while the incidences of grades II-III nausea and vomiting were relatively high in the DDP group. The incidence of renal toxicity, mainly grades I-II, was significantly lower in the NDP group than in the DDP group (8.8% vs 38.2%, P < 0.05). There was no significant difference in the incidence of liver toxicity between the two groups (P > 0.05). The incidence of leukopenia, mainly grades I-II, was slightly higher in the NDP group than in the DDP group, but the difference was not statistically significant (P > 0.05). The incidence of thrombocytopenia (grades I-II: 38.2%; grades III-IV: 17.6%) was also slightly higher in the NDP group. Thrombocytopenia occurred mainly 7 to 10 d after treatment and resolved in all cases 14 d after treatment. These results indicate that the incidence of gastrointestinal reactions such as nausea and vomiting was significantly lower in the NDP group. The liver and kidney toxicity was mild. The main dose-limiting toxicity was myelosuppression, especially thrombocytopenia, which can be managed by symptomatic and supportive treatment or dosage adjustment.
In conclusion, NDP is an effective drug for treatment of esophageal carcinoma. NDP combined with 5-FU is superior to DDP plus 5-FU in terms of reducing the incidences of gastrointestinal and renal toxicity and improving clinical tolerance. Since the sample size is small in the present study, further large-sample trials are required to evaluate the long-term efficacy and toxicity of NDP-based regimens.
Radiotherapy given concurrently with chemotherapy can improve the efficacy of radiotherapy in esophageal carcinoma. Concurrent chemoradiotherapy has been recommended as the standard treatment for locally advanced esophageal carcinoma, and conventional fractionated radiotherapy plus cisplatin (DDP) and 5-fluorouracil (5-FU) has been advocated as a standard regimen for this malignancy. However, the risk of gastrointestinal and renal toxicity associated with DDP-based PF regimen (DDP + 5-FU) limits its use.
In the present study, the authors designed a randomized controlled phase II trial to compare the efficacy, acute adverse reactions and late toxicity of three-dimensional conformal radiotherapy plus nedaplatin (NDP) and 5-FU vs plus the PF regimen in the treatment of locally advanced esophageal carcinoma, with an aim to find a regimen that has fewer adverse reactions and better efficacy than the PF regimen.
The survival and local control as well as the side effects during follow-up were analyzed by comparing with cisplatin. The authors found NDP-based concurrent chemoradiotherapy is effective and well-tolerated. Compared with DDP, NDP-based concurrent chemoradiotherapy exhibits favorable efficacy with lower toxicity.
The study results suggest that NDP-based concurrent chemoradiotherapy is a potential therapeutic regimen that could be used in locally advanced esophageal carcinoma.
Cisplatin, a common chemotherapeutic drug, has been one of doctors’ first lines of defense against tumors, especially those of the lung, ovary, testes and locally advanced esophageal carcinoma. Nedaplatin is a new platinum derivative, selected from a series of platinum analogues based on its pronounced preclinical antitumor activity against various solid tumors with lower nephrotoxicity and gastrointestinal reactions.
This is a good clinical study in which the authors evaluated the efficacy and safety of three-dimensional conformal radiotherapy plus NDP and 5-FU versus plus the PF regimen in the treatment of locally advanced esophageal carcinoma. The results suggest that NDP-based concurrent chemoradiotherapy exhibits favorable efficacy with lower toxicity.
P- Reviewers: Meyers BM, Pernetti R, Thiele M S- Editor: Wang JL L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Yeh AM, Mendenhall WM, Morris CG, Zlotecki RA, Desnoyers RJ, Vogel SB. Factors predictive of survival for esophageal carcinoma treated with preoperative radiotherapy with or without chemotherapy followed by surgery. J Surg Oncol. 2003;83:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, Cooper J, Byhardt R, Davis L, Emami B. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1514] [Cited by in RCA: 1445] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 3. | Suntharalingam M, Moughan J, Coia LR, Krasna MJ, Kachnic L, Haller DG, Willett CG, John MJ, Minsky BD, Owen JB. The national practice for patients receiving radiation therapy for carcinoma of the esophagus: results of the 1996-1999 Patterns of Care Study. Int J Radiat Oncol Biol Phys. 2003;56:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Ruhstaller T, Pless M, Dietrich D, Kranzbuehler H, von Moos R, Moosmann P, Montemurro M, Schneider PM, Rauch D, Gautschi O. Cetuximab in combination with chemoradiotherapy before surgery in patients with resectable, locally advanced esophageal carcinoma: a prospective, multicenter phase IB/II Trial (SAKK 75/06). J Clin Oncol. 2011;29:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1261] [Cited by in RCA: 1372] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 6. | Bedenne L, Michel P, Bouché O, Milan C, Mariette C, Conroy T, Pezet D, Roullet B, Seitz JF, Herr JP. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 878] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 7. | Yamada K, Murakami M, Okamoto Y, Okuno Y, Nakajima T, Kusumi F, Takakuwa H, Matsusue S. Treatment results of chemoradiotherapy for clinical stage I (T1N0M0) esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2006;64:1106-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Wong RK, Malthaner R. Combined chemotherapy and radiotherapy (without surgery) compared with radiotherapy alone in localized carcinoma of the esophagus. Cochrane Database Syst Rev. 2010;CD002092. [PubMed] |
| 9. | Seung SK, Smith JW, Molendyk J, Bader SB, Phillips M, Regan J, Louie J, Soo E, Seligman M, Ruzich J. Selective dose escalation of chemoradiotherapy for esophageal cancer: role of treatment intensification. Semin Oncol. 2004;31:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Desoize B, Madoulet C. Particular aspects of platinum compounds used at present in cancer treatment. Crit Rev Oncol Hematol. 2002;42:317-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 272] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Heike Y, Takahashi M, Ohira T, Arioka H, Funayama Y, Nishio K, Ogasawara H, Saijo N. In vivo screening models of cisplatin-resistant human lung cancer cell lines using SCID mice. Cancer Chemother Pharmacol. 1995;35:200-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Takigawa N, Segawa Y, Ueoka H, Kiura K, Tabata M, Shibayama T, Takata I, Miyamoto H, Eguchi K, Harada M. Combination of nedaplatin and vindesine for treatment of relapsed or refractory non-small-cell lung cancer. Cancer Chemother Pharmacol. 2000;46:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Hartmann JT, Lipp HP. Toxicity of platinum compounds. Expert Opin Pharmacother. 2003;4:889-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 436] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 14. | Kato H, Fukuchi M, Manda R, Nakajima M, Miyazaki T, Sohda M, Masuda N, Fukai Y, Tsukada K, Kuwano H. Efficacy and toxicity of nedaplatin and 5-FU with radiation treatment for advanced esophageal carcinomas. Anticancer Res. 2003;23:3493-3498. [PubMed] |
| 15. | Guo K, Cai L, Zhang Y, Zhu JF, Rong TH, Lin P, Hao CL, Wang WP, Li Z, Zhang LJ. The predictive value of histological tumor regression grading (TRG) for therapeutic evaluation in locally advanced esophageal carcinoma treated with neoadjuvant chemotherapy. Chin J Cancer. 2012;31:399-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Watanabe T, Chinen T, Nakachi N, Nakamoto M, Uchima N, Hirata T, Hokama A, Kinjo N, Kinjo F, Fujita J. [Recurrent esophageal cancer with complete response to TS-1 chemotherapy]. Gan To Kagaku Ryoho. 2007;34:419-422. [PubMed] |