Published online Dec 21, 2013. doi: 10.3748/wjg.v19.i47.9133
Revised: September 24, 2013
Accepted: October 19, 2013
Published online: December 21, 2013
Processing time: 226 Days and 21.2 Hours
Approximately 80%-95% of gastrointestinal stromal tumors (GISTs) show positive staining for KIT, while the other 5%-20% show negative staining. If the tumor is negative for KIT, but is positive for CD34, a histological diagnosis is possible. However, if the tumor is negative for KIT, CD34, S-100, and SMA, a definitive diagnosis is often challenging. Recently, Discovered on GIST-1 (DOG1) has received considerable attention as a useful molecule for the diagnosis of GIST. DOG1, a membrane channel protein, is known to be overexpressed in GIST. Because the sensitivity and specificity of DOG1 are higher than those of KIT, positive staining for DOG1 has been reported, even in KIT-negative GISTs. KIT-negative GISTs most commonly arise in the stomach and are mainly characterized by epithelioid features histologically. We describe our experience with a rare case of a KIT-negative GIST of the stomach that was diagnosed by positive immunohistochemical staining for DOG1 in a patient who presented with severe anemia. Our findings suggest that immunohistochemical staining for DOG1, in addition to gene analysis, is useful for the diagnosis of KIT-negative tumors that are suspected to be GISTs.
Core tip: We describe our experience with a rare case of a KIT-negative gastrointestinal stromal tumor (GIST) of the stomach that was diagnosed by positive immunohistochemical staining for Discovered on GIST-1 (DOG1) in a patient who presented with severe anemia. Our findings suggest that immunohistochemical staining for DOG1, in addition to gene analysis, is useful for the diagnosis of KIT-negative tumors that are suspected to be GISTs.
- Citation: Wada T, Tanabe S, Ishido K, Higuchi K, Sasaki T, Katada C, Azuma M, Naruke A, Kim M, Koizumi W, Mikami T. DOG1 is useful for diagnosis of KIT-negative gastrointestinal stromal tumor of stomach. World J Gastroenterol 2013; 19(47): 9133-9136
- URL: https://www.wjgnet.com/1007-9327/full/v19/i47/9133.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i47.9133
A gastrointestinal stromal tumor (GIST) is a mesenchymal tumor derived from the mesoderm that arises in the gastrointestinal tract. The estimated incidence is 2 cases per 100000 people per year. The most common age at diagnosis is 50-60 years. KIT protein is characteristically expressed by immunohistochemical staining. Gain-of-function mutations of the c-kit gene (approximately 90%) or the platelet-derived growth factor receptor alpha (PDGFRA) gene (approximately 5%) are the major causes of GISTs[1]. Immunohistochemical staining and gene analysis are considered useful for diagnosis, but if the tumor is negative for KIT, CD34, S-100, and smooth muscle actin (SMA), a definitive diagnosis is often challenging. We describe our experience with a patient in whom immunohistochemical staining for Discovered on GIST-1 (DOG1) enabled the diagnosis of a KIT-negative GIST[2].
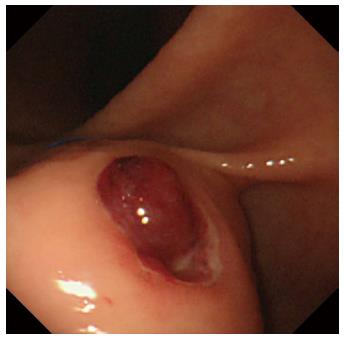
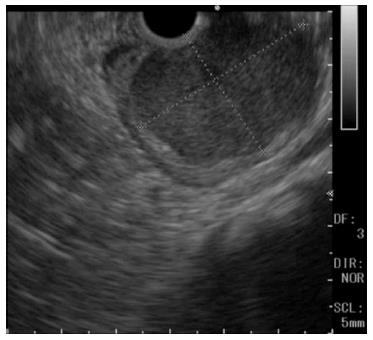
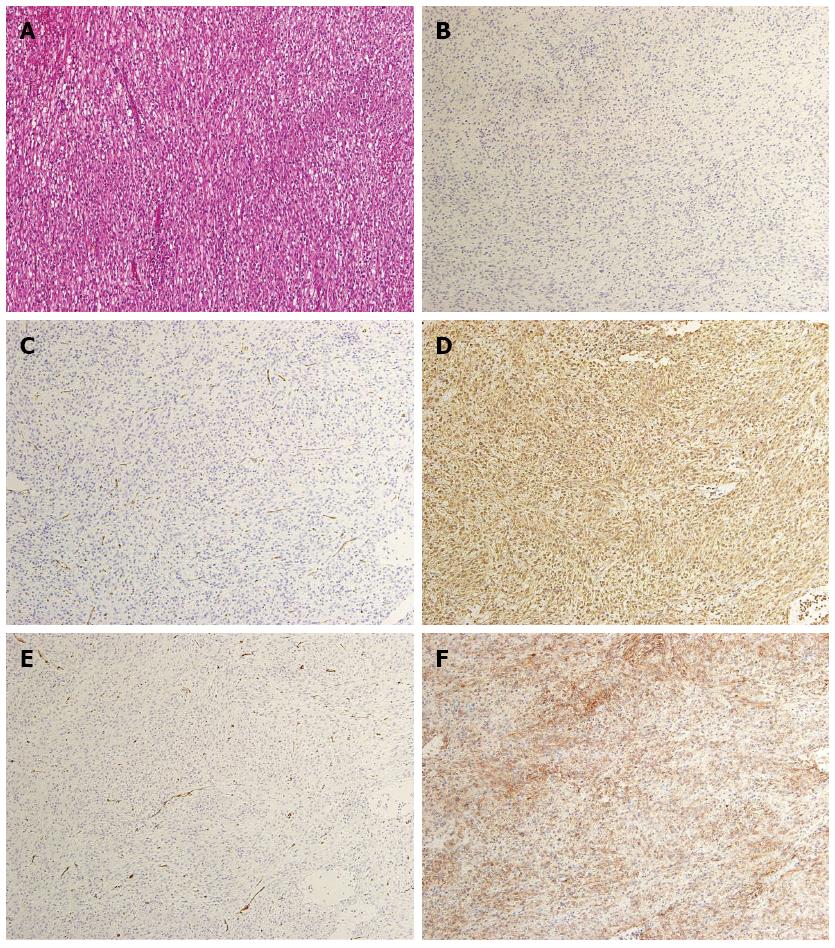
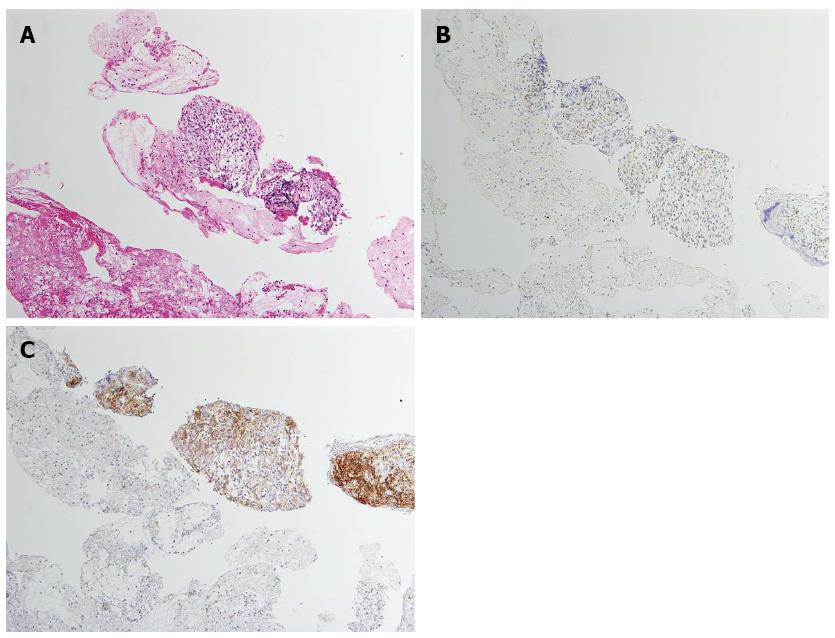
A 60-year-old man was referred to the Department of Gastroenterology of our hospital because of wooziness, shortness of breath on effort, and tarry stools. A blood test showed that the hemoglobin level was 3.6 g/dL, indicating severe anemia. Upper gastrointestinal endoscopy disclosed a submucosal tumor accompanied by an ulcer with an adherent clot, arising in the superior portion of the anterior wall of the gastric antrum (Figure 1). Endoscopic ultrasonography (EUS) revealed a well-demarcated, homogeneous, hypoechoic mass with a flat border. The mass was approximately 4 cm in diameter and arose from the fourth layer of the gastric wall (Figure 2). Endoscopic ultrasound-guided fine needle aspiration biopsy (EUS-FNAB)[3,4] was performed to obtain a definitive diagnosis and showed aggregations of cells with spindle-like or polygonal nuclei. However, immunohistochemical staining was negative for KIT, CD34, S-100, and SMA. A GIST was strongly suspected, but a definite diagnosis was not reached. Gene analysis could not be performed because the tissue sample was too small. However, the patient had a symptomatic, submucosal tumor with no distinct evidence of distant metastasis or direct invasion on enhanced computed tomography of the chest and abdomen. Surgery was, therefore, indicated according to the clinical practice guidelines for GIST in Japan[5], and a distal gastrectomy was performed. On macroscopic examination, the surgically resected specimen showed no evidence of bleeding or necrosis. The tumor measured 45 mm in diameter, and the resection margins were tumor negative. Histopathological examination showed that the tumor consisted of mixed components, including diffuse proliferations of spindle cells with eosinophilic cytoplasm, as well as epithelioid cells in some regions. One mitosis was found per 50 high-power fields, and the MIB-1 index was 3%. Immunohistochemical staining was negative for KIT, CD34, S-100, and SMA, but it was positive for vimentin and DOG1, a membrane channel protein (Figure 3). The tissue specimen obtained by EUS-FNA also stained positively for DOG1 (Figure 4). Genetic analysis showed a mutation in exon 18 (D842V) of the PDGFRA gene, with no mutation in the c-kit gene. On the basis of these results, a KIT-negative GIST with low risk according to Fletcher’s classification[6], and very low risk according to Miettinen’s classification, was diagnosed[7]. The patient recovered uneventfully after surgery. As of 3 years after surgery, the patient has been followed up on an outpatient basis and remains free of metastasis and recurrence.
GIST is a mesenchymal tumor of the mesoderm arising from the interstitial cells of Cajal in the gastrointestinal tract. The most common site is the stomach (60%), followed by the small intestine (30%), duodenum (5%), and large intestine (4%)[8]. GIST can be associated with diverse clinical symptoms, such as gastrointestinal bleeding, abdominal pain, and tumor obstruction. Histopathologically, GIST can be classified into 3 categories: spindle-cell type, epithelioid-cell type, and mixed type. Epithelioid-cell type accounts for approximately 70% of all GISTs, epithelioid-cell type accounts for approximately 20%, and mixed type, as was found in our patient, accounts for approximately 10%[8].
At present, specific tumor markers for the diagnosis of GIST are unavailable. A definite diagnosis is established by immunostaining tissue specimens obtained by EUS-FNAB or at surgery for KIT, CD34, SMA, desmin, S-100, and Ki-67[4,6]. Approximately 80%-95% of GISTs show positive staining for KIT, while the other 5%-20% show negative staining. If the tumor is negative for KIT but positive for CD34, a histological diagnosis is possible; however, if the tumor is negative for KIT, CD34, S-100, and SMA, similar to our patient, a definitive diagnosis is often challenging.
Recently, DOG1 has received considerable attention as a useful molecule for the diagnosis of GIST[2]. DOG1, a membrane channel protein, is known to be overexpressed in GIST. Because the sensitivity and specificity of DOG1 are higher than those of KIT, positive staining for DOG1 has been reported even in KIT-negative GIST[9-11]. KIT-negative GISTs most commonly arise in the stomach and are mainly characterized by epithelioid features histologically. KIT-negative GISTs are often associated with PDGFRA gene mutations[8]. Rizzardi et al[12] genetically analyzed a DOG1-positive, KIT-negative GIST of the stomach and reported the presence of a deletion in exon 14 of the PDGFRA gene, with no mutation in the c-kit gene.
In our patient, pathological examination of the surgically resected specimen showed a mixed-type GIST, including epithelioid cells. Immunostaining was negative for both KIT and CD34 but was positive for DOG1. Consistent with these findings, a mutation was found in exon 18 (D842V) of the PDGFRA gene, with no mutation in the c-kit gene. Because the D842V mutation of the PDGFRA gene is resistant to imatinib, sunitinib is prescribed when recurrence is found[1,13].
In histological specimens obtained by EUS-FNAB before surgery, immunostaining was negative for KIT. A definite diagnosis could not be made. Immunohistochemical staining for DOG1 was additionally performed and showed that the cytoplasm of the tumor cells was positively stained. Hwang et al[14] reported that DOG1 was a useful marker for the cytologic diagnosis of GIST in tissue specimens obtained by EUS-FNAB. However, one study reported that approximately 30% of KIT-negative GISTs are negative for DOG1, suggesting that tumors suspected to be GIST should be comprehensively evaluated, including analysis of other genes[10].
We have described our experience with a rare case of KIT-negative GIST of the stomach that was diagnosed by positive immunostaining for DOG1 in a patient who presented with severe anemia. Our findings suggest that immunostaining for DOG1, in addition to gene analysis, is useful for the diagnosis of KIT-negative tumors suspected to be GISTs.
P- Reviewers: Borges BD, Guo J, Tetsu O S- Editor: Zhai HH L- Editor: Logan S E- Editor: Ma S
| 1. | Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342-4349. [PubMed] |
| 2. | Sepe PS, Moparty B, Pitman MB, Saltzman JR, Brugge WR. EUS-guided FNA for the diagnosis of GI stromal cell tumors: sensitivity and cytologic yield. Gastrointest Endosc. 2009;70:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009;69:1218-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 4. | Liegl B, Hornick JL, Corless CL, Fletcher CD. Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am J Surg Pathol. 2009;33:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Nishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, Otani Y, Shimada Y, Takahashi F, Kubota T. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008;13:416-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 6. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [PubMed] |
| 7. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [PubMed] |
| 8. | Liegl-Atzwanger B, Fletcher JA, Fletcher CD. Gastrointestinal stromal tumors. Virchows Arch. 2010;456:111-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | West RB, Corless CL, Chen X, Rubin BP, Subramanian S, Montgomery K, Zhu S, Ball CA, Nielsen TO, Patel R. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165:107-113. [PubMed] |
| 10. | Espinosa I, Lee CH, Kim MK, Rouse BT, Subramanian S, Montgomery K, Varma S, Corless CL, Heinrich MC, Smith KS. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol. 2008;32:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 298] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 11. | Lee CH, Liang CW, Espinosa I. The utility of discovered on gastrointestinal stromal tumor 1 (DOG1) antibody in surgical pathology-the GIST of it. Adv Anat Pathol. 2010;17:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Rizzardi C, Marzinotto S, Avellini C, Melato M, Mariuzzi L. A KIT-negative, DOG1-positive epithelioid GIST of the stomach harboring a novel PDGFRA exon 14 single nucleotide deletion. Anticancer Res. 2012;32:1775-1778. [PubMed] |
| 13. | Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329-1338. [PubMed] |
| 14. | Hwang DG, Qian X, Hornick JL. DOG1 antibody is a highly sensitive and specific marker for gastrointestinal stromal tumors in cytology cell blocks. Am J Clin Pathol. 2011;135:448-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |