Published online Dec 28, 2013. doi: 10.3748/wjg.v19.i48.9439
Revised: November 5, 2013
Accepted: November 12, 2013
Published online: December 28, 2013
Processing time: 110 Days and 4.5 Hours
AIM: To evaluate human gastric submucosal vascular dysfunction and its mechanism during the aging process.
METHODS: Twenty male patients undergoing subtotal gastrectomy were enrolled in this study. Young and elderly patient groups aged 25-40 years and 60-85 years, respectively, were included. Inclusion criteria were: no clinical evidence of cardiovascular, renal or diabetic diseases. Conventional clinical examinations were carried out. After surgery, gastric submucosal arteries were immediately dissected free of fat and connective tissue. Vascular responses to acetylcholine (ACh) and sodium nitroprusside (SNP) were measured by isolated vascular perfusion. Morphological changes in the gastric mucosal vessels were observed by hematoxylin and eosin (HE) staining and Verhoeff van Gieson (EVG) staining. The expression of xanthine oxidase (XO) and manganese-superoxide dismutase (Mn-SOD) was assessed by Western blotting analysis. The malondialdehyde (MDA) and hydrogen peroxide (H2O2) content and the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) were determined according to commercial kits.
RESULTS: The overall structure of vessel walls was shown by HE and EVG staining, respectively. Disruption of the internal elastic lamina or neointimal layers was not observed in vessels from young or elderly patients; however, cell layer number in the vessel wall increased significantly in the elderly group. Compared with submucosal arteries in young patients, the amount of vascular collagen fibers, lumen diameter and media cross-sectional area were significantly increased in elderly patients. Ach- and SNP-induced vasodilatation in elderly arterioles was significantly decreased compared with that of gastric submucosal arterioles from young patients. Compared with the young group, the expression of XO and the contents of MDA and H2O2 in gastric submucosal arterioles were increased in the elderly group. In addition, the expression of Mn-SOD and the activities of SOD and GSH-Px in the elderly group decreased significantly compared with those in the young group.
CONCLUSION: Gastric vascular dysfunction and senescence may be associated with increased oxidative stress and decreased antioxidative defense in the aging process.
Core tip: Aging is usually accompanied by a high risk of gastric disease. It is currently thought that adequate mucosal blood flow plays an important role in maintaining mucosal integrity. This study showed that oxidative stress induces gastric submucosal vascular structure dysfunction during the aging process. Vascular aging of gastric mucosa may lead to blood supply insufficiency, and thus increase the incidence of gastric diseases.
- Citation: Liu L, Liu Y, Cui J, Liu H, Liu YB, Qiao WL, Sun H, Yan CD. Oxidative stress induces gastric submucosal arteriolar dysfunction in the elderly. World J Gastroenterol 2013; 19(48): 9439-9446
- URL: https://www.wjgnet.com/1007-9327/full/v19/i48/9439.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i48.9439
Although it is difficult to define the term “aging” in medical fields, it usually means the progressive accumulation of irreversible degenerative changes leading to loss of homeostasis. It is thought that there is also a modest decline in the structure and function of several digestive organs.
The most common stomach diseases in elderly individuals are atrophic gastritis and peptic ulcer disease[1]. The former is significantly associated with Helicobacter pylori infection and reduced acid secretion[2]. Hyposecretion of gastric acid reduces the absorption of vitamin B12, iron and calcium, and these deficits can lead to megaloblastic or iron-deficiency anemia and a higher frequency of osteoporosis[3]. Peptic ulcers in older patients are often caused by the use or overuse of nonsteroidal anti-inflammatory drugs[4].
It is currently thought that an adequate mucosal blood flow plays an important role in maintaining mucosal integrity. The blood supplies oxygen, nutrients and gastrointestinal hormones to support the correct structure, function and turnover of gastric mucosa. Blood flow is also important in the production and secretion of mucus and helps to maintain the mucosal barrier. In addition, the blood circulating in the surface mucosa removes waste materials and back-diffusing hydrogen ions and maintains the secretion of bicarbonate ions, protecting the mucosa by maintaining the neutral status of regional mucosa[5]. Numerous experimental studies have demonstrated the importance of mucosal blood flow in the defense of gastric mucosa against injury[6-13].
The structure and function of gastric blood vessels are important for determining blood flow, which plays an important role in maintaining mucosal integrity. There is considerable evidence showing that vascular aging is associated with an increased production of reactive oxygen species (ROS)[14]. There is a balance between the generation and elimination of ROS in vessels[15]. If the balance is destroyed, excess ROS will be produced, resulting in cellular dysfunction and vascular aging[16,17]. In the present study, human gastric submucosal arterioles from subtotal gastrectomy specimens were used to determine the relationship between gastric vascular dysfunction and oxidative stress, and to further explain the reason for the higher risk of gastric diseases in the elderly.
Acetylcholine (ACh) and sodium nitroprusside (SNP) were purchased from Sigma (St. Louis, MO, United States). The kits for assessing lipid oxidation injury including superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), hydrogen peroxide (H2O2) and malondialdehyde (MDA) were purchased from Nanjing Jiancheng Bioengineering Institute (Jiangsu Province, China). Polyclonal antibodies for xanthine oxidase (XO), manganese-superoxide dismutase (Mn-SOD) and the alkaline phosphorylase tagged goat anti-rabbit IgG antibody were purchased from Santa Cruz Biotechnology (CA, United States). The 5-bromo-4-chloro-3-indolyl phosphate/nitrotetrazolium blue chloride (BCIP/NBT) kit was purchased from Promega (Madison, WI, United States).
The study was approved by the Ethics Committee of Xuzhou Medical College. Twenty male patients undergoing subtotal gastrectomy for gastric cancer in the Affiliated Hospital of Xuzhou Medical College were enrolled in this study. Patients with diabetes, hypertension or other cardiovascular diseases were excluded. All enrolled patients underwent conventional clinical examinations, including fasting blood glucose, total cholesterol, triglycerides and blood pressure, which were all found within normal ranges for clinic.
The patients were divided into two groups according to age: 10 patients aged 25-40 years were included in the young group and 10 patients aged 60-85 years were included in the elderly group. Gastric tissues, which were confirmed by pathology to be normal tissue, were obtained a distance from the cancer tissue and were undamaged due to surgical instruments. Gastric submucosal arterioles were immediately dissected free of fat and connective tissue in order to measure vascular function and changes in biochemistry and molecular biology.
Body mass index was calculated using body weight in kilograms divided by the square of the height in meters (kg/m2). Fasting plasma total cholesterol, triglyceride concentration, systolic blood pressure, diastolic blood pressure and fasting plasma glucose concentration were measured. Arterial blood pressure was measured over the brachial artery during supine rest using a semiautomatic device (Dynamap XL, Johnson and Johnson). Fasting plasma metabolic parameters and oxidized low-density lipoprotein were determined by standard assays. Plasma samples were also analyzed for oxidized low-density lipoprotein[18].
After surgery, sections of arteries were placed in phosphate buffered formaldehyde (4%) overnight, then stored in ethanol and embedded in paraffin. Cross sections (4 μm) were stained with hematoxylin and eosin (HE) staining and Verhoeff van Gieson (EVG) staining.
Similar to our previous study[19], gastric submucosal arterioles, approximately 200 μm in maximal diameter and approximately 10 mm in length, were isolated and cannulated in a water-jacketed (37 °C) perfusion device, intravascular pressure was maintained at 80 mmHg, and the changes in vascular diameter were recorded using a video monitor system. Dilations due to ACh (10-7 to 10-5 mol/L) and SNP (10-7 to 10-5 mol/L) were assessed in the arterioles from the young and elderly groups. Vasodilatation responses were expressed as the percentage of basal diameter at 80 mmHg.
Gastric arteries were isolated and crushed with liquid nitrogen and a homogenate was prepared. The homogenate was centrifuged, and the supernatant was used for biochemical analyses. The protein concentration in the supernatant was determined by the bicinchoninic acid assay (BCA assay, Nanjing Jiancheng Bioengineering Institute).
The MDA concentration in the homogenate was determined using a commercially available kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) based on thiobarbituric acid (TBA) reactivity. Briefly, after mixing trichloroacetic acid with the homogenate and centrifuging, a supernatant was obtained, and TBA was added. The developed red color of the resulting reaction was measured at 532 nm with a spectrophotometer. Other procedures were carried out following the manufacturer’s protocols.
The content of H2O2 in gastric submucosal arteries was assessed using a commercially available kit (Nanjing Jiancheng Bioengineering Institute). H2O2 bound with molybdenic acid to form a complex, which was measured at 405 nm and the content of H2O2 was then calculated.
SOD activity in gastric submucosal arteries was assessed using a commercially available kit (Nanjing Jiancheng Bioengineering Institute) based on the auto-oxidation of hydroxylamine. The developed blue color was measured at 550 nm.
GSH-Px activity was determined by the velocity method using a GSH-Px kit (Nanjing Jiancheng Bioengineering Institute). The reaction was initiated by the addition of H2O2. A series of enzymatic reactions was activated by GSH-Px in the homogenate which subsequently led to the conversion of GSH (reduced glutathione) to oxidized glutathione (GSSG). The change in absorbance during the conversion of GSH to GSSG was recorded spectrophotometrically at 412 nm.
Gastric submucosal arteries from young and elderly patients were isolated and pooled in liquid nitrogen, respectively. Samples were solubilized in lysis buffer containing 1% protease inhibitor cocktail (Sigma) in ice for 30 min, followed by sonication for 1 min which was carried out twice at a 5-min interval. The supernatants were collected after centrifugation at 10000 ×g for 15 min at 4 °C. Protein concentrations were determined using the BCA protein assay kit. Samples (50 μg protein) were separated on 10% SDS-PAGE gels and transferred to a polyvinylidene fluoride membrane. The blots were incubated with 5% bovine serum albumin in TBST (10 mmol/L Tris, pH 7.5; 150 mmol/L NaCl, 0.05% Tween-20) at room temperature for 2 h, then incubated with primary antibodies (anti-Mn-SOD, polyclonal antibody 1:500, anti-XO polyclonal antibody 1:500) at 4 °C overnight. After washing with TBST, the blots were incubated with secondary antibody for 2 h and were determined using a BCIP/NBT assay kit. β-actin was used to normalize loading variations.
Data were expressed as mean ± SE. Comparisons between two groups were made using the Student’s t test. Statistical analyses were performed using SPSS for Windows version 13.0. P < 0.05 was considered statistically significant.
Patients with diabetes, hypertension or other cardiovascular diseases which could affect the structure and function of vessels were excluded from the study. The results of conventional clinical examinations in the young and elderly patients are shown in Table 1. Fasting plasma total cholesterol, triglyceride concentrations, fasting plasma glucose concentrations, systolic blood pressure, diastolic blood pressure and mean arterial pressure were higher in the elderly group compared with the young group (P < 0.05). However, these values were within normal ranges for clinic. There were no differences in body mass index between the two groups.
| Young group | Elderly group | |
| No. of patients | 10 | 10 |
| Age, yr | 34.2 ± 4 | 71.6 ± 7a |
| Body mass index, kg/m2 | 21.5 ± 2.6 | 20.5 ± 3 |
| Fasting blood glucose, mmol/L | 4.2 ± 0.7 | 5.4 ± 0.6a |
| Total cholesterol, mmol/L | 3.87 ± 0.3 | 4.89 ± 0.17a |
| Triglycerides, mmol/L | 1.03 ± 0.01 | 1.23 ± 0.11a |
| Systolic blood pressure, mmHg | 112.2 ± 9.6 | 126.8 ± 8.6a |
| Diastolic blood pressure, mmHg | 67.7 ± 3.7 | 77.9 ± 5.5a |
| Mean arterial pressure, mmHg | 82.53 ± 2.98 | 94.2 ± 5.05a |
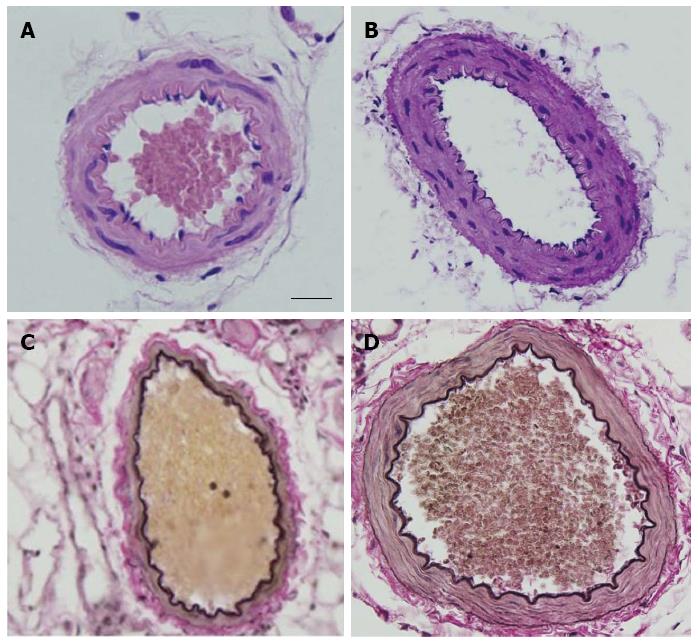
The overall structure of vessel walls was examined by HE and EVG staining, respectively (Figure 1). Disruption of the internal elastic lamina or neointimal layers was not observed in young or aged vessels; however, the cell layer number in the vessel walls increased significantly in the elderly group (Figure 1A and B). Compared with submucosal arteries in young patients, the amount of vascular collagen fibers, lumen diameter and media cross-sectional area were significantly increased in the elderly group (Figure 1C and D).
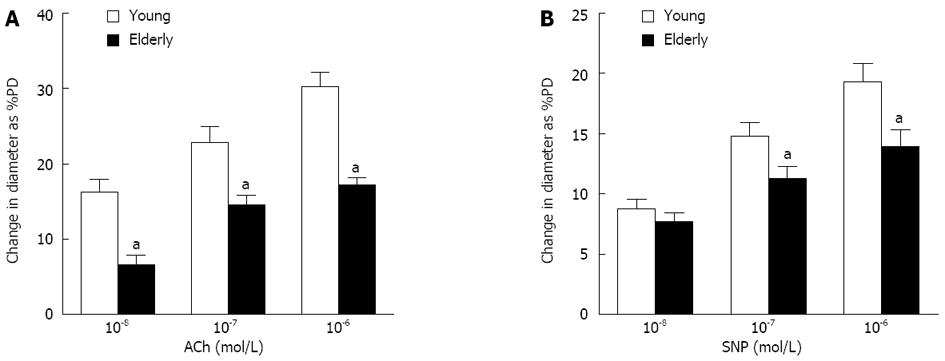
ACh (10-7, 10-6, and 10-5 mol/L)- and SNP (10-7, 10-6, and 10-5 mol/L)-induced dilations were compared in gastric submucosal arterioles. Basal diameter and passive diameter at 80 mmHg of intravascular pressure in the arterioles from young and elderly patients were 142.8 ± 3.6 and 144.8 ± 2.8 μm, and 192.8 ± 4.1 and 189.0 ± 2.27 μm, respectively. As shown in Figure 2, dilation due to ACh, which stimulates NO synthesis and release from endothelium, was greatly reduced in the vessels from the elderly group compared with the young group (P < 0.05). Endothelium-independent vasodilatation was determined using SNP (a NO donor). Dilation due to SNP was also reduced in the vessels from the elderly group (P < 0.05), but the magnitude of this reduction was less.
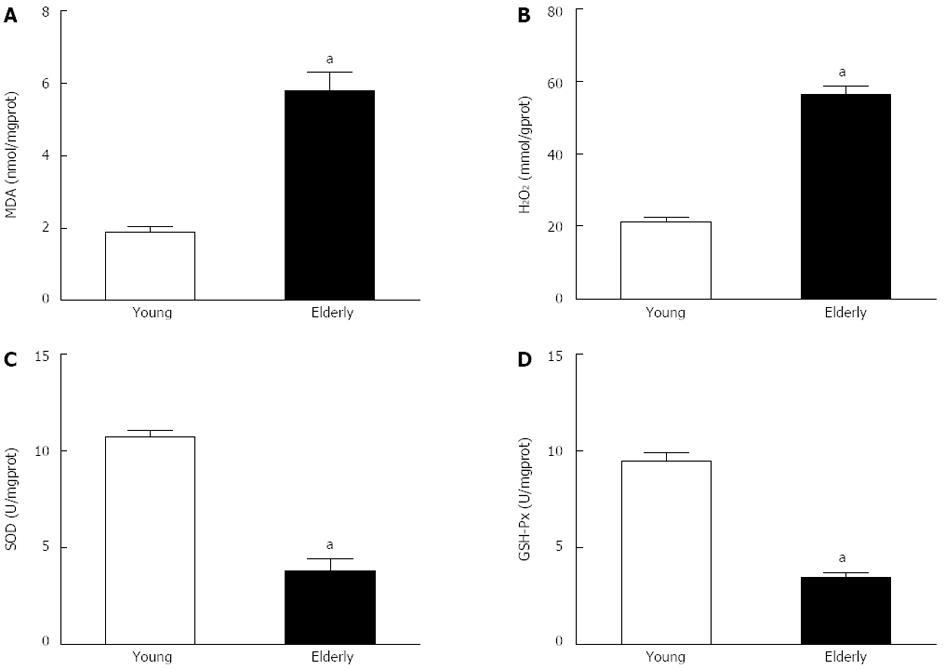
To determine whether oxidative stress participates in the aging process, we measured the contents of MDA and H2O2 and the activities of GSH-Px and SOD in gastric submucosal arteries, these are key markers of oxidative stress. Compared with the young group, there was a significant increase in MDA and H2O2 content (P < 0.05), and a decrease in SOD and GSH-Px activities (P < 0.05) in the elderly group (Figure 3).
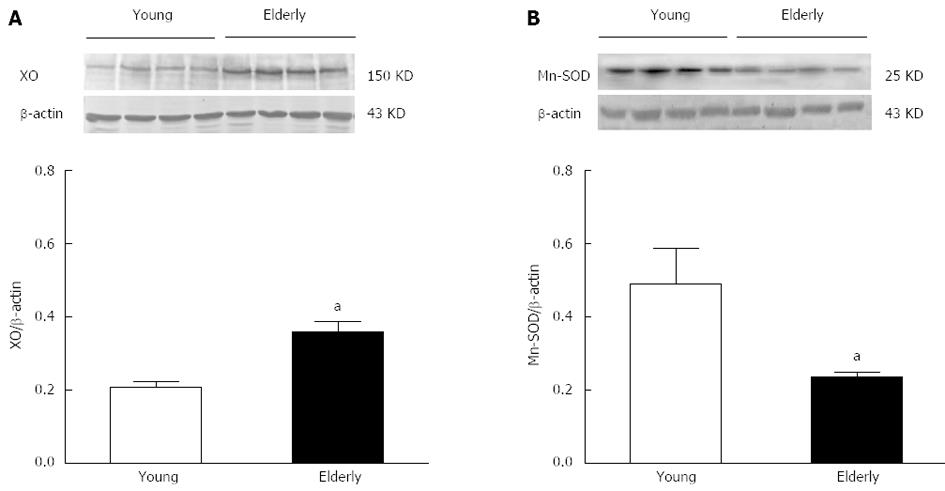
Gastric submucosal arteries were isolated and the expression of XO and Mn-SOD was assessed. Figure 4 shows a representative Western blotting of XO and Mn-SOD in arteries. The XO protein was significantly increased (Figure 4A), whereas the expression of Mn-SOD (Figure 4B) was significantly reduced in the elderly group compared with the young group (P < 0.05).
Vascular aging is a key factor in accelerating the aging process and increasing the incidence of disease in humans. In this study, we demonstrated a marked deterioration in the structure and function of gastric blood vessels during aging. The underlying mechanisms may be associated with increased oxidative stress and decreased antioxidative defense, which induce an imbalance in oxidative stress during the aging process, thus accelerating vascular dysfunction and senescence in the elderly.
Aging is considered the leading cause of morbidity and mortality worldwide, and the proportion of elderly people is steadily growing[20]. With increasing age, the vasculature undergoes functional and structural impairment. Vascular changes during aging are manifested in various ways in many experimental animals; the most obvious changes noted are thickening of less compliant vessel walls[21]. In the present study, we evaluated the walls of gastric submucosal arteries from elderly patients, which were much thicker than the arteries from young patients. In addition, the collagen content was increased in aged gastric submucosal arteries (Figure 1). Aging can radically transform the endothelial layers lining the vessel wall in response to shear and stretch stress[22], and prompt them to thicken, as observed in many vascular models[23,24]. The changes in gastric submucosal arteries may be due to the recruitment of vascular smooth muscle cells for increased synthesis of interstitial, extra-cellular matrix proteins[25,26].
As expected, endothelium-dependent and -independent dilation were significantly attenuated in aged gastric submucosal arterioles compared with arterioles from young patients (Figure 2). Endothelial cell dysfunction has been observed in the elderly[27], and the production of endothelium-derived vasodilator substances is decreased[28,29]. In the present study, ACh-induced dilation of the gastric submucosal arteries from elderly patients was reduced, which was related to endothelial dysfunction during the aging process. Endothelium-independent dilation was also detected. SNP (a NO donor) induced dilation in the arterioles of elderly patients was reduced compared with that in arterioles from young patients. This change may be due to the thick vessel walls and increased collagen content. The changes in gastric submucosal vascular structure and function may lead to gastric blood supply insufficiency in the elderly.
Adequate mucosal blood flow plays an important role in maintaining mucosal integrity. Decreased gastric blood flow causes acute gastric mucosal lesions in animals and humans[9]. Gastric blood flow is important in the development of gastric ulceration and healing. Furthermore, the speed of ulcer healing is affected by the speed of blood flow at the ulcer edge[30]. Our group has recently shown that aspirin-induced injury of gastric mucous membrane was inhibited by increasing gastric mucosal blood flow[31]. The changes in gastric submucosal vascular structure and function which induced blood supply insufficiency may be one of the most important reasons for the higher incidence of gastric diseases in elderly subjects.
The mechanism involved in these changes in vascular structure and function in the elderly was also investigated in the present study. MDA is a marker of free radical species-related injury, H2O2 is the main reactive oxygen species produced, and these are by-products of mitochondrial respiration. In our experiments, MDA and H2O2 were significantly increased in aged arteries compared to arteries from young patients. Age-related increases in oxidative stress may result in changes in vascular structure and function in aged gastric submucosal arteries.
Oxidative stress is associated with increased production of oxidizing species or a significant decrease in antioxidant defense capability[32]. XO is an important potential source of superoxide generation, it catalyzes the conversion reactions of hypoxanthine to xanthine and xanthine to uric acid, the last reaction in purine catabolism, with the byproduct of toxic superoxide radical[33]. XO protein levels in gastric submucosal arteries were significantly higher in elderly patients than in young patients. Increased expression of oxidases is the main source of reactive oxygen species in humans[34].
SOD, a major antioxidant enzyme, contributes to the destruction of free superoxide radicals and other reactive oxygen species, and blocks free radical-induced damage in the body[35,36]. There are three isoforms of SOD, cytosolic SOD or copper zinc SOD (CuZn-SOD or SOD-1), mitochondrial SOD or manganese SOD (Mn-SOD or SOD-2), and extracellular CuZn-SOD (EC-SOD or SOD-3). Mn-SOD, which is found in mitochondria, plays an important role in the maintenance of vascular function. Previous animal studies found that the antioxidant enzyme content was decreased, while the expression of oxidative enzymes was significantly increased with aging[37]. The Mn-SOD protein level (Figure 4B) and SOD activity (Figure 3C) were significantly decreased in this study, which was consistent with previously reported findings.
GSH-Px is a free radical scavenging enzyme similar to SOD, which converts H2O2 to water independently[38]. In the present study, GSH-Px and total SOD were significantly lower in the elderly group compared with the young group. These results suggest that elderly patients have a higher risk of oxidative stress than younger patients and consequently greater vulnerability for chronic disease in old age. The decreased expression and activity of antioxidant enzymes will accelerate oxidative stress damage in aging vessels.
In summary, we demonstrated that oxidative stress and a decreased antioxidative defense induce vascular aging and enhance vascular dysfunction. Vascular aging of gastric mucosa may lead to blood supply insufficiency and an increased incidence of gastric diseases. This research has provided theoretical evidence suggesting that a decrease in oxidative stress during the aging process and improvement in the function of gastric submucosal vessels may be beneficial in the treatment of gastric disease in the elderly.
Aging is usually accompanied by a higher risk of gastric disease. Current opinion suggests that adequate mucosal blood flow plays an important role in maintaining mucosal integrity. The structure and function of gastric submucosal arteries are important for regulating gastric blood flow.
Mucosal blood flow plays an important role in maintaining mucosal structure, function and turnover of gastric mucosa. Numerous experimental studies have demonstrated the importance of mucosal blood flow in the defense of gastric mucosa against injury. However, few studies have directly studied the structural and functional changes in gastric submucosal vessels.
This study showed that oxidative stress and a decreased antioxidative defense induce gastric vascular aging and enhance vascular dysfunction in the elderly. The structure and function of gastric submucosal arteries are important for regulating gastric blood flow. Vascular aging of gastric mucosa leads to blood supply insufficiency and an increase in the incidence of gastric disease.
This research provides theoretical evidence to suggest that a decrease in oxidative stress during the aging process and improvement in the function of gastric submucosal arteries may be beneficial in the treatment of gastric diseases in the elderly.
Vascular aging involves vascular structure changes and dysfunction during the aging process. The aging of submucosal arteries leads to changes in mucosal blood flow which plays an important role in maintaining mucosal integrity. Inadequate blood flow will increase the incidence of gastric diseases.
The authors demonstrated that aging induces vascular dysfunction through increasing oxidative stress in isolated human gastric submucosal arterioles. This study has provided theoretical evidence that a decrease in oxidative stress during the aging process and improvement in the function of gastric submucosal arterioles may be beneficial in the treatment of gastric disease in the elderly.
P- Reviewers: Cullen JJ, Naito Y, Koch TR S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
| 1. | Grassi M, Petraccia L, Mennuni G, Fontana M, Scarno A, Sabetta S, Fraioli A. Changes, functional disorders, and diseases in the gastrointestinal tract of elderly. Nutr Hosp. 2011;26:659-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 2. | Feldman M, Cryer B, McArthur KE, Huet BA, Lee E. Effects of aging and gastritis on gastric acid and pepsin secretion in humans: a prospective study. Gastroenterology. 1996;110:1043-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 180] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | van Asselt DZ, van den Broek WJ, Lamers CB, Corstens FH, Hoefnagels WH. Free and protein-bound cobalamin absorption in healthy middle-aged and older subjects. J Am Geriatr Soc. 1996;44:949-953. [PubMed] |
| 4. | Somerville K, Faulkner G, Langman M. Non-steroidal anti-inflammatory drugs and bleeding peptic ulcer. Lancet. 1986;1:462-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 351] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Petersson J, Phillipson M, Jansson EA, Patzak A, Lundberg JO, Holm L. Dietary nitrate increases gastric mucosal blood flow and mucosal defense. Am J Physiol Gastrointest Liver Physiol. 2007;292:G718-G724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Abdel-Salam OM, Czimmer J, Debreceni A, Szolcsányi J, Mózsik G. Gastric mucosal integrity: gastric mucosal blood flow and microcirculation. An overview. J Physiol Paris. 2001;95:105-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Cheung LY, Chang N. The role of gastric mucosal blood flow and H+ back-diffusion in the pathogenesis of acute gastric erosions. J Surg Res. 1977;22:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Guth PH. Current concepts in gastric microcirculatory pathophysiology. Yale J Biol Med. 1992;65:677-688. [PubMed] |
| 9. | Kawano S, Tsuji S. Role of mucosal blood flow: a conceptional review in gastric mucosal injury and protection. J Gastroenterol Hepatol. 2000;15 Suppl:D1-D6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Leung FW, Itoh M, Hirabayashi K, Guth PH. Role of blood flow in gastric and duodenal mucosal injury in the rat. Gastroenterology. 1985;88:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 117] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Sato N, Kawano S, Tsuji S, Ogihara T, Yamada S. Gastric blood flow in ulcer diseases. Scand J Gastroenterol Suppl. 1995;208:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Stein HJ, Bauerfeind P, Hinder RA, Koerfer J, Blum AL. Luminal acid reduces gastric mucosal blood flow in the ischemic stomach. J Surg Res. 1989;46:616-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Whittle BJ, Kauffman GL, Moncada S. Vasoconstriction with thromboxane A2 induces ulceration of the gastric mucosa. Nature. 1981;292:472-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 106] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Cosentino F, Francia P, Camici GG, Pelicci PG, Lüscher TF, Volpe M. Final common molecular pathways of aging and cardiovascular disease: role of the p66Shc protein. Arterioscler Thromb Vasc Biol. 2008;28:622-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Rodriguez R, Redman R. Balancing the generation and elimination of reactive oxygen species. Proc Natl Acad Sci USA. 2005;102:3175-3176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Aslan M, Ozben T. Reactive oxygen and nitrogen species in Alzheimer’s disease. Curr Alzheimer Res. 2004;1:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Thomas MP, Chartrand K, Reynolds A, Vitvitsky V, Banerjee R, Gendelman HE. Ion channel blockade attenuates aggregated alpha synuclein induction of microglial reactive oxygen species: relevance for the pathogenesis of Parkinson’s disease. J Neurochem. 2007;100:503-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension. 2005;45:1107-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Yan C, Huang A, Wu Z, Kaminski PM, Wolin MS, Hintze TH, Kaley G, Sun D. Increased superoxide leads to decreased flow-induced dilation in resistance arteries of Mn-SOD-deficient mice. Am J Physiol Heart Circ Physiol. 2005;288:H2225-H2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Voelker R. IOM: focus on care for aging population. JAMA. 2008;299:2611-2613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Marín J. Age-related changes in vascular responses: a review. Mech Ageing Dev. 1995;79:71-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 151] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Yang JL, Weissman L, Bohr VA, Mattson MP. Mitochondrial DNA damage and repair in neurodegenerative disorders. DNA Repair (Amst). 2008;7:1110-1120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Slemmer JE, Shacka JJ, Sweeney MI, Weber JT. Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging. Curr Med Chem. 2008;15:404-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 292] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 24. | Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 455] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 25. | Rajapakse AG, Yepuri G, Carvas JM, Stein S, Matter CM, Scerri I, Ruffieux J, Montani JP, Ming XF, Yang Z. Hyperactive S6K1 mediates oxidative stress and endothelial dysfunction in aging: inhibition by resveratrol. PLoS One. 2011;6:e19237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 26. | Romano AD, Serviddio G, de Matthaeis A, Bellanti F, Vendemiale G. Oxidative stress and aging. J Nephrol. 2010;23 Suppl 15:S29-S36. [PubMed] |
| 27. | Oeseburg H, Iusuf D, van der Harst P, van Gilst WH, Henning RH, Roks AJ. Bradykinin protects against oxidative stress-induced endothelial cell senescence. Hypertension. 2009;53:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Alvarez de Sotomayor M, Mingorance C, Andriantsitohaina R. Fenofibrate improves age-related endothelial dysfunction in rat resistance arteries. Atherosclerosis. 2007;193:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Trott DW, Gunduz F, Laughlin MH, Woodman CR. Exercise training reverses age-related decrements in endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol (1985). 2009;106:1925-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Kamada T, Kawano S, Sato N, Fukuda M, Fusamoto H, Abe H. Gastric mucosal blood distribution and its changes in the healing process of gastric ulcer. Gastroenterology. 1983;84:1541-1546. [PubMed] |
| 31. | Liu L, Cui J, Song CJ, Bian JS, Sparatore A, Soldato PD, Wang XY, Yan CD. H(2)S-releasing aspirin protects against aspirin-induced gastric injury via reducing oxidative stress. PLoS One. 2012;7:e46301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3353] [Cited by in RCA: 3218] [Article Influence: 134.1] [Reference Citation Analysis (0)] |
| 33. | Metwally NS, Ali SA, Mohamed AM, Khaled HM, Ahmed SA. Levels of certain tumor markers as differential factors between bilharzial and non-biharzial bladder cancer among Egyptian patients. Cancer Cell Int. 2011;11:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Nishino T, Okamoto K, Eger BT, Pai EF, Nishino T. Mammalian xanthine oxidoreductase - mechanism of transition from xanthine dehydrogenase to xanthine oxidase. FEBS J. 2008;275:3278-3289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 266] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 35. | Vacek TP, Gillespie W, Tyagi N, Vacek JC, Tyagi SC. Hydrogen sulfide protects against vascular remodeling from endothelial damage. Amino Acids. 2010;39:1161-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Nishiyama SK, Wray DW, Richardson RS. Aging affects vascular structure and function in a limb-specific manner. J Appl Physiol (1985). 2008;105:1661-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Ozbay B, Dülger H. Lipid peroxidation and antioxidant enzymes in Turkish population: relation to age, gender, exercise, and smoking. Tohoku J Exp Med. 2002;197:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Rattan SI. Theories of biological aging: genes, proteins, and free radicals. Free Radic Res. 2006;40:1230-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |