Published online Dec 14, 2013. doi: 10.3748/wjg.v19.i46.8647
Revised: September 21, 2013
Accepted: September 29, 2013
Published online: December 14, 2013
Processing time: 182 Days and 4.4 Hours
AIM: To assess adherence with the the Society for Healthcare Epidemiology of America (SHEA)/ the Infectious Diseases Society of America (IDSA) guidelines for management of Clostridium difficile (C. difficile)-associated disease (CDAD) at a tertiary medical center.
METHODS: All positive C. difficile stool toxin assays in adults between May 2010 and May 2011 at the University of Maryland Medical Center were identified. CDAD episodes were classified as guideline adherent or non-adherent and these two groups were compared to determine demographic and clinical factors predictive of adherence. Logistic regression analysis was performed to assess the effect of multiple predictors on guideline adherence.
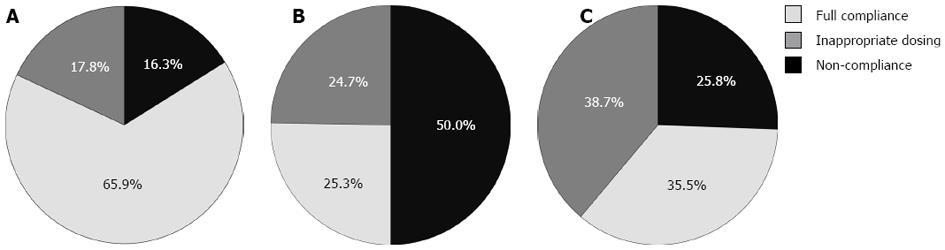
RESULTS: 320 positive C. difficile stool tests were identified in 290 patients. Stratified by disease severity criteria set forth by the SHEA/IDSA guidelines, 42.2% of cases were mild-moderate, 48.1% severe, and 9.7% severe-complicated. Full adherence with the guidelines was observed in only 43.4% of cases. Adherence was 65.9% for mild-moderate CDAD, which was significantly better than in severe cases (25.3%) or severe-complicated cases (35.5%) (P < 0.001). There was no difference in demographics, hospitalization, ICU exposure, recurrence or 30-d mortality between adherent and non-adherent groups. A multivariate model revealed significantly decreased adherence for severe or severe-complicated episodes (OR = 0.18, 95%CI: 0.11-0.30) and recurrent episodes (OR = 0.46, 95%CI: 0.23-0.95).
CONCLUSION: Overall adherence with the SHEA/IDSA guidelines for management of CDAD at a tertiary medical center was poor; this was most pronounced in severe, severe-complicated and recurrent cases. Educational interventions aimed at improving guideline adherence are warranted.
Core tip: This study assesses a tertiary care medical center’s adherence with updated guidelines on the management of Clostridium difficile (C. difficile)-associated diseases in adults. We found that overall adherence is poor, especially in patients with severe disease. Factors associated with poor adherence and limitations of current guidelines are identified. Our data suggests that educational interventions aimed at improving C. difficile guideline adherence are warranted.
-
Citation: Curtin BF, Zarbalian Y, Flasar MH, Rosenvinge EV.
Clostridium difficile -associated disease: Adherence with current guidelines at a tertiary medical center. World J Gastroenterol 2013; 19(46): 8647-8651 - URL: https://www.wjgnet.com/1007-9327/full/v19/i46/8647.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i46.8647
Clostridium difficile (C. difficile) is the major infectious cause of nosocomial diarrhea and can cause prolonged hospital stays, renal failure, toxic megacolon, and death[1-3]. In 1995, the Society for Healthcare Epidemiology of America (SHEA) published a clinical position paper on C. difficile-associated disease (CDAD)[4]. Based on data from small, randomized, controlled studies showing no outcome-difference when comparing metronidazole and vancomycin, the 1995 position paper considered them equally effective; however, it stated, “metronidazole may be preferred to reduce the risk of vancomycin resistance among other organisms in hospitals”.
Updated clinical practice guidelines for the management of CDAD in adults were published in 2010 by SHEA and the Infectious Diseases Society of America (IDSA)[5]. The 15-year interval between the two sets of recommendations was marked by dramatic changes in CDAD epidemiology and outcomes, with increases in prevalence, severity, and therapy resistance; emergence of hypervirulent strains may have contributed to these trends[6-8]. Additionally, new data suggested vancomycin might be superior for CDAD treatment in some cases. Zar et al[9] prospective, randomized, comparative efficacy study of metronidazole vs vancomycin demonstrated superiority of vancomycin for the treatment of severe CDAD. These results influenced the 2010 SHEA/IDSA guidelines that recommended vancomycin as first-line treatment for severe CDAD, while maintaining a recommendation for metronidazole in mild-moderate cases. These guidelines recommend treating an initial recurrence in the same manner as the initial episode, and a second recurrence with vancomycin in a tapering and/or pulsed regimen[5].
The 2010 SHEA /IDSA recommendations promote significant clinical practice changes. Since adherence to the guidelines may affect patient outcomes and infection control, we sought to determine adherence with the updated SHEA/IDSA CDAD guidelines at a tertiary care medical center.
The Institutional Review Board of the University of Maryland Baltimore approved this study and waived the requirement for informed consent. All positive C. difficile stool tests (Quick Check A/B Toxin Assay; Wampole Laboratories, Princeton, New Jersey) in adults between May 2010 and May 2011 at the University of Maryland Medical Center were retrospectively identified. Medical charts were reviewed for demographics, clinical information, and adherence to CDAD guidelines.
Classifications defined in the updated 2010 SHEA/IDSA guidelines were used. These guidelines define mild-moderate CDAD as the presence of a white blood cell count ≤ 15000/mm3 and a serum creatinine level ≤ 1.5 times the premorbid level. Conversely, severe CDAD is defined by the presence of a white blood cell count ≥ 15000/mm3 or a serum creatinine level ≥ 1.5 times the premorbid level. Severe-complicated CDAD is defined by the presence of hypotension, shock, ileus, or megacolon. According to the guidelines, the correct treatment for mild-moderate CDAD is metronidazole 500 mg orally three times per day for 10-14 d. For treatment of severe CDAD, recommended treatment is oral vancomycin 125 mg four times per day for 10-14 d. For severe-complicated CDAD, the recommended treatment is oral vancomycin 500 mg four times per day in addition to intravenous metronidazole 500 mg every eight hours. If complete ileus exists, then rectal administration of vancomycin should be considered. Patients with a first recurrence are recommended to receive the same treatment as per their initial episode. For a second recurrence, vancomycin in a tapered and/or pulsed regimen is recommended.
Specific data collected included age, gender, disease severity as defined by the 2010 SHEA/IDSA guidelines, location of treatment (stratified into outpatient, hospital ward or intensive care unit), non-CDAD antibiotic treatment during the month preceding diagnosis, presence of immunosuppression, if the episode was a recurrence, 30 d mortality, and agent selection and dosage of CDAD treatment. CDAD episodes were classified as guideline adherent if treatment provided was with the correct agent(s) at the correct dosage(s). If one of these parameters was not in accordance with the guidelines, then the treatment regimen was deemed non-adherent. Partial adherence was defined as the patient receiving the correct antibiotic, but at the wrong dose. Patients stratified into adherent and non-adherent groups were compared to determine demographics and clinical factors predictive of guideline adherence. Logistic regression analysis was performed to assess the effect of multiple predictors on guideline adherence (SAS, version 9.2).
About 320 positive C. difficile stool tests were identified in 290 patients (average age 57.6 years, 43.1% female). Of the cases, 95.9% were in hospitalized patients and 15.6% were identified as a recurrence. Stratified by disease severity criteria set forth by the SHEA/IDSA guidelines, 42.2% of cases were mild-moderate, 48.1% severe, and 9.7% severe-complicated. Most (80.6%) of the severe-complicated cases met this criterion due to hypotension or shock. Full adherence with the guidelines was observed in 43.4% of cases; 65.9% for mild-moderate, which was significantly better than in severe (25.3%) and severe-complicated cases (35.5%) (P < 0.001) (Figure 1). Of the severe CDAD cases, 55.3% were managed incorrectly with metronidazole. Partial adherence, where the correct drug was given at the incorrect dose, occurred in 17.8% of mild-moderate, 24.7% of severe, and 38.7% of severe-complicated cases (Figure 1).
On bivariate analysis (Table 1), factors significantly associated with adherence included disease severity, immunosuppression (IS), and documented receipt of antibiotics in the preceding 30 d. There was no difference in age, gender, hospitalization, ICU exposure, recurrence or 30-d mortality between adherent and non-adherent groups. IS patients were classified as mild-moderate more often than non-IS patients (60.0% vs 32.9%, P < 0.001). A multivariate model controlling for disease severity, prior antibiotics, IS, and recurrence status revealed significantly decreased adherence for severe/severe-complicated episodes (OR = 0.18, 95%CI: 0.11-0.30) and recurrent episodes (OR = 0.46, 95%CI: 0.23-0.95) but no significant difference for prior antibiotics or IS status.
| Guideline | Guideline | Unadjusted | Adjusted | |
| Compliant, | Non-compliant, | P value | P value | |
| n = 139 | n = 181 | |||
| Demographics | ||||
| Mean ± SD, yr | 56.8 ± 14.1 | 59.4 ± 16.2 | 0.13 | |
| Female | 61 (44.2) | 77 (55.8) | 0.81 | |
| Disease severity | ||||
| Mild-moderate | 89 (65.9) | 46 (34.1) | < 0.0011 | < 0.0011 |
| Severe | 39 (25.3) | 115 (74.7) | ||
| Severe-complicated | 11 (35.5) | 20 (64.5) | ||
| Severe + severe-complicated | 50 (27.0) | 135 (73.0) | ||
| Other factors | ||||
| Hospitalized | 133 (43.3) | 174 (56.7) | 0.84 | |
| ICU | 60 (40.0) | 90 (60.0) | 0.24 | |
| Prior antibiotics (< 30 d) | 88 (39.1) | 137 (60.9) | 0.02 | 0.08 |
| Recurrence | 17 (34.0) | 33 (66.0) | 0.14 | 0.04 |
| Immunosuppressed | 57 (51.8) | 53 (49.2) | 0.03 | 0.49 |
| 30-d mortality | 15 (41.7) | 21 (58.3) | 0.82 |
Our results reveal poor overall adherence with the 2010 SHEA/IDSA guidelines for management of CDAD at a tertiary care academic medical center. Guideline adherence is worst in severe, severe-complicated, and recurrent CDAD. Our data suggests a lack of familiarity with current guidelines, as most providers continue to treat all initial episodes of CDAD with metronidazole, which was suggested as preferable by the 1995 SHEA clinical position paper on CDAD management. In fact, over half of our severe CDAD population, which should be treated with vancomycin, was incorrectly treated with metronidazole. This also explains the significantly improved adherence observed in mild-moderate patients whose treatment was not changed by the updated guidelines. We considered other possible causes of guideline non-adherence, such as the high cost of vancomycin and concern for vancomycin-resistance in other organisms, which has been shown to be significant in other nosocomial settings[10,11]. While the cost of branded oral vancomycin is approximately fifty-fold higher than oral metronidazole, our pharmacy routinely administers the generic intravenous formulation orally, which reduces the cost-difference dramatically[12], and makes cost concerns negligible. This finding also suggests an increased need for more intensive antibiotic stewardship, as not all incidences of non-adherence are likely due to knowledge. Antibiotic stewardship has been proposed as an effective method of increasing compliance at medical centers[13-15]. The exact impact of concerns over vancomycin resistance in other organisms on prescribing practices at our institution is unknown. We suspect this impact is small, as research on vancomycin use for CDAD has been conflicting with regards to rates of colonization and infection with resistant organisms[16-18].
Partial adherence with the guidelines, where the correct drug was chosen but an incorrect dosage was administered, occurred frequently as noted in Figure 1. The dosage of vancomycin chosen was often higher than recommended by the guidelines. While this is a form of non-adherence, it may be appropriate, as patients given the recommended dosage (125 mg four times daily) can have low fecal levels during the first day of treatment[19]. Additionally, one would expect a higher dose to be equally effective and, given poor systemic absorption, little difference in side effects.
Interestingly, we found clusters of cases in specific wards of the hospital, such as the Trauma Unit or the Cancer Center, suggesting nosocomial spread of the infection, which is a major source of morbidity, mortality, and cost from the disease[20-22]. This finding highlights the need for improved hand hygiene and infection control measures such as contact precautions.
Two patient populations expose limitations of applying the SHEA/IDSA guidelines: immunosuppressed patients and patients with end-stage renal disease. As noted in our results, immunosuppressed patients were significantly more likely to have their disease severity classified as mild-moderate. This may reflect an inability to mount a severe-defining white blood cell count. The frequent incidence of neutropenia among cancer patients in particular suggests a limitation of applying the guidelines to this group. Similarly, the end-stage renal disease population, in whom serum creatinine fluctuations cannot be used as indicators of disease severity, present a problem with applying the SHEA/IDSA guidelines.
The present work has limitations. Our study was retrospective, took place in a single tertiary medical center, and 95.9% of CDAD cases were in hospitalized patients. Therefore, our results may not be applicable to community hospitals or outpatients. Additionally, our study was not powered to detect differences in 30-d mortality. Our institution was also using a toxin assay for diagnosis at the time of data collection, which is less sensitive than PCR[23,24]. Furthermore, the study by Zar et al[9] that was used to develop the treatment guidelines has had questions raised with regards to its methodology[25,26]. Further studies that directly survey providers about their treatment practices and measure the effects of guideline adherence on mortality and morbidity factors such as length of stay and complications of CDAD are warranted.
In conclusion, our results suggest that many providers are unfamiliar with the 2010 SHEA/IDSA C. difficile guidelines. Educational interventions and antibiotic stewardship may prove beneficial to improve adherence, and potentially patient outcomes.
Clostridium difficile (C. difficile) remains a major cause of nosocomial diarrhea, resulting in prolonged hospital stays, renal failure, toxic megacolon, and death. This also contributes to a significant economic burden on medical facilities across the globe. While the number of nosocomial infections increases, the correct treatment for this disease is of paramount importance.
In 1995, the Society for Healthcare Epidemiology of America (SHEA) published guidelines that suggested that Metronidazole is the preferred agent for treatment of C. difficile. Recently, updated clinical practice guidelines for the management of C. difficile colitis have been published by SHEA and the Infectious Diseases Society of America (IDSA) suggesting that oral Vancomycin be preferred in cases of severe and severe-complicated disease, but adherence to these new guidelines is unclear at this time. In this study, the authors observe compliance to the new 2010 guidelines at a tertiary medical center.
Despite advances in health care sanitation technique, Clostridium infections continue to increase. In this study, the authors observed that compliance to the updated 2012 SHEA/IDSA guidelines is poor at the tertiary care hospital, suggesting a need for increased education and antibiotic stewardship for providers. The authors also identified specific areas that the guidelines fail to address clearly; end-stage renal disease patients and patients who are significantly immunosuppressed.
By recognizing poor compliance at our tertiary care facility, steps can be made to increase education and antibiotic stewardship at other facilities. In addition, the study suggests the guidelines should be updated to include the aforementioned patient populations with specific guidelines pertaining to their management.
Compliance in the study is defined as using the proper dosage (both strength and frequency) in the proper duration for a specific C. difficile-associated diarrhea (CDAD) infection. The guidelines define mild to moderate CDAD as the presence of a white blood cell count ≤ 15000/mm3 and a serum creatinine level ≤ 1.5 times the premorbid level. Conversely, severe CDAD is defined by the presence of a white blood cell count ≥ 15000/mm3 or a serum creatinine level ≥ 1.5 times the premorbid level. Severe-complicated CDAD is defined by the presence of hypotension, shock, ileus, or megacolon.
The authors report the results of a study conducted to assess adherence with the SHEA/IDSA guidelines for management of CDAD at a tertiary medical center. The study is well-designed, includes sufficient number of patients and the paper is well written. Despite the fact that the study is single-centered, and includes only hospitalized patients which reduces its generalizability (as mentioned by the authors), the results are considerable. This is a worthy study and appears of high clinical interest.
P- Reviewers: De Francesco V, Hadianamrei R, Soki J, Tarchini G S- Editor: Wen LL L- Editor: A E- Editor: Wang CH
| 1. | McFarland LV. Epidemiology of infectious and iatrogenic nosocomial diarrhea in a cohort of general medicine patients. Am J Infect Control. 1995;23:295-305. [PubMed] |
| 2. | Miller MA, Hyland M, Ofner-Agostini M, Gourdeau M, Ishak M. Morbidity, mortality, and healthcare burden of nosocomial Clostridium difficile-associated diarrhea in Canadian hospitals. Infect Control Hosp Epidemiol. 2002;23:137-140. [PubMed] |
| 3. | Marra AR, Edmond MB, Wenzel RP, Bearman GM. Hospital-acquired Clostridium difficile-associated disease in the intensive care unit setting: epidemiology, clinical course and outcome. BMC Infect Dis. 2007;7:42. [PubMed] |
| 4. | Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16:459-477. [PubMed] |
| 5. | Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2285] [Cited by in RCA: 2201] [Article Influence: 220.1] [Reference Citation Analysis (0)] |
| 6. | Jury LA, Sitzlar B, Kundrapu S, Cadnum JL, Summers KM, Muganda CP, Deshpande A, Sethi AK, Donskey CJ. Outpatient healthcare settings and transmission of Clostridium difficile. PLoS One. 2013;8:e70175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Zerey M, Paton BL, Lincourt AE, Gersin KS, Kercher KW, Heniford BT. The burden of Clostridium difficile in surgical patients in the United States. Surg Infect (Larchmt). 2007;8:557-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Nylund CM, Goudie A, Garza JM, Fairbrother G, Cohen MB. Clostridium difficile infection in hospitalized children in the United States. Arch Pediatr Adolesc Med. 2011;165:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307. [PubMed] |
| 10. | Dong D, Zhang L, Chen X, Jiang C, Yu B, Wang X, Peng Y. Antimicrobial susceptibility and resistance mechanisms of clinical Clostridium difficile from a Chinese tertiary hospital. Int J Antimicrob Agents. 2013;41:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Weber I, Riera E, Déniz C, Pérez JL, Oliver A, Mena A. Molecular epidemiology and resistance profiles of Clostridium difficile in a tertiary care hospital in Spain. Int J Med Microbiol. 2013;303:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Pham P, Bartlett JG. Vancomycin and Metronidazole in Johns Hopkins poc-it HIV guide. Available from: http://www.hopkinsguides.com/hopkins/ub/view/Johns_Hopkins_HIV_Guide/545217/all/Vancomycin. Accessed on 9 July 2012. |
| 13. | Drew RH. Antimicrobial stewardship programs: how to start and steer a successful program. J Manag Care Pharm. 2009;15:S18-S23. [PubMed] |
| 14. | Goff DA. Antimicrobial stewardship: bridging the gap between quality care and cost. Curr Opin Infect Dis. 2011;24 Suppl 1:S11-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Van Schooneveld T. Antimicrobial stewardship: attempting to preserve a strategic resource. J Community Hosp Intern Med Perspect. 2011;1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Miller M, Bernard L, Thompson M, Grima D, Pepin J. Lack of increased colonization with vancomycin-resistant enterococci during preferential use of vancomycin for treatment during an outbreak of healthcare-associated Clostridium difficile infection. Infect Control Hosp Epidemiol. 2010;31:710-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Fujitani S, George WL, Morgan MA, Nichols S, Murthy AR. Implications for vancomycin-resistant Enterococcus colonization associated with Clostridium difficile infections. Am J Infect Control. 2011;39:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Grima DT, Webb GF, D’Agata EM. Mathematical model of the impact of a nonantibiotic treatment for Clostridium difficile on the endemic prevalence of vancomycin-resistant enterococci in a hospital setting. Comput Math Methods Med. 2012;2012:605861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Gonzales M, Pepin J, Frost EH, Carrier JC, Sirard S, Fortier LC, Valiquette L. Faecal pharmacokinetics of orally administered vancomycin in patients with suspected Clostridium difficile infection. BMC Infect Dis. 2010;10:363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Weber DJ, Rutala WA, Miller MB, Huslage K, Sickbert-Bennett E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control. 2010;38:S25-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 510] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 21. | Salgado CD, Mauldin PD, Fogle PJ, Bosso JA. Analysis of an outbreak of Clostridium difficile infection controlled with enhanced infection control measures. Am J Infect Control. 2009;37:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Vonberg RP, Reichardt C, Behnke M, Schwab F, Zindler S, Gastmeier P. Costs of nosocomial Clostridium difficile-associated diarrhoea. J Hosp Infect. 2008;70:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Baker I, Leeming JP, Reynolds R, Ibrahim I, Darley E. Clinical relevance of a positive molecular test in the diagnosis of Clostridium difficile infection. J Hosp Infect. 2013;84:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Wilcox MH. Overcoming barriers to effective recognition and diagnosis of Clostridium difficile infection. Clin Microbiol Infect. 2012;18 Suppl 6:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Drekonja DM. Vancomycin “telephone”. Clin Infect Dis. 2011;53:211-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Tarchini G. Clostridium difficile disease and vancomycin--questionable clinical superiority. Clin Infect Dis. 2011;53:212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |