Published online Dec 21, 2013. doi: 10.3748/wjg.v19.i47.9012
Revised: July 17, 2013
Accepted: September 16, 2013
Published online: December 21, 2013
Processing time: 328 Days and 18.2 Hours
AIM: To study the epidemiologic changes of gastroenteropancreatic neuroendocrine tumors (GEP-NET) in Germany, we analyzed two time periods 1976-1988 and 1998-2006.
METHODS: We evaluated epidemiological data of GEP-NET from the former East German National Cancer Registry (DDR Krebsregister, 1976-1988) and its successor, the Joint Cancer Registry (GKR, 1998-2006), which was founded after German reunification. Due to a particularly substantial database the epidemiological data from the federal states of Mecklenburg-Western Pomerania, Saxony, Brandenburg and Thuringia, covering a population of more than 10.8 million people, were analyzed. Survival probabilities were calculated using life table analysis. In addition, GEP-NET patients were evaluated for one or more second (non-GEP-NET) primary malignancies.
RESULTS: A total of 2821 GEP neuroendocrine neoplasms were identified in the two registries. The overall incidence increased significantly between 1976 and 2006 from 0.31 (per 100.000 inhabitants per year) to 2.27 for men and from 0.57 to 2.38 for women. In the later period studied (2004-2006), the small intestine was the most common site. Neuroendocrine (NE) neoplasms of the small intestine showed the largest absolute increase in incidence, while rectal NE neoplasms exhibited the greatest relative increase. Only the incidence of appendiceal NET in women showed little change between 1976 and 2006. Overall survival of patients varied for sex, tumor site and the two periods studied but improved significantly over time. Interestingly, about 20% of the GEP-NET patients developed one or more second malignancies. Their most common location was the gastrointestinal tract. GEP-NET patients without second malignancies fared better than those with one or more of them.
CONCLUSION: The number of detected GEP-NET increased about 5-fold in Germany between 1976 and 2006. At the same time, their anatomic distribution changed, and the survival of GEP-NET patients improved significantly. Second malignancies are common and influence the overall survival of GEP-NET patients. Thus, GEP-NET warrant our attention as well as intensive research on their tumorigenesis.
Core tip: Modern endoscopic and radiological tumor imaging have been implicated in the rise of the incidence of detected neuroendocrine tumors (NET) in Western countries. The particularities of German history, which resulted in two German states with two different health care systems from 1949-1989, allowed to study the epidemiological changes of NET in Germany on the background of two health care systems in 1949-1989. The number of detected gastroenteropancreatic-NET increased about 5-fold between 1976 and 2006. Most likely, the general availability of endoscopy after German reunification contributed to the major rise in frequency of detected rectal, gastric and duodenal NET in the new federal states of reunified Germany.
- Citation: Scherübl H, Streller B, Stabenow R, Herbst H, Höpfner M, Schwertner C, Steinberg J, Eick J, Ring W, Tiwari K, Zappe SM. Clinically detected gastroenteropancreatic neuroendocrine tumors are on the rise: Epidemiological changes in Germany. World J Gastroenterol 2013; 19(47): 9012-9019
- URL: https://www.wjgnet.com/1007-9327/full/v19/i47/9012.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i47.9012
Gastroenteropancreatic neuroendocrine tumors (GEP-NET) are infrequent, constituting only 1%-2% of all neoplasms. Most commonly they present as indolent, slow-growing tumors[1-4]. Their anatomic distribution reflects that of the neuroendocrine cells from which they derive: up to 65% in the gastrointestinal tract, about 25% in the bronchopulmonary system, and the remaining 10% at other sites[1,5].
In Western countries, pancreatic neuroendocrine tumors are diagnosed in 0.5-1 per 100000 inhabitants and represent 1%-2% of all clinically manifest pancreatic neoplasms[6-8]. GEP-NET occur in approximately 2.0-2.5 per 100000, carcinoid syndrome being most frequently associated with NET of the jejunum and ileum[9-13].
Previous and current WHO classifications distinguish between well-differentiated and poorly differentiated neoplasms. All well-differentiated neoplasms, whether benign or metastatic, are now called neuroendocrine (NE) and are graded as G1 (Ki-67 ≤ 2%) or G2 (Ki-67: 3%-20%). The poorly differentiated neoplasms are called neuroendocrine carcinomas and are graded as G3 (Ki-67 > 20%). The term “carcinoid” is now synonymous with G1 well-differentiated neuroendocrine tumor.
Population-based data from the Third National Cancer Survey and the United States Surveillance Epidemiology and End Results (SEER) Program, covering 10%-14% of the United States population, show a steady increase in the incidence of NET throughout the 35-year period between 1969 and 2004[14-17]. The overall incidence of GEP-NET increased two- to three-fold during this period, and there were significant changes in the anatomic distribution. Thus, the proportion of GEP-NET located in the appendix decreased from 43% to 4% with corresponding increases in the stomach (from 2% to 9%), small intestine (from 31% to 42%), and rectum (from 15% to 27%)[8,16]. The observed changes may in part reflect the increased number of asymptomatic GEP-NET incidentally identified thanks to increased availability of modern endoscopic and radiological imaging[3,8,18].
There have been few studies on GEP-NET epidemiology in Germany[17], and no comprehensive and comparative epidemiological studies have as yet been published on GEP-NET at various locations.
Therefore we evaluated epidemiological data from the former East German National Cancer Registry (DDR Krebsregister) for 1976-1988 and from its successor, the Joint Cancer Registry (GKR) for 1998-2006. After German reunification, the East German registry was renamed and thus became the GKR of the new federal states of Germany and Berlin. After an interruption of several years, it continued to collect epidemiological data. Thanks to a particularly substantial database we analyzed epidemiological data from the federal states of Mecklenburg-Western Pomerania, Saxony, Brandenburg and Thuringia, covering a population of over 10.8 million people.
The official population statistics for Germany as reported by the German government were used to estimate the incidence rate of GEP-NET. Absolute numbers were used to estimate the crude incidence rate both in former East Germany and - after German reunification - in the new federal states, including Berlin. Since other federal states, including Berlin, had a less extensive database, their data were not used in the analysis presented here.
The study included all persons (living in Mecklenburg-Western Pomerania, Saxony, Brandenburg or Thuringia) diagnosed with GEP-NET between 1976 and 1988 or between 1998 and 2006. Mortality data from 1976 to 1990 and from 1998 to 2009 were used. We included all patients with NE tumors at the following tumor sites according to ICD10: C15-C25, D37.1-D37.5 (esophagus, stomach, small intestine, large intestine, appendix, rectosigmoid, rectum, anus and pancreas), C26.0, C26.8-9, D37.7, D37.9 (unspecified location in the digestive tract) and an NE morphology code according to ICD-O-3: 8150-8153, 8155-8157, 8240-8246, 8249 or 8574.
Annual incidence rates were calculated for the periods 1976-1988 and 1998-2006. Incidence rates are presented for each tumor site, sex and age group. Trends in incidence are presented as overall change throughout the two periods and between two representative 2-year periods, i.e., 1976-1978 and 2004-2006. The absolute number of GEP-NET reported per 100.000 inhabitants per year is referred to as the “crude incidence rate”.
Age-adjusted incidence rates were calculated using the World Standard Population published in 1966[5] and the 1987 German standard population.
Survival probabilities were calculated using life table analysis. Since data were available only on the time but not the cause of death, only the overall survival of the registered GEP-NET patients could be determined. Tumor-specific survival could not be analyzed. The Wilcoxon-Gehan test was used to determine significance when comparing the survival rates.
In addition, all GEP-NET patients were evaluated for one or more second primary malignancies. Second (non-GEP-NET) neoplasms were analyzed for location of the second primary.
From 1976 to 1988 and from 1998 to 2006, a total of 2821 cases of GEP-NET were registered in Mecklenburg-Western Pomerania, Saxony, Brandenburg and Thuringia - 1001 cases from 1976 to 1988 and 1820 cases from 1998 to 2006. The total patient population comprised 1291 men (45.8%) and 1530 women (54.2%).
In 2006, a total of 10837539 persons lived in Mecklenburg-Western Pomerania, Saxony, Brandenburg and Thuringia - 5329539 men (49.2%) and 5508000 women (50.8%).
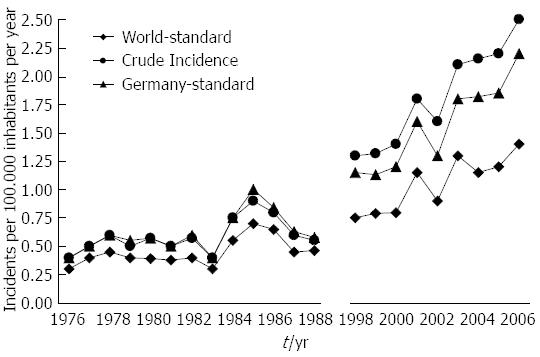
The crude incidence rate of GEP-NET (per year and 100.000 population) rose from 0.45 in 1976 to 2.53 in 2006, which corresponds to a 462% increase. The incidence rate increased by 342% when age-adjusted to the 1966 world population and by 270% when adjusted to the 1987 population of Germany (for details, Figure 1).
The crude incidence rate increased more prominently in men (from 0.31 in 1976 to 2.7 in 2006) than in women (from 0.57 in 1976 to 2.38 in 2006).
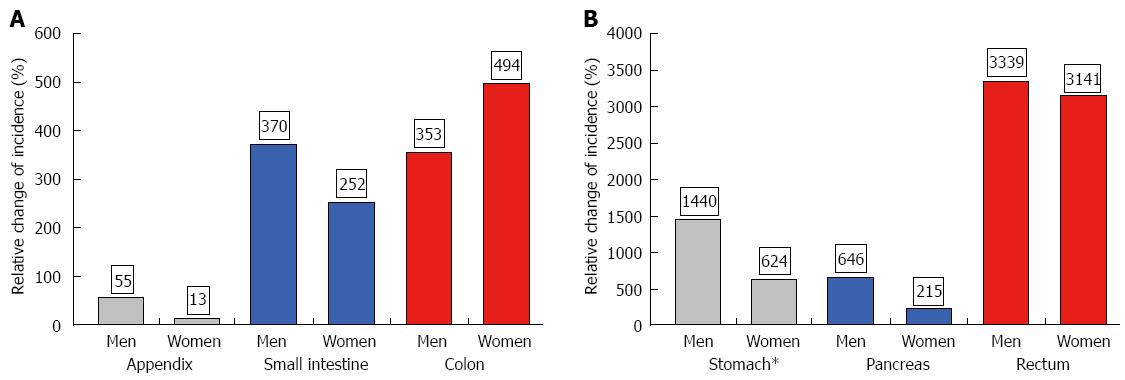
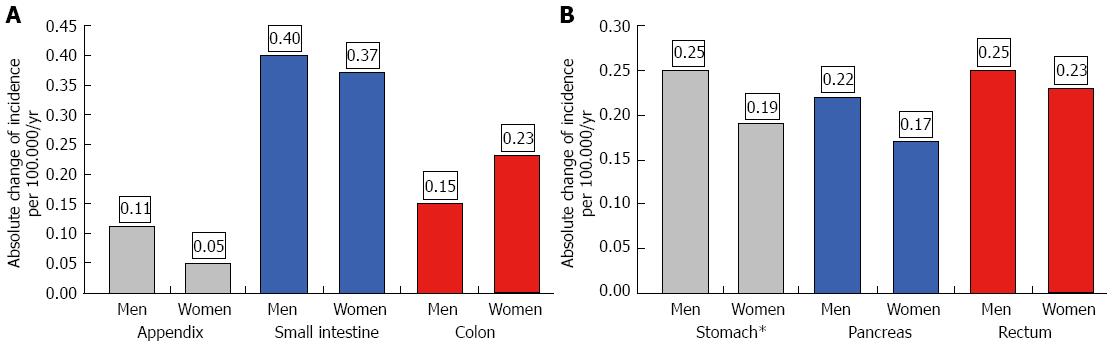
The age-adjusted (population of Germany in 1987) incidence is given in Table 1 for the periods 1976-1988 and 1998-2006. Both age-adjusted and crude incidence rates increased at all tumor sites. When comparing representative time intervals such as 1976-1978 and 2004-2006, the largest increment in absolute numbers was found for the small intestine; the absolute increase in crude incidence was 0.56 in men and 0.48 in women. Focusing on relative changes revealed the largest increase for rectal NE neoplasms; the crude incidence rose by about 6000% in men and by more than 2700% in women. In contrast, hardly any change was observed for appendiceal NE neoplasms in the female population between 1976 and 2006 (see Figures 2 and 3).
| Period | Stomach (M/F)1 | Small intestine (M/F)1 | Pancreas (M/F)1 | Appendix (M/F)1 | Colon (M/F)1 | Rectum (M/F)1 |
| 1976-1978 | 0.02/0.00 | 0.11/0.15 | 0.03/0.08 | 0.20/0.35 | 0.04/0.05 | 0.01/0.01 |
| 1979-1981 | 0.04/0.03 | 0.10/0.11 | 0.07/0.014 | 0.15/0.34 | 0.04/0.07 | 0.01/0.03 |
| 1982-1984 | 0.04/0.04 | 0.16/0.12 | 0.11/0.08 | 0.15/0.35 | 0.04/0.07 | 0.03/0.02 |
| 1985-1988 | 0.05/0.04 | 0.20/0.18 | 0.10/0.10 | 0.24/0.35 | 0.08/0.15 | 0.03/0.07 |
| 1989-1997 | ||||||
| 1998-2000 | 0.16/0.12 | 0.39/0.31 | 0.22/0.18 | 0.13/0.28 | 0.19/0.19 | 0.09/0.10 |
| 2001-2003 | 0.18/0.18 | 0.44/0.40 | 0.25/0.25 | 0.23/0.46 | 0.22/0.23 | 0.16/0.15 |
| 2004-2006 | 0.27/0.23 | 0.51/0.52 | 0.25/0.25 | 0.31/0.39 | 0.20/0.28 | 0.26/0.24 |
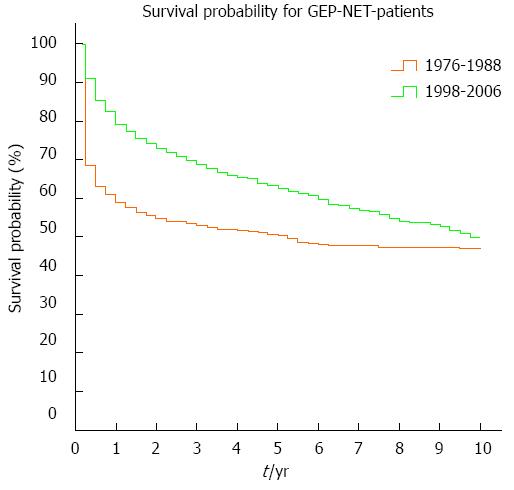
Overall survival increased significantly between the time periods 1976-1988 and 1998-2006 (P < 0.001). The 1-, 5- and 10-year overall survival rates were 59%, 50% and 47% for the earlier period and 79%, 63% and 50% for the later period (Figure 4).
Overall survival differed significantly (P < 0.001) between men and women. In the earlier period (1976-1988), 51% of men and 64% of women were alive after 1 year; 43% of men and 55% of women stayed alive after 5 years, and 41% of men and 51% of women were alive after 10 years. In the later period (1998-2006), 1-year survival was 75% for men and 83% for women; 5-year survival had increased to 57% for men and 68% for women, and 10-year survival was 42% for men and 58% for women. Overall survival differed not only for sex but also for the primary tumor site. Table 2 shows significant differences in survival for various anatomic locations of the primary as well as for the two time periods (1976-1988 and 1998-2006).
| Overall survival/period | Stomach | Small intestine | Pancreas | Appendix | Colon | Rectum |
| 1-yr/1976-1988 | 22% | 30% | 26% | 95% | 35% | 50% |
| 5-yr/1976-1988 | 11% | 18% | 11% | 92% | 16% | 37% |
| 10-yr/1976-1988 | 5% | 10% | 8% | 90% | 13% | 37% |
| 1-yr/1998-2006 | 71% | 85% | 74% | 95% | 68% | 74% |
| 5-yr/1998-2006 | 53% | 68% | 52% | 86% | 48% | 65% |
| 10-yr/1998-2006 | 43% | 53% | 35% | 81% | 34% | 50% |
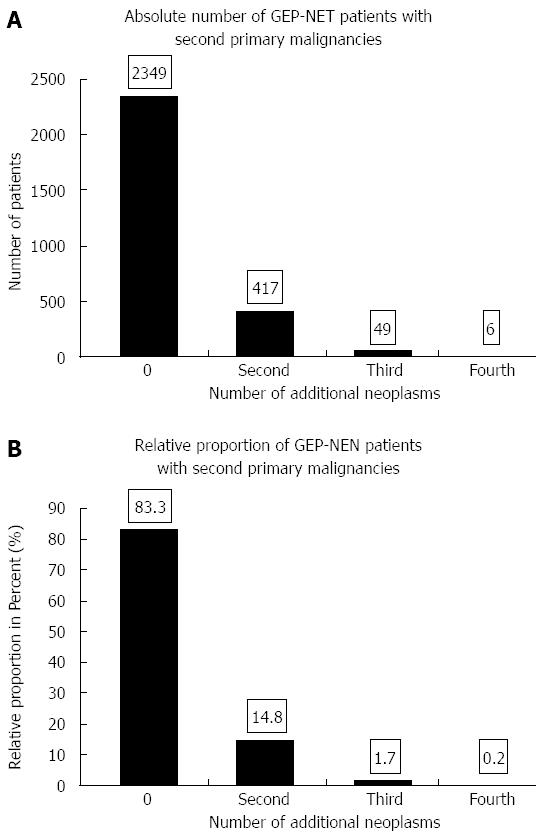
Of the 2821 NE tumor patients diagnosed between 1976-1988 and 1998-2006, 472 developed 533 second malignancies (Figure 5). The 533 second malignancies were diagnosed as pre-existing, synchronous or metachronous malignancies during the same time intervals as the GEP-NET (1976-1988 or 1998-2006); 16.7% of the GEP-NET patients suffered from second neoplasms.
GEP-NET patients without second malignancies fared better than those with one or more of them. An analysis of all 2821 GEP-NET patients showed significantly better 5- and 10-year overall survival for those without than for those with one or more second malignancies (5-year survival of 60% vs 53%; 10-year survival of 52% vs 42%, P < 0.05).
The main locations of second malignancies were the digestive tract (28%), the female genital organs (12%), the skin (12%), the breasts (7%) and the male genital organs (7%).
Analysis of DDR Krebsregister for the period 1976-1988 and its successor, the GKR, for the period 1998-2006 revealed major increases in the incidence of detected GEP-NET in Germany. Our findings are in line with a recent report from the United States American SEER registry[8,16]. Recent epidemiological data from England and Norway also showed increases[8,14]. The current incidence of GEP-NET in Germany compares well with the incidence rates found in the United States and Australia as well as in several other European countries, i.e., 1.33 to 3.8 per 100.000[8,14,16]. Despite these similarities, there are a number of important differences.
The nomenclature and classifications of NE neoplasms have not been uniform in the last 35 years[17]. Thus the low incidence rates from 1976-1988 may in part reflect differences between the classification and nomenclature used by East German pathologists (of the DDR Krebsregister) and those applied elsewhere. On the other hand, recent improvements in the general awareness and immunohistological diagnosis of NE neoplasms may have contributed to increased incidences in the last 20 years.
Moreover, colorectal cancer screening and the general availability of high-resolution endoscopy and radiological imaging may well have facilitated the detection of early asymptomatic GEP-NET. The hypothesis is supported by studies demonstrating that the incidence and anatomic distribution differ significantly between tumors detected post mortem and those diagnosed clinically[1,7,8]. These studies suggest that most small (≤ 1 cm) GEP-NET remain asymptomatic and were generally not diagnosed in the era before the widespread availability of high-resolution endoscopy and computed tomography (CT) imaging.
The question arises whether the increased detection of early (asymptomatic) NET disease has contributed to recent epidemiological trends. In recent years, localized NET constitute by far the largest subgroup in the SEER registry and are largely responsible for the increased incidence of GEP-NET[3,18]. Consistent with this notion, Japanese, South Korean and Polish endoscopy screening programs detected rectal NET in 50-70 of 100000 persons screened[19-21]. The vast majority of rectal NE neoplasms detected by screening are 1 cm or smaller in diameter. Comparison with historical registries shows that screening is associated with a shift to smaller-sized rectal NET[3,18]. A national program of endoscopic colorectal cancer screening was introduced in Germany in October 2002. Screening colonoscopy is now offered free of charge to any person aged 55 years or older. Both colonoscopy and esophagogastroduodenoscopy are now readily available in Germany. In former East Germany (up to 1989), on the other hand, gastrointestinal endoscopy was available only at 3-4 centers.
The now widespread availability of endoscopy and radiological imaging may well have contributed to the observed increases in gastroduodenal, rectal and pancreatic NET[18,22-26]. On the other hand, the incidence of appendiceal NET remained quite stable in the Eastern parts of Germany from 1976 to 2006. Even today, they are generally diagnosed postoperatively in patients who undergo appendectomy for suspected appendicitis. Endoscopy and radiological imaging probably do not have much impact on their early detection (≤ 1 cm). Instead, they are found incidentally in one out of 200-300 appendectomy specimens. Appendectomies are among the most common surgical procedures performed in Germany. They account for 135.000 procedures per year. This contributes to the frequent detection of early appendiceal NET[27].
In line with recent reports from England[28] and Austria[27], we observed a large increase in the incidence of gastric NET. In the current analysis, however, the nature of the epidemiological data does not enable an examination of underlying causes. Noteworthy is the fact that the incidence rates of both gastric and rectal carcinoids are most likely underestimated in DDR Krebsregister as well as in its successor registry, the GKR of the new federal states, including Berlin. This is due to the fact that only malignant NE neoplasms had to be reported to either registry. Thus, well-differentiated small (< 1-2 cm) carcinoids of the stomach or rectum were probably not consistently documented in the past. Recent prospective data from Austria identify the stomach and colorectum as the most common sites of GEP-NET[27]. The Austrian observation has been confirmed by a retrospective study including 150 consecutive GEP-NET patients diagnosed at the Vivantes Hospitals in Berlin between 2005 and 2009. The stomach was the most frequent site of GEP-NET in the Vivantes Hospitals, closely followed by the small intestine and colorectum (data not shown).
Overall survival of GEP-NET patients has improved significantly in Germany during the last 35 years. This applies to both sexes and all examined anatomic sites except the appendix. In the latter location, overall survival decreased in women and remained unchanged in men. But even in the period 1998-2006, the 5- and 10-year survival reached 86% and 81%. The significant improvement in overall survival of GEP-NET patients can probably be attributed to earlier diagnosis, the greater effectiveness of modern multimodal treatment strategies, and the general increase in life expectancy.
Only a few studies have reported on the frequency of one or more second malignancies in GEP-NET patients. Second primaries were found in 16.7% of our GEP-NET patients. This is consistent with data from Florida (23.6%), a meta-analysis from 13 studies including more than 5000 GEP-NET patients (17%), and the SEER registry (22.4%)[15,29-31]. At 28%, the incidence of these second primaries was highest in the gastrointestinal tract and much lower at 12% in both the female genital tract and the skin. These data on second malignancies are consistent with the observations reported in the studies mentioned above.
GEP-NET patients appear to have an increased risk of second malignancies, although there is an ongoing debate. In their review, Habal et al[29] summarize several theories regarding the influence of NET on the emergence and growth of second malignancies. They estimated that the risk of developing a second tumor is twice as high for patients with GEP-NET than for those with other neoplasms[29]. Several studies have examined amines, peptides, growth hormones and other compounds secreted by NET for their relation to the formation and growth of neoplasms in the breast or gastrointestinal tract[31-33].
Due to the high prevalence of second neoplasms, screening for other malignancies seems advisable in GEP-NET patients. Remarkably, Zar et al[34] observed that many GEP-NET patients died of their second malignancies but not of their GEP-NET. Consistent with the data of Zar et al[34], our GEP-NET patients with one or more second malignancies did not fare as well as those without them.
We conclude that both the frequency of detected GEP-NET and the overall survival of GEP-NET patients have increased significantly in Germany between 1976 and 2006. These epidemiological changes warrant our attention. Future research efforts will focus on the carcinogenesis of GEP-NET.
This publication is part of the medical thesis (“Doktorarbeit”) submitted by Zappe SM to the Charité-Universitätsmedizin Berlin.
Neuroendocrine tumors belong to the three malignancies that increase most in frequency in Western countries.
The genetic footprints of neuroendocrine neoplasias are about to be unravelled.
The frequency of second malignancies in neuroendocrine tumors (NET) patients is highlighted in this paper. The power of screening endoscopy in detecting small neuroendocrine tumors (e.g., of the rectum) is evidenced by its availability in former East Germany after German reunification in 1989.
The general availability of modern endoscopy enables the (early) detection of small neuroendocrine tumors of the stomach, duodenum and rectum. Detection of small asymptomatic neuroendocrine tumors appears to have contributed to their epidemiological rise.
The authors describe an increase of gastroenteropancreatic-NET (GEP-NET) for the time period 1977-1988 to 1998-2006 by five-fold. However, they quite clearly demonstrated in the discussion section that this increase is mainly due to different reasons: Nomenclature has been changed; improvement of general awareness and immunological diagnoses; availability of the German National Programme of Colorectal Cancer Screening since October 2002; better imaging diagnoses. An important finding of the project is that almost 17 percent of GEP-NEN patients showed second primary malignancies and therefore screening for other malignancies in those patients should be important for the future.
P- Reviewers: Bloomston M, Filsch UR S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Berge T, Linell F. Carcinoid tumours. Frequency in a defined population during a 12-year period. Acta Pathol Microbiol Scand A. 1976;84:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 320] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 3. | Scherübl H, Jensen RT, Cadiot G, Stölzel U, Klöppel G. Management of early gastrointestinal neuroendocrine neoplasms. World J Gastrointest Endosc. 2011;3:133-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer. 2005;103:1587-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 220] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Doll R, Payne PM, Waterhouse JAH. Cancer incidence in five countries. International Union Against Cancer. Berlin: Springer-Verlag 1966; . |
| 6. | Eriksson B, Oberg K. Neuroendocrine tumours of the pancreas. Br J Surg. 2000;87:129-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 7. | Lam KY, Lo CY. Pancreatic endocrine tumour: a 22-year clinico-pathological experience with morphological, immunohistochemical observation and a review of the literature. Eur J Surg Oncol. 1997;23:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1183] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 9. | Quaedvlieg PF, Visser O, Lamers CB, Janssen-Heijen ML, Taal BG. Epidemiology and survival in patients with carcinoid disease in The Netherlands. An epidemiological study with 2391 patients. Ann Oncol. 2001;12:1295-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Oberg K, Astrup L, Eriksson B, Falkmer SE, Falkmer UG, Gustafsen J, Haglund C, Knigge U, Vatn MH, Välimäki M. Guidelines for the management of gastroenteropancreatic neuroendocrine tumours (including bronchopulmonary and thymic neoplasms). Part I-general overview. Acta Oncol. 2004;43:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Oberg K, Astrup L, Eriksson B, Falkmer SE, Falkmer UG, Gustafsen J, Haglund C, Knigge U, Vatn MH, Välimäki M. Guidelines for the management of gastroenteropancreatic neuroendocrine tumours (including bronchopulmonary and thymic neoplasms). Part II-specific NE tumour types. Acta Oncol. 2004;43:626-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Plöckinger U, Rindi G, Arnold R, Eriksson B, Krenning EP, de Herder WW, Goede A, Caplin M, Oberg K, Reubi JC. Guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumours. A consensus statement on behalf of the European Neuroendocrine Tumour Society (ENETS). Neuroendocrinology. 2004;80:394-424. [PubMed] |
| 13. | Tomassetti P, Campana D, Piscitelli L, Casadei R, Nori F, Brocchi E, Santini D, Pezzilli R, Corinaldesi R. Endocrine tumors of the ileum: factors correlated with survival. Neuroendocrinology. 2006;83:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Hauso O, Gustafsson BI, Kidd M, Waldum HL, Drozdov I, Chan AK, Modlin IM. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer. 2008;113:2655-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 385] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 15. | Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1852] [Article Influence: 84.2] [Reference Citation Analysis (1)] |
| 16. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3246] [Article Influence: 190.9] [Reference Citation Analysis (0)] |
| 17. | Stang A, Stegmaier C, Eisinger B, Stabenow R, Metz KA, Jöckel KH. Descriptive epidemiology of small intestinal malignancies: the German Cancer Registry experience. Br J Cancer. 1999;80:1440-1444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Scherübl H. Rectal carcinoids are on the rise: early detection by screening endoscopy. Endoscopy. 2009;41:162-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Kaminski M, Polkowski M, Regula J. Prevalence and endoscopic features of rectal neuroendocrine tumors (carcinoids) among 50148 participants of the Polish colorectal-cancer screening programe. Gut. 2007;56:A310. |
| 20. | Matsui K, Iwase T, Kitagawa M. Small, polypoid-appearing carcinoid tumors of the rectum: clinicopathologic study of 16 cases and effectiveness of endoscopic treatment. Am J Gastroenterol. 1993;88:1949-1953. [PubMed] |
| 21. | Shim KN, Yang SK, Myung SJ, Chang HS, Jung SA, Choe JW, Lee YJ, Byeon JS, Lee JH, Jung HY. Atypical endoscopic features of rectal carcinoids. Endoscopy. 2004;36:313-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Klöppel G, Scherübl H. [Neuroendocrine neoplasms of the appendix and colorectum]. Pathologe. 2011;32:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Ito T, Sasano H, Tanaka M, Osamura RY, Sasaki I, Kimura W, Takano K, Obara T, Ishibashi M, Nakao K. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 261] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 24. | Kvols LK, Brendtro KL. The North American Neuroendocrine Tumor Society (NANETS) guidelines: mission, goals, and process. Pancreas. 2010;39:705-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Scherübl H, Cadiot G, Jensen RT, Rösch T, Stölzel U, Klöppel G. Neuroendocrine tumors of the stomach (gastric carcinoids) are on the rise: small tumors, small problems? Endoscopy. 2010;42:664-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Scherübl H, Jensen RT, Cadiot G, Stölzel U, Klöppel G. Neuroendocrine tumors of the small bowels are on the rise: Early aspects and management. World J Gastrointest Endosc. 2010;2:325-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 27. | Niederle MB, Hackl M, Kaserer K, Niederle B. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. Endocr Relat Cancer. 2010;17:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 323] [Article Influence: 21.5] [Reference Citation Analysis (1)] |
| 28. | Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am J Gastroenterol. 2010;105:2563-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 29. | Habal N, Sims C, Bilchik AJ. Gastrointestinal carcinoid tumors and second primary malignancies. J Surg Oncol. 2000;75:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Perez EA, Koniaris LG, Snell SE, Gutierrez JC, Sumner WE, Lee DJ, Hodgson NC, Livingstone AS, Franceschi D. 7201 carcinoids: increasing incidence overall and disproportionate mortality in the elderly. World J Surg. 2007;31:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Gerstle JT, Kauffman GL, Koltun WA. The incidence, management, and outcome of patients with gastrointestinal carcinoids and second primary malignancies. J Am Coll Surg. 1995;180:427-432. [PubMed] |
| 32. | Rehfeld JF. The new biology of gastrointestinal hormones. Physiol Rev. 1998;78:1087-1108. [PubMed] |
| 33. | Zucker KA, Longo WE, Modlin IM, Bilchik AJ, Adrian TE. Malignant diathesis from jejunal-ileal carcinoids. Am J Gastroenterol. 1989;84:182-186. [PubMed] |
| 34. | Zar N, Garmo H, Holmberg L, Hellman P. Risk of second primary malignancies and causes of death in patients with adenocarcinoma and carcinoid of the small intestine. Eur J Cancer. 2008;44:718-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |