Published online Dec 21, 2013. doi: 10.3748/wjg.v19.i47.9034
Revised: October 7, 2013
Accepted: November 3, 2013
Published online: December 21, 2013
Processing time: 172 Days and 4.4 Hours
AIM: To determine if there is consistency between endoscopic ultrasound (EUS) findings and pathological results for detecting lesions of different depth in the esophageal mucosa.
METHODS: A canine (Beagle) model was established in which lesions of different depths were created in the esophageal mucosa by thermal burning. Seventy-two hours later, these lesions and adjacent tissue in the esophagus were examined by EUS. EUS findings including infiltrating depth, strength of echogenicity and homogeneity were recorded. Dogs were sacrificed and tissue specimens were obtained. We then compared the EUS findings with the pathology reports.
RESULTS: Thermal burns created at different power settings caused lesions of different depth in the esophageal mucosa. When the echo strength was shifted from high, medium, to low echogenicity, an increase in the infiltrating depth of the lesion was noted, which coincided with results of the pathology examination. Obvious submucosal edema visualized by EUS was also detected by pathology. Furthermore, because of the enhancement caused by the submucosal edema, the lesions invading into the submucosa were easily visualized by EUS.
CONCLUSION: There is consistency between EUS findings and pathological results of esophageal lesions with different depths. Submucosal edema can serve as an ultrasonic contrast agent.
Core tip: Nowadays, endoscopic ultrasound (EUS) is an optimal modality to detect early esophageal cancer (EC); however, it is still unknown whether there is correlation between EUS findings and pathological results. In this animal study, superficial esophageal lesions with different infiltrating depth in dogs were created by thermal burning. There is consistency between EUS imaging and pathology. The accompanied submucosa edema can sever as an ultrasonic contrast agent.
- Citation: Li JJ, He LJ, Shan HB, Wang TD, Xiong H, Chen LM, Xu GL, Li XH, Huang XX, Luo GY, Li Y, Zhang R. Superficial esophageal lesions detected by endoscopic ultrasound enhanced with submucosal edema. World J Gastroenterol 2013; 19(47): 9034-9042
- URL: https://www.wjgnet.com/1007-9327/full/v19/i47/9034.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i47.9034
Treatment options for esophageal cancer (EC) differ according to the depth of the lesion[1-4]. According to a recent edition of the American Joint Committee On Cancer (AJCC) and the International Union Against Cancer (UICC) staging system and guidelines, patients with early EC confined solely to the mucosa (substaged as T1a or Tm) are able to undergo endoscopic mucosal resection; however, patients with lesions invading into the submucosa (sub-staged as T1b or Tsm) require esophagectomy[5,6]. Therefore, differentiating the depth or stage of the disease is of great importance preoperatively.
Depending on the frequency strength of the probe that is placed into the lumen of the esophagus, three to seven histological layers of the esophageal wall can be discerned[7-10]. In the clinic, endoscopic ultrasonography (EUS) is superior to other modalities such as computed tomography (CT) and positron emission tomography (PET) for distinguishing the tissue layers of the esophageal wall, and it has become the method of choice for determining the depth of esophageal lesions[11-18].
However, EUS has several limitations for detecting esophageal lesions. First, the mucosal layers (including squamous epithelium, lamina propria, and muscularis mucosa) have similar echoic characteristics, especially acoustic impedance. Thus, these layers have similar echogenicity, and it is very difficult to distinguish one from another. Second, because ultrasound propagates a similar speed through the different layers of the esophageal wall, it is difficult for EUS to detect minor differences in ultrasonic energy loss (also presenting as echoic gray scale) among the layers[19,20]. Other factors that may influence the efficacy of EUS are the size of the lumen in the esophagus (which prevents the ultrasonic probe from pressing close to the mucosa), the motility of the esophagus, and the experience of the endoscopist[21,22]. Therefore, the accuracy of EUS for determining the T stage of EC is poor and diverse in the literatures[5,6]. In addition, previous reports did not provide information on the accuracy of EUS for identifying the T1 sub-stage of EC, which is an important factor by which physicians determine treatment. Our team tried to sequentially combine submucosal saline injection (SSI) with EUS to detect the T1 sub-stage of EC, and our preliminary data revealed that this technique is nearly 90% accurate. Therefore, SSI may enhance EUS for early EC diagnosis[23,24].
However, many questions about the efficacy of EUS for EC diagnosis remain[25,26]. For example, does the echo in the EUS reflect the actual structure or component of the esophageal wall? Is there consistency between EUS findings and the results of pathological examinations? What are the echoic characteristics of the water/liquid in the submucosa? Can the water/liquid enhance EUS, and if so, how? To answer these questions, we used different doses of thermal burns to create superficial lesions with different infiltrating depth in the esophageal mucosa, and EUS examinations were conducted to detect these lesions in a canine model.
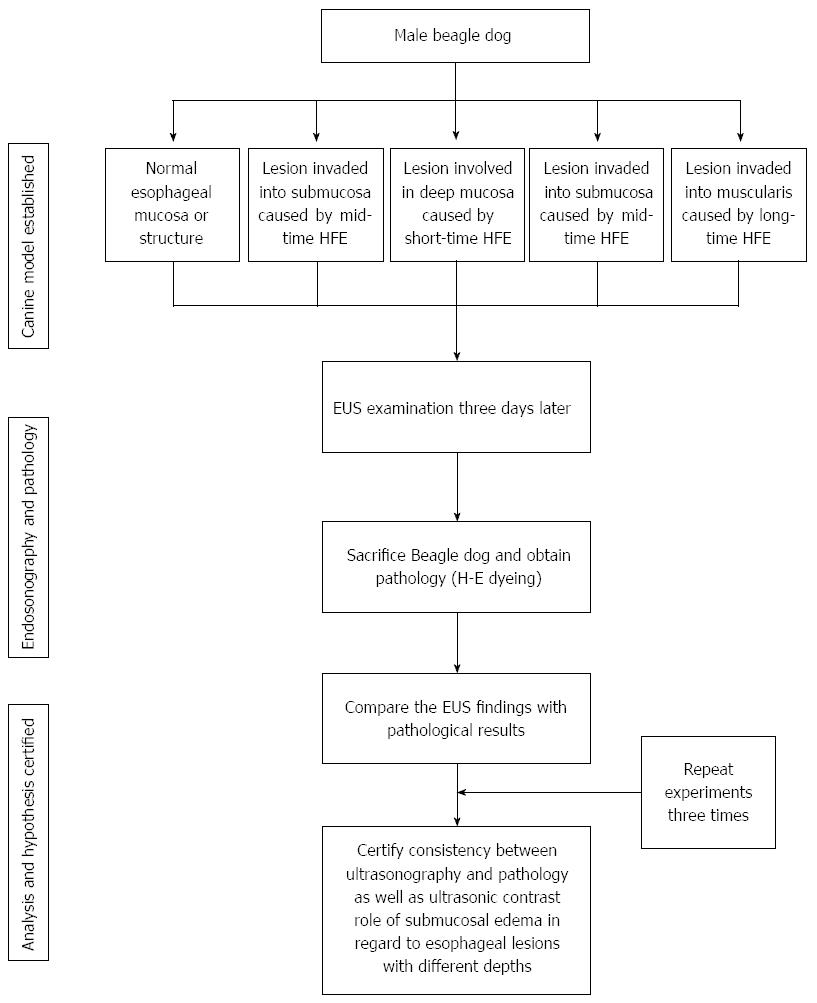
The experimental protocol used in this study was approved by the animal welfare and ethics committee of Sun Yat-sen University Cancer Center (approval number: GZR2012- 114). Male adult dogs (10 kg) were provided by the medical animal center in the north campus of Sun Yat-sen University. The flow diagram of this study is shown in Figure 1. The dogs were kept separately with an absolute diet for 8 h and dehydrated for 6 h before the experiment. Dogs were then injected intraperitoneally with 0.03 mg/kg pentobarbital sodium for premedication and then injected peritoneally with 0.03 mg/kg pentobarbital sodium per hour for maintenance.
EUS examinations were performed using an Olympus GF-UM2000 endoscopy system with a 12 MHz ultrasonic probe (Olympus Co. Ltd., Japan). An Endoscopic Electrosurgical Workstation was purchased from ERBE Co. Ltd., Germany, which included an argon plasma coagulation (APC) system and a high frequency electro-coagulation generator (HFE).
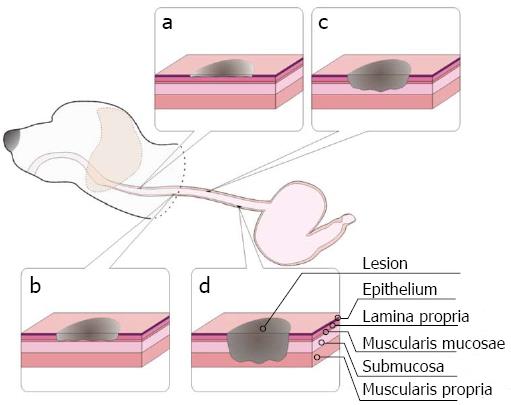
Guided by endoscopy, esophageal lesions of variable infiltration depths were induced in anesthetized dogs using APC (40 W, 1.4 L/min, 2 s each time), short-time HFE (40 W, 2 s each time), medium-time HFE (40 W, 5 s each time), and long-time HFE (40 W, 10 s each time). Superficial round lesions of approximately 1 cm × 1 cm were formed, and the gap between lesions was approximately 5 cm. Seventy-two hours later, the dogs underwent EUS examination. The echoic characteristics of the lesions with different infiltrating depths (including the echogenicity of the lesions, leading and trailing edge, the echogenicity of each layer in the esophageal wall, etc.) and submucosal edema were recorded. Then, the dogs were sacrificed, and samples of the normal and abnormal esophagus containing superficial lesions with variable infiltrating depths were extracted and stored in 10% formaldehyde solution. The specimens were stained with hematoxylin and eosin (HE). In addition, we focused on submucosal edema and its role as an ultrasonic contrast agent. We compared the EUS findings with the corresponding pathological results to determine whether both were in agreement. Details regarding the creation of this canine are presented in Figure 2. Examinations were performed by an endoscopic expert with over 10 years of experience. Similarly, pathological examinations were performed by an expert with over 10 years of experience. The above experiment was repeated three times.
After exposure to APC and HFE at different power levels, lesions in the esophageal mucosa could be observed, although the clarity was poor due to obvious congestion and edema in the adjacent mucosa. Seventy-two hours later, the edema in the surrounding mucosa decreased, and the lesions became more apparent. However, we only observed lesions in the lumen and could not confirm the exact burn depths by ordinary endoscopy. Therefore, we proceeded with EUS examination.
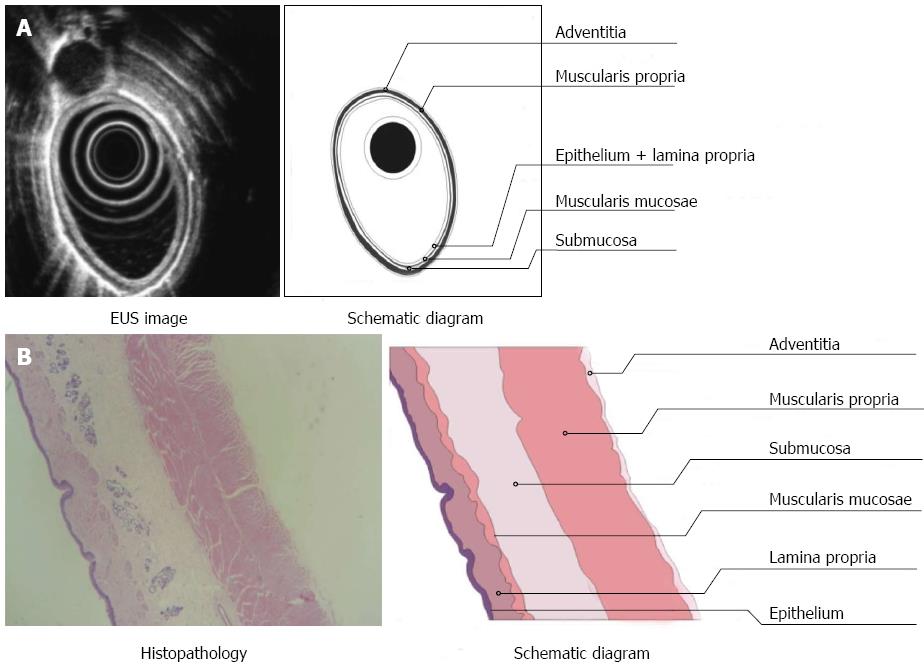
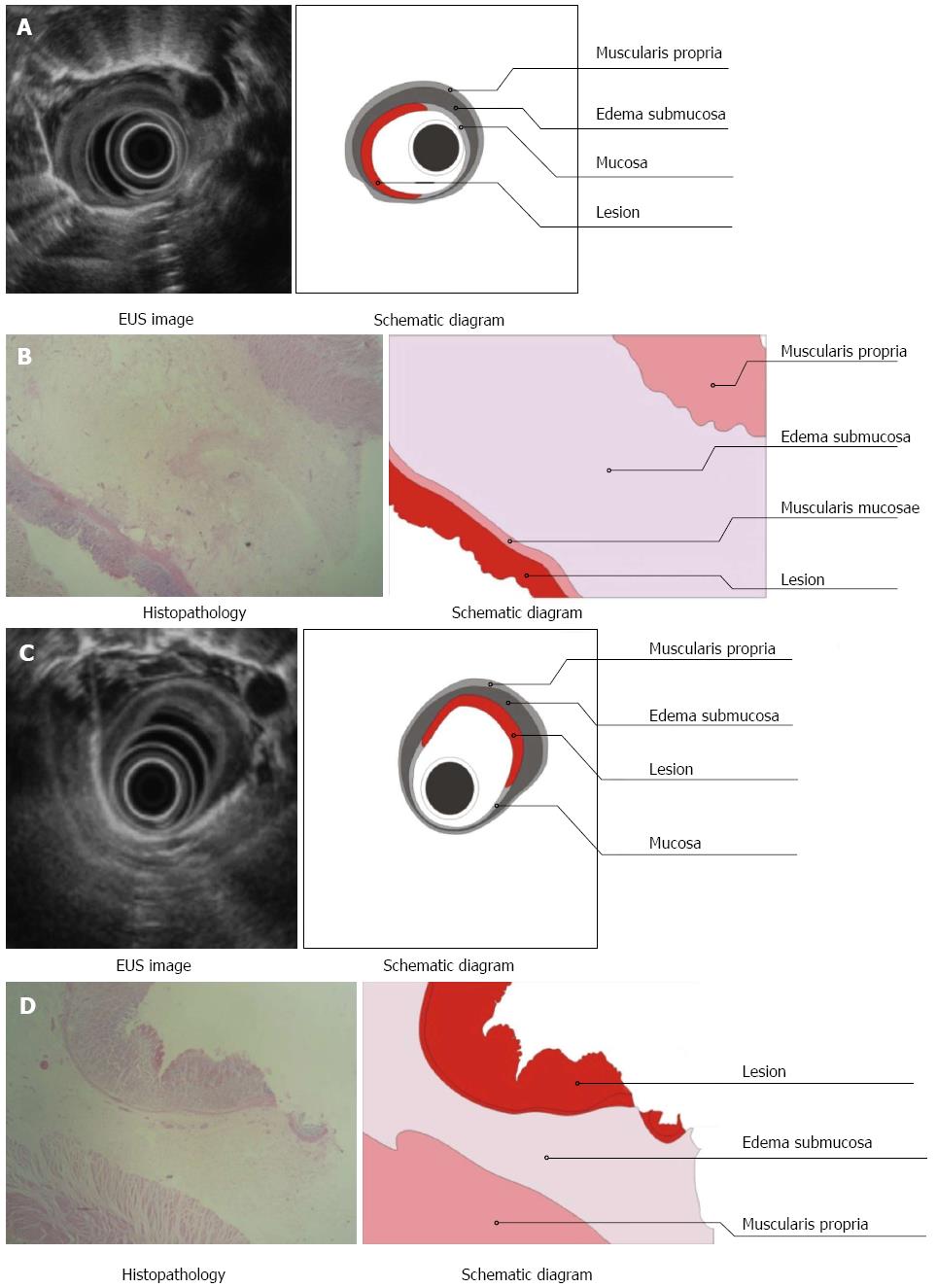
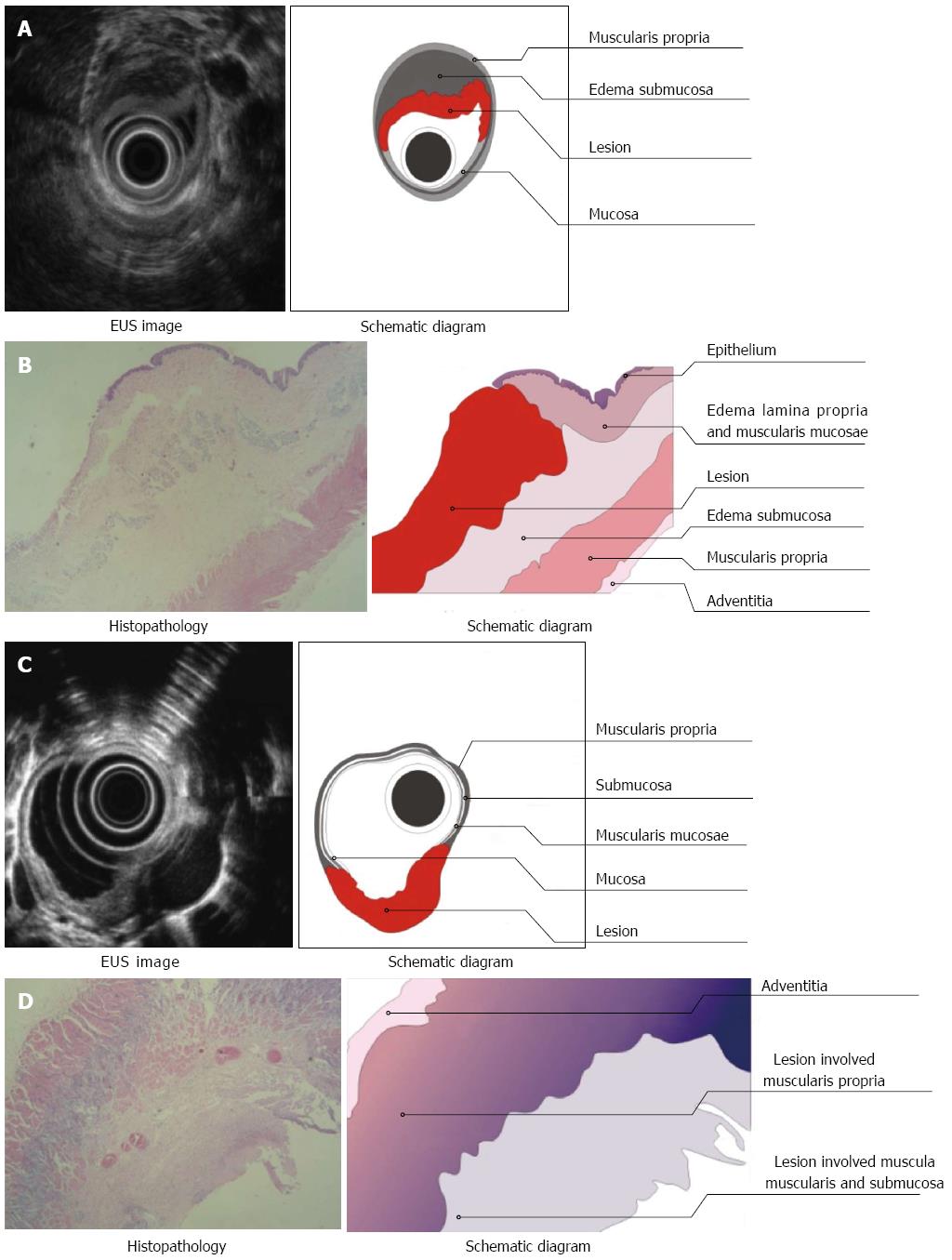
Three layers of the normal esophagus can be visualized using EUS with a 10 MHz ultrasonic probe: the thickest, high echoic belt is revealed as the first layer, which corresponds to the mucosa and submucosa; a thick, low echoic belt corresponding to muscularis propria; and a narrow, high echoic belt that is thought to be the adventitia and other dense connective tissue (Figure 3A). In the canine model, different power levels can cause different burns with variable infiltration depths, as visualized by EUS. APC (Figure 4A), short-time HFE (Figure 4C), medium-time HFE (Figure 5A), and long-time HFE (Figure 5C) resulted in superficial mucosal injury, deep mucosa injury, injury involving the submucosa, and injury invading into the muscularis propria, respectively. The lesions presented as middle to low echogenicity, which were lower and more asymmetrical echoic compared to the normal mucosa on sonography, particularly in the case of lesions invading into the muscularis propria. Additionally, we found that the high echogenicity of the mucosa was related to the integrity of the squamous epithelium, especially the keratin pearl and intercellular bridge. Once these keratin pearls or intercellular bridges disappeared, the echo of the lesion decreased as shown in Figures 3B, 4B and 5B. Furthermore, a low echoic belt was the evidence of edema in the submucosa of the lesions, except in the lesions invading the muscularis propria. The edema was observed as a low echoic belt with diverse light spots, which separated the mucosa and submucosa, as shown in Figures 4A, C, and 5C. In addition, echo enhancement was observed in the trailing edge of the low echoic belt, confirming that the low echoic belt was water-filled tissue in the submucosa. Therefore, due to the contrast of the low echoic edema, the layer of muscularis propria was displayed as a smooth, middle echo belt. However, in the case of lesions involving the muscularis propria, there was no low echoic belt in the submucosa and no obvious boundary among the layers of the esophagus.
There were, in successive order, squamous epithelium, lamina propria, muscularis mucosa, submucosa, and muscularis propria and adventitia in the normal esophageal wall of dogs. The squamous mucosa was thick, with a distinct keratin pearl, intercellular bridges, and thin lamina propria and muscularis mucosa. The submucosa was also thick, and it was characterized by blood vessels, lymphatic and connective tissues. The muscularis propria contained a ring muscle layer (inner) and a longitudinal muscle layer (outer), divided by thin connective tissue. The adventitia was composed of fibrous connective tissue (Figure 3B). Tissue sections of the lesions containing superficial mucosa showed partial epithelial degeneration with a complete squamous component and intact muscularis mucosa. Prominent submucosal edema was observed, and there were no obvious inflammatory cells in the submucosa (Figure 4B). Similarly, total degeneration was observed in the mucosa, with a partial squamous cell component and broken muscularis mucosa. Prominent submucosal edema was also found in the lesions containing deep mucosa. There were no obvious inflammatory cells in the submucosa (Figure 4D). In lesions that invaded into the submucosa, the squamous cell structure and muscularis mucosa disappeared with submucosal edema, and inflammatory cells were clearly present in the submucosa (Figure 5B). For lesions that invaded into the muscularis propria, the squamous cell structure and muscularis mucosa disappeared; however, this was not accompanied by submucosal edema and inflammatory cells in the submucosa (Figure 5D).
In this study, echogenicity reflected characteristics of tissues propagated by ultrasound (Table 1). First, there was a correlation between the echoic belts or layers in the tissue sections. The esophageal mucosa and submucosa of dogs presented as a high echoic belt on sonography with a 10 MHz probe; the second low echoic belt corresponded to the muscularis propria; and the adventitia (mainly composed of dense fibrous connective tissue) was observed as a thin high echoic belt by EUS. Second, the ultrasonic echo decreased with increasing infiltration depth, and the inner echogenicity of the lesion changed from homogeneous to heterogeneous. The echoic changes (high-middle-low) corresponded to gradual changes of the lesions with intact squamous epithelium (complete, incomplete, and totally broken) and the different infiltrating depths of the lesions (located in the mucosa, involving the submucosa, and invading into the muscularis propria). Third, the identification of submucosal edema by sonography and pathology was highly correlated. Submucosal edema was obvious in lesions which were located in the mucosa, whereas the submucosal edema was narrow in lesions involving the submucosa. However, there was no submucosal edema in lesions involving the muscularis propria. With the help of submucosal edema, the infiltrating margin of the lesion was significantly distinguished. Therefore, we were able to easily judge whether a lesion invaded into the submucosa. After long-time HFE thermal burns, a lesion with a low echoic belt extending from the lumen to the second layer was difficult to distinguish from the adjacent tissue. Pathology revealed that the lesion already invaded into the muscularis propria, and it contained a complex composition of inflammatory cells and blood/lymphatic vessels. In addition, using the contrast of submucosal edema or low echoic belt, the layers of the esophagus were apparent using both sonography and pathology.
| Tissue echogenicity | Echogenicity of submucosal edema | |||||||
| Echoic belts of esophagus | Echo strength of lesion | Involved layers | Homogeneity of lesion | Boundary among layers | Submucosal edema belt | Front edge of edema belt | Width of edema belt | |
| EUS findings | H-L-H | / | / | / | Clear | / | / | / |
| Normal mucosa | ||||||||
| Superficial mucosa | H-L-H | H | 1st | Homogeneous | Clear | YES | Smooth | Wide |
| Deep mucosa | H-L-H | H | 1st | Homogeneous | Clear | YES | Less Smooth | Middle |
| Submucosa | M-L-H | M | 1st | Heterogeneous | Clear | YES | Unsmooth | Narrow |
| Muscularis propria | L- H | L | 1st-2nd | Chaotic | Dim | None | None | None |
| Pathology results | ||||||||
| Normal | Mu/SM-MS -AD | / | / | / | Clear | / | / | / |
| Superficial mucosa | Mu/SM-MS -AD | Mu | MU | Homogeneous | Clear | Yes | Smooth | Wide |
| Deep mucosa | Mu/SM-MS -AD | Mu | MU | Homogeneous | Clear | Yes | Less smooth | Middle |
| Submucosa | SM-MS -AD | SM | Mu-SM | Heterogeneous | Clear | Yes | Unsmooth | Narrow |
| Muscularis propria | MS-AD | MS | MU-SM-MS | Chaotic | Dim | None | None | None |
As stated above, thermal burns in the esophageal mucosa of dogs caused lesions with different depths and different degrees of submucosal edema (except in the case of lesions invading into the muscularis propria). Submucosal edema displayed as a smooth, low echoic belt between the lesions and the layer of muscularis propria. Because water or liquid is a good medium for ultrasound, with trivial loss in ultrasonic energy, the mucosa was easily distinguished from the submucosa under condition of submucosal edema. The smooth leading edge of the submucosal edema identified lesions that did not invade into the submucosa, whereas an irregular leading edge of the submucosal edema indicated that the lesion invaded into the submucosa.
The strength of echogenicity depends on the energy of the reflection to the ultrasonic probe; when the reflection energy is higher, the gray scale of echogenicity is stronger[27,28]. In addition, the energy of the reflected ultrasound is correlated to the difference in acoustic impedance between the interfaces; when the difference is larger, the reflection is stronger and the echogenicity is higher. The difference of acoustic impedance depends on the tissue gradient. Generally, a homogeneous gradient in tissue reflects a small acoustic impedance difference, and the reflection of ultrasonic energy is small and the echoic gray scale is low. Alternatively, complicated tissue composition means that there are large acoustic impedance differences between interfaces, the ultrasonic energy is high and the echogenicity is strong[20,22]. It has been well established that the thickness of the ultrasonic image is in direct proportion to ultrasonic propagation time. Under conditions of similar ultrasonic transmission speed in soft tissue, we can assume that the thickness of the ultrasonic image is equal to the actual thickness. In fact, the ultrasonic image corresponds to the histological characteristics of the tissue due to the pathway of ultrasound propagation, such as extracellular water, tissue density, histological type, vessels, adipose tissue, keratin pearls, and intercellular bridges in squamous cell epithelium[23].
Therefore, the high echo belt on sonography means that there are complicated gradients and a significant difference in acoustic impedance, thus corresponding to the mucosa (including the squamous cell epithelium with keratin pearl and intercellular bridges and muscularis mucosa, which is thin and has just one-fold muscular tissue) and the submucosal layer (composed of a complex gradient of vessels, lipid, and other soft connective tissue). The second low echoic belt indicates that the homogeneous tissue is mainly composed of muscular cells, with only a trivial difference in acoustic impedance and no significant difference between interfaces; thus, this low echoic echo corresponds to the muscularis propria. The thin, third strong echoic belt is indicative of dense tissue with a significant difference in the acoustic impedance between tissues; thus, it corresponds to the adventitia and other dense connective tissue[4].
The results of this study not only confirmed that thermal burns created at different energy levels caused superficial lesions with different infiltration depths but also that the EUS findings corresponded with pathological results in a canine model. Furthermore, our results indicate that submucosal edema separates the mucosa and submucosa, which caused drastic changes in acoustic impedance between the layers of the esophagus. Moreover, submucosal edema increased the thickness of the esophagus, allowing the layers to be definitively identified by sonography. Therefore, extracellular water or edema served as an ultrasonic contrast agent (negative role). Although the lesions caused by thermal burns in the canine model differ from actual EC, we can detect EC lesions using submucosal extracellular saline or fluid injection to enhance the accuracy of EUS. This is especially useful to distinguish T1a and T1b EC in the clinic. Our team tried to combine SSI with EUS examination to increase the accuracy of EUS for the staging and sub-staging of early esophageal squamous cell carcinoma preoperatively.
The esophagus of dogs has a thicker squamous cell epithelium and muscularis propria, as well as a thinner lamina propria and muscularis mucosa than that of human beings[25]. The large number of interfaces in the squamous epithelium, such as keratin pearls, extracellular bridges, vessels and lipid tissue in the submucosa, can cause strong ultrasonic reflection. Therefore, the first layer on sonography displayed as a high echoic belt with a 10 MHz ultrasonic probe; the mucosa and the submucosa were present as a high echoic belt, and they were difficult to distinguish from each other. Hence, the first low echoic belt includes the mucosa and submucosa in the normal esophagus of dogs. Once the lesion invades the submucosa, sonography cannot easily distinguish the lesion from the submucosa[15,16]. In fact, physicians are predominantly concerned with determining whether the lesions have already invaded into the submucosa or into deeper layers because EC patients with submucosa invasion are not eligible for endoscopic resection and must have esophagectomy[29]. With the contrast of fluid in the submucosa, lesions invading into the submucosa were easily identified by EUS.
In this study, we planned to perform SSI sequentially with EUS after thermal burning at different energy levels created superficial lesions of different infiltration depths. However, in pre-experiments, we found that lesions caused by thermal burning led to significant submucosal edema, so SSI was not needed to perform in order to enhance EUS. Furthermore, as the mucosa recovered (generally longer than two weeks), the submucosal edema gradually vanished. Therefore, performing SSI after the disappearance of submucosal edema was unnecessary because the mucosa had already recovered, with no remaining lesions. Furthermore, at 72 h post-thermal burning, the superficial lesions were obvious, whereas the mucosal edema had subsided, and the submucosal edema produced an effect similarly to the saline cushion caused by SSI.
The identification of esophageal lesions with different depths using ultrasonic technology is consistent with pathological results, demonstrating that the submucosal edema can serve as an ultrasonic contrast agent.
It is well known that treatment for esophageal cancer (EC) differs according to the depth of the lesion since the T1 stage or sub-stage of EC depends on the invading depth in early EC. Endoscopic ultrasonography (EUS) is the most common modality to stage early EC preoperatively.
There are many questions about the effectiveness of EUS for EC diagnosis especially in consistency between sonographic and pathological results.
This study confirmed that there was consistency between EUS findings and pathological results of esophageal lesions with different depths. Submucosal edema can serve as an ultrasonic contrast agent.
Using submucosal saline as an ultrasonic contrast agent, the physicians may employ a novel technique-submucosal saline injection (SSI) to enhance the accuracy of EUS for staging or sub-staging early EC in clinic.
EUS is a medical procedure in which endoscopy (insertion of a probe into a hollow organ) is combined with ultrasound to obtain images of the internal organs in the chest and abdomen. It can be used to visualize the walls of these organs, or to look at adjacent structures. Endoscopic ultrasonography is most commonly used in the upper digestive tract. SSI is a technique prior to endoscopic treatment for early EC to avoid damage to adjacent tissues.
The authors present interesting data on the accuracy of EUS assessment of thermal esophageal burns, facilitated by submucosal edema in a canine model. Further studies will be required to determine the utility of submucosal fluid enhanced EUS examination of esophageal carcinoma.
P- Reviewers: Ahmed F, Tellez-Avila FI S- Editor: Zhai HH L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol. 2007;8:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 388] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 2. | Lightdale CJ. Esophageal cancer. American College of Gastroenterology. Am J Gastroenterol. 1999;94:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Moretó M. Diagnosis of esophagogastric tumors. Endoscopy. 2003;35:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Maish MS, DeMeester SR. Endoscopic mucosal resection as a staging technique to determine the depth of invasion of esophageal adenocarcinoma. Ann Thorac Surg. 2004;78:1777-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Sobin LH, Gospodarowicz MK, Wittekind Ch. TNM Classification of Malignant Tumors. 7th edn. New York: Wiley-Blackwell 2010; . |
| 6. | NCCN Practice Guidelines in Oncology - Esophageal Cancer. version 2. 2011; Available from: http://www.jnccn.org/content/9/8/830.full.pdf. |
| 7. | Lightdale CJ, Kulkarni KG. Role of endoscopic ultrasonography in the staging and follow-up of esophageal cancer. J Clin Oncol. 2005;23:4483-4489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Pfau PR, Perlman SB, Stanko P, Frick TJ, Gopal DV, Said A, Zhang Z, Weigel T. The role and clinical value of EUS in a multimodality esophageal carcinoma staging program with CT and positron emission tomography. Gastrointest Endosc. 2007;65:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Rampado S, Bocus P, Battaglia G, Ruol A, Portale G, Ancona E. Endoscopic ultrasound: accuracy in staging superficial carcinomas of the esophagus. Ann Thorac Surg. 2008;85:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Mennigen R, Tuebergen D, Koehler G, Sauerland C, Senninger N, Bruewer M. Endoscopic ultrasound with conventional probe and miniprobe in preoperative staging of esophageal cancer. J Gastrointest Surg. 2008;12:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | de Geus-Oei LF, Vriens D, Arens AI, Hutchings M, Oyen WJ. FDG-PET/CT based response-adapted treatment. Cancer Imaging. 2012;12:324-335. [PubMed] |
| 12. | Marzola MC, De Manzoni G, Grassetto G, Cordiano C, Al-Nahhas A, Alavi A, Rubello D. Extended staging of oesophageal cancer using FDG-PET - a critical appraisal. Eur J Radiol. 2012;81:21-30. [PubMed] |
| 13. | Caputo FM, Buquicchio GL. Esophageal cancer staging: the role of radiology. Rays. 2005;30:309-314. [PubMed] |
| 14. | Gretschel S, Moesta KT, Hünerbein M, Lange T, Gebauer B, Stroszczinski C, Bembenek A, Schlag PM. New concepts of staging in gastrointestinal tumors as a basis of diagnosis and multimodal therapy. Onkologie. 2004;27:23-30. [PubMed] |
| 15. | Stein HJ, Brücher BL, Sendler A, Siewert JR. Esophageal cancer: patient evaluation and pre-treatment staging. Surg Oncol. 2001;10:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Wu LF, Wang BZ, Feng JL, Cheng WR, Liu GR, Xu XH, Zheng ZC. Preoperative TN staging of esophageal cancer: comparison of miniprobe ultrasonography, spiral CT and MRI. World J Gastroenterol. 2003;9:219-224. [PubMed] |
| 17. | Crabtree TD, Yacoub WN, Puri V, Azar R, Zoole JB, Patterson GA, Krupnick AS, Kreisel D, Meyers BF. Endoscopic ultrasound for early stage esophageal adenocarcinoma: implications for staging and survival. Ann Thorac Surg. 2011;91:1509-1515; discussion 1515-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Noble F, Bailey D, Tung K, Byrne JP. Impact of integrated PET/CT in the staging of oesophageal cancer: a UK population-based cohort study. Clin Radiol. 2009;64:699-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Rösch T, Classen M. Endosonography--what are the limits in gastroenterological diagnostics? Endoscopy. 1991;23:144-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Noce JP. Fundamentals of diagnostic ultrasonography. Biomed Instrum Technol. 1990;24:456-459. [PubMed] |
| 21. | Goldstein A. Overview of the physics of US. Radiographics. 1993;13:701-704. [PubMed] |
| 22. | Taylor KJ, Wells PN. Tissue characterisation. Ultrasound Med Biol. 1989;15:421-428. [PubMed] [DOI] [Full Text] |
| 23. | Li JJ, Shan HB, Gu MF, He L, He LJ, Chen LM, Luo GY, Xu GL. Endoscopic ultrasound combined with submucosal saline injection for differentiation of T1a and T1b esophageal squamous cell carcinoma: a novel technique. Endoscopy. 2013;45:667-670. [PubMed] |
| 24. | Li JJ, Shan HB, Xu GL, He LJ, Xia JC. Submucosal saline solution injection combined with endosonography for distinguishing between stages T1a and T1b of early esophageal cancer. Gastrointest Endosc. 2013;77:159-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Rösch T. Progress in endoscopy: areas of current interest and topics to watch out for. Endoscopy. 2012;44:1148-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Hiele M, De Leyn P, Schurmans P, Lerut A, Huys S, Geboes K, Gevers AM, Rutgeerts P. Relation between endoscopic ultrasound findings and outcome of patients with tumors of the esophagus or esophagogastric junction. Gastrointest Endosc. 1997;45:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Pavlov K, Maley CC. New models of neoplastic progression in Barrett’s oesophagus. Biochem Soc Trans. 2010;38:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Shinkai M, Niwa Y, Arisawa T, Ohmiya N, Goto H, Hayakawa T. Evaluation of prognosis of squamous cell carcinoma of the oesophagus by endoscopic ultrasonography. Gut. 2000;47:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Ell C, May A, Pech O, Gossner L, Guenter E, Behrens A, Nachbar L, Huijsmans J, Vieth M, Stolte M. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc. 2007;65:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 342] [Article Influence: 19.0] [Reference Citation Analysis (0)] |