Published online Dec 14, 2013. doi: 10.3748/wjg.v19.i46.8562
Revised: March 25, 2013
Accepted: April 27, 2013
Published online: December 14, 2013
Processing time: 277 Days and 15.8 Hours
Celiac disease (CD) is an autoimmune disease of the small bowel induced by ingestion of wheat, rye and barley. Current guidelines indicate histological analysis on at least four duodenal biopsies as the only way to diagnose CD. These indications are based on the conception of the inability of standard endoscopy to make diagnosis of CD and/or to drive biopsy sampling. Over the last years, technology development of endoscopic devices has greatly ameliorated the accuracy of macroscopic evaluation of duodenal villous pattern, increasing the diagnostic power of endoscopy of CD. The aim of this paper is to review the new endoscopic tools and procedures proved to be useful in the diagnosis of CD, such as chromoendoscopy, Fujinon Intelligent Chromo Endoscopy, Narrow Band Imaging, Optical Coherence Tomography, Water-Immersion Technique, confocal laser endomicroscopy, high-resolution magnification endoscopy, capsule endoscopy and I-Scan technology.
Core tip: Celiac disease (CD) is an autoimmune disorder induced, in genetically predisposed people, by the ingestion of proteins rich in proline and glutamine. The aim of this review is to focus on the new endoscopic tools and techniques developed over the last years which can be useful in the diagnosis and the follow-up of CD.
- Citation: Ianiro G, Gasbarrini A, Cammarota G. Endoscopic tools for the diagnosis and evaluation of celiac disease. World J Gastroenterol 2013; 19(46): 8562-8570
- URL: https://www.wjgnet.com/1007-9327/full/v19/i46/8562.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i46.8562
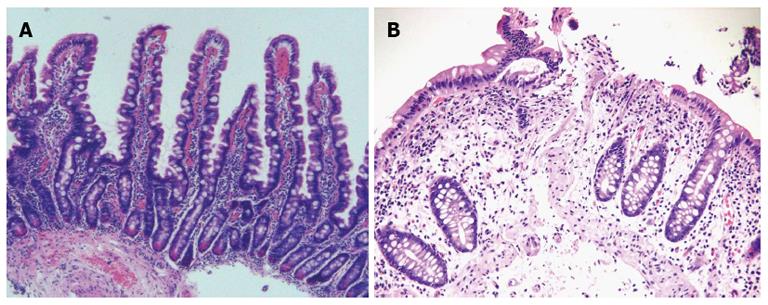
Celiac disease (CD) is an autoimmune disorder induced, in genetically predisposed people, by the ingestion of proteins rich in proline and glutamine. It occurs in adults and children with an average prevalence of about 1% of the population. CD is characterized by an inflammatory reaction, primarily in the upper small intestine, with features of infiltration of the lamina propria and the epithelium with chronic inflammatory cells and progressive villous atrophy[1,2]. At the state of the art the role of serology is becoming more and more important, so that, according to the European Society for Paediatric Gastro-enterology, Hepatology, and nutrition guidelines, diagnosis of celiac disease can be performed without histology in some selected situations-such as the presence, in children, of human leukocyte antigen-DQ2, high titers of anti-tissue transglutaminase antibodies and the positivity of anti-endomysial antibodies[3]. However, current guidelines indicate histological analysis as the gold standard for the diagnosis of CD: specific pathological features are infiltration of the lamina propria, crypt hyperplasia and villous atrophy, classified according to the Marsh classification and its modifications[4-8] (Figure 1). To perform a correct diagnosis, biopsy specimens have to be well oriented, and of good quality. From 4 to 6 duodenal biopsies, including a bulb biopsy, are required to make diagnosis of CD, even because villous atrophy can be unequally distributed -that is the so-called “patchy atrophy”[7,9-13].
Anyway, the diagnosis of CD can also be missed if the disease is not suspected and biopsy sampling not performed. So, in such situations, the role of the endoscopist becomes crucial, because of the strong importance of the macroscopic appearance of the duodenum[14-16].
A number of macroscopic endoscopic markers of CD has been identified over the years, and they include the following: “scalloping” -that is a dented aspect- of the duodenal folds; an absence or a reduction in number of duodenal folds; evidence of submucosal vascular pattern; the so-called “mosaicism”, which is a micronodular look of the mucosa; finally, grooves and fissurations of the mucosa[9-10,14,15]. Results about the value of these markers, however, are conflicting: among different studies, the overall specificity and sensitivity sways from 83% to 100%, and from 6% to 94%, respectively[14,15,17-26].
This happens probably because endoscopic markers cannot be present in milder degrees of the disease. (such as partial villous atrophy) and absent in case of patchy disease[12,18,19]. On the other hand, scalloped feature of duodenal folds has a positive predictive value of 69% for celiac disease and 96% for any duodenal mucosal disease[27]. So, the contradictory evidences and the low sensitivity of endoscopic markers implicates that bioptic sampling should always be performed when the disease is suspected, because their absence does not exclude the diagnosis[16].
The water-immersion technique is a easy, prompt and safe procedure of enhancement of duodenal villous pattern during a conventional upper endoscopy. Our group developed this technique as a method to emphasize the visualization of duodenal villi[28], and then modified it to make it helpful in clinical practice[29]. The mechanism of the water-immersion technique is very simple, comprising, at first, the removal of air from the duodenal lumen by suction, quickly followed by the injection of 90-150 mL of water[29]. The procedure requests about 25-30 s more than a standard upper endoscopy, resulting very fast. Our group proved the high accuracy of the water-immersion technique in highlighting the duodenal villous pattern in patients undergoing upper endoscopy for the investigation of dyspepsia[29]. This procedure was also trialed in the follow-up of celiac patients after gluten-free diet[30], and also in cases with patchy villous atrophy or villous abnormality limited to the duodenal bulb[11,30], and moreover in children with suspected CD, achieving the same optimal diagnostic accuracy for in vivo prediction of areas of the duodenum with villous damage[31]. The water-immersion technique has the potential to reduce the number of biopsy specimens, because of his power of enhancing visualization of areas with villous atrophy (Figure 2A, B); moreover, in patients strongly suspected from CD and with total villous atrophy at water-immersion visualization during upper endoscopy, the high specificity of the procedure could allow to avoid biopsy sampling, with a considerable cost saving[32]. Furthermore, water-immersion technique shows excellent results in terms of operator learning curve, safety, tolerability, and diagnostic accuracy[11,29-32]. In conclusion, for its facility and quickness of performance, and because of its high reliability in evaluating the duodenal villous pattern, the water-immersion technique could potentially be used as a routine procedure during conventional upper gastrointestinal endoscopy, potentially pulling down the number of misdiagnosis of CD, especially when not suspected. Trials with the water-immersion technique has not been replicated by other groups: therefore, further data, with larger population trials, including large multicenter studies, are required to strengthen this evidence.
The efficacy of dye-staining chromoendoscopy with indigo carmine or methylene blue in enhancing the visualization of the mucosal surface is nowadays well known[33,34]. The usefulness of chromoendoscopy with indigo carmine for the evaluation of celiac disease was proved yet in 1976[35]. However, this evidence was not confirmed in a latter study[36]. A new generation of endoscopic tools-the ‘‘magnification’’ or ‘‘zoom’’ endoscopes-can produce magnified, high-resolution images (up to 100-135 ×), enhancing details compared to conventional endoscopy[33,37]. They own charged computed device chips with a density of more than 850000 pixels; standard instruments, instead, have charged computer device chips with a density of 100000-300000 pixels. Video endoscopes can provide more and more details about the mucosal surface than conventional ones[38]. The association of indigo carmine-chromoendoscopy and magnification endoscopy in the evaluation of duodenal villous pattern was experienced by Siegel et al[39]: this combination showed a sensitivity and specificity of 94% and 88%, respectively for the detection of any villous alteration, and was especially helpful in documenting partial villous atrophy. In a following study, neither this combination technique nor each technology alone showed advantage compared to standard endoscopy in identifying duodenal lesions such as polyps or hyperplastic Brunner’s glands, but anyway authors recognized the role of this combination in case of suspected CD[40]. The role of zoom endoscopy, with a total immersion technique (instillation of 10 mL of water), in the diagnosis of CD was analyzed in 2005[41]: a sensitivity of 90.7%, specificity of 62.9%, a positive predictive value of 83% and a negative predictive value of 77.2% for the diagnosis of any degree of villous atrophy resulted; diagnosis of total villous atrophy was better performed than diagnosis of partial villous atrophy. Cammarota et al[42] investigated the combination of magnification endoscopy and water-immersion technique in subjects with suspected duodenal disease, showing a concordance of 100% with histopathology for detecting the absence or the presence of villi. The sensitivity, specificity, positive predictive value and negative predictive value for the detection of total villous atrophy were all 100%, and quite lower for the diagnosis of partial villous atrophy and normal villous patterns. According to other reports, magnification endoscopy could play a role in the detection of patchy villous atrophy[43,44]. In conclusion, enhanced magnification endoscopy, a technique that combines use of acetic acid instillation with magnification endoscopy, has showed a better accuracy in the evaluation of duodenal mucosal pattern than conventional endoscopy[45].
Fujinon intelligent chromo endoscopy system or optimal band imaging (also known as multiband imaging) is able to assure the same contrast enhancement power of the standard chromoendoscopy, but in a virtual manner. This technology is based on the selection of particular wavelengths from a reflected light signal, resulting in an establishment of digitally created, enhanced images[46]. The usefulness of FICE technology has been successfully proved in Barrett’s metaplasia, early gastric cancer, small colorectal tumors[47-49]; moreover, it has showed a great accuracy (100%) for the evaluation of duodenal villi and for the depiction of duodenal villous patterns in CD[50] (Figure 2C, D).
Narrow-band imaging (NBI) is a new endoscopic technique that allows evaluation of minimal mucosal alterations. NBI uses a narrowed wavelength of light, deriving from the narrowing of the bandwidths of the blue and green filters. This particular wavelength of light is greatly absorbed by hemoglobin, enhancing the visualization of microvascular pattern. It also has a quite deeper superficial penetration than standard white light[51,52]. The efficacy of NBI has been proved in the endoscopic evaluation of a number of diseases, among which also in CD[53,54]. According to Singh et al[54], NBI technique is able to detect and grade villous atrophy, with a sensitivity and specificity in detecting villous atrophy of 93.3% and 97.8% respectively, and a sensitivity and specificity in grading villous atrophy of 83.3% and 100%.
Optical coherence tomography (OCT) had its debut in medicine in 1991, and nowadays is a cornerstone in ophthalmology, for the usefulness in the evaluation of the retina and atheromasic plaques[55]. The mechanism of OCT is very similar to that of B-mode ultrasonography: OCT detects the echo time delay and the magnitude of back-scattered light waves from various structural tissue features, using interferometry to measure back-scattered light because the delays of reflected light are too little for a direct electronic measurement[55-57]. The images performed by OCT resemble those generated by B-mode ultrasound and endoscopic ultrasonography; however, the resolution of OCT is better (5-10 mm)-because of the use of light instead of sound waves-, closer to the histological images[55,56,58]. So, OCT allows the study of the proximal layers of gastrointestinal (GI) wall, and may be helpful in the early diagnosis of neoplasms[57]. The usefulness of OCT has been proved yet in the study of GI malignancies[59,60], Barrett’s esophagus and dysplasia[61-67], pancreatic and biliary ducts[68,69], and other diseases. Preliminary reports from Masci et al[70-72], the use of OCT in vivo during real-time endoscopic imaging generated promising results for the evaluation of duodenal villous morphology. These authors, in fact, found total concordance between OCT and histology results for the evaluation of villous morphology in both patients with CD and healthy individuals, also in children, exactly identifying, furthermore, different degrees of villous atrophy.
Confocal laser endomicroscopy, or confocal endomicroscopy, is a novel technology that allows an in-vivo microscopy of the human gastrointestinal mucosa during upper or lower endoscopy[73,74]. Endomicroscopy has been applied in a number of gastrointestinal diseases, and also in CD[73-77]. In particular, in the experience of Zambelli et al[76], the images obtained by confocal endomicroscopy and histology were similar, both for negative subjects and for celiac patients; moreover, in celiac patients confocal endomicroscopy was able to identify moderate-to-severe villous atrophy, but quite less to visualize the crypt hyperplasia and flogistic infiltration. In a case report, CD was diagnosed in vivo by confocal endomicroscopy on the basis of the presence of complete villous atrophy and a rise of intraepithelial lymphocytes[77].
Capsule endoscopy is a useful, patient-friendly method for the evaluation of the whole small bowel. Obscure gastrointestinal bleeding is the strongest indication for capsule endoscopy[78]; however, recent evidences point out new, intriguing purposes and indications: in particular, regarding the object of this review, the role of capsule endoscopy in the diagnosis and follow-up of CD is growing up quickly[79-91]. The optical system of the capsule possesses a 8-folds magnification power, that allows to easily evaluate the duodenal villous pattern (Figure 2E, F). Moreover, it allows an evaluation of the small intestine along its whole length. Capsule endoscopy seems to be able to recognize the endoscopic markers of celiac disease described in the literature, such as scalloping and reduction in number of duodenal plicae, nodularity and mosaic pattern of mucosa[81,82,86,87].
In an initial multicenter trial, capsule endoscopy had an excellent reported sensitivity and specificity of 87.5% and 90.9%, respectively, for the detection of villous atrophy as compared with the criterion standard of duodenal histology[84], but such promising data have not been confirmed in the series presented by the same group[85]. Summarizing the most important studies about the role of capsule endoscopy in CD, it counts a high sensitivity (range, 70%-95.2%), a quite less high specificity (range, 63.6%-100%) and high positive predictive value (96.5%-100%), but a lower negative predictive value (71.4%-88.9%)[82,83,85,88]. These results are cheerful, but the relatively low negative predictive value indicates that CD can’t be surely excluded by a negative evaluation at capsule endoscopy.
It should be noted that there is not an overall high degree of agreement between investigators (range 0.41-0.87), and it probably denotes a difficulty in evaluating correctly villous atrophy even if operators are well-experienced in video capsule enteroscopy.
However, the use of capsule endoscopy could be considered in patients with positive tissue transglutaminase or anti-endomysial antibodies who are unable or unwilling to perform an upper endoscopy[89], and also for the evaluation of the whole small bowel in patients with positive antibodies and duodenal histology negative for CD, even if regarding evidences don’t confirm this hypothesis[90]. More realistically, capsule endoscopy can be very useful in case of suspected refractory or complicated CD. In particular, capsule endoscopy can detect alterations such as malignancy or ulcerative jejunitis in refractory celiac disease (RCD) type II, but evidences are not so bright regarding RCD type I[91].
I-scan technology is an image enhanced endoscopy technology recently developed by Pentax Medical®, Japan[92]. It can be classified among digital contrast methods. It allows three different modalities of image enhancement: surface enhancement (SE), contrast enhancement (CE), and tone enhancement (TE). SE enhances light-dark contrast by obtaining luminance intensity data for each pixel. CE digitally adds blue color in relatively dark areas, enhancing minute irregularities on the mucosal depressed areas. Both enhancement functions work in real time without impairing the original color of the organ. TE separates and analyzes the individual red, green and blue components of a normal image; the algorithm then alters the color frequencies of each component, recombining the components to a single, new color image. For SE and CE, it is possible to switch among three enhancement levels (low, medium and high). At now, three types of TE are available: TE-e (for esophagus), TE-g (for stomach) and TE-c (for intestine). Switching the levels or modes of enhancements can be done on a real-time basis, without any time lag, by pushing a relevant button.
I-scan technology has been applied to several field of interest in gastrointestinal endoscopy, such as colorectal lesions[93-97], Whipple’s disease[98], gastroesophageal reflux disease[99-101], Barrett’s esophagus[102]. Recently, our group has experienced I-scan technology for the evaluation of duodenal villous pattern[103], with the following results: I-scan system was demonstrated to have great accuracy (100%) in the detection of marked villous atrophy patterns and quite lower accuracy in determining partial villous atrophy or normal villous patterns (respectively, 90% for both items) (Figure 2G).
Therefore, I-scan technology seems to be a reliable tool also for the diagnosis of CD. Obviously, further, larger studies are needed to confirm this feeling.
The recent advances in terms of technology and techniques of endoscopy, reviewed above, can certainly improve our diagnostic possibilities in the evaluation of CD, and should not be ignored, but accepted with wisdom. Surely, it is important to perform these tools in appropriate endoscopic centers, owning good equipment and enough expertise. Moreover, in a hypothetic world without biopsy sampling, but with a virtual histological analysis, a gastroenterologist can not absolutely brush aside a solid histological training. Therefore the most realistic scenario is not a replacement, but an interaction between endoscopic and histological analysis: a similar “joint-venture” might knock down misdiagnoses and reduce overall costs of diagnostic course of CD: large, randomized trials, also with cost analyses and clinical outcome evaluations, are needed to carry out this concept.
P- Reviewer: Sezgin O S- Editor: Zhai HH L- Editor: A E- Editor: Liu XM
| 1. | Di Sabatino A, Corazza GR. Coeliac disease. Lancet. 2009;373:1480-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 461] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 2. | Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 1272] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 3. | Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1708] [Cited by in RCA: 1837] [Article Influence: 141.3] [Reference Citation Analysis (5)] |
| 4. | Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 480] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 5. | Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology. 1992;102:330-354. [PubMed] |
| 6. | Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1205] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 7. | Vogelsang H, Hänel S, Steiner B, Oberhuber G. Diagnostic duodenal bulb biopsy in celiac disease. Endoscopy. 2001;33:336-340. [PubMed] |
| 8. | United European Gastroenterology. When is a coeliac a coeliac? Report of a working group of the United European Gastroenterology Week in Amsterdam, 2001. Eur J Gastroenterol Hepatol. 2001;13:1123-1128. [PubMed] |
| 9. | Hopper AD, Cross SS, Sanders DS. Patchy villous atrophy in adult patients with suspected gluten-sensitive enteropathy: is a multiple duodenal biopsy strategy appropriate? Endoscopy. 2008;40:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Brocchi E, Corazza GR, Brusco G, Mangia L, Gasbarrini G. Unsuspected celiac disease diagnosed by endoscopic visualization of duodenal bulb micronodules. Gastrointest Endosc. 1996;44:610-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Cammarota G, Cesaro P, La Mura R, Martino A, Cazzato A, Miele L, Lupascu A, Vecchio FM, Larocca LM, Grieco A. Role of the “immersion technique” in diagnosing celiac disease with villous atrophy limited to the duodenal bulb. J Clin Gastroenterol. 2007;41:571-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Bonamico M, Mariani P, Thanasi E, Ferri M, Nenna R, Tiberti C, Mora B, Mazzilli MC, Magliocca FM. Patchy villous atrophy of the duodenum in childhood celiac disease. J Pediatr Gastroenterol Nutr. 2004;38:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Rodrigo L. Celiac disease. World J Gastroenterol. 2006;12:6585-6593. [PubMed] |
| 14. | Mauriño E, Capizzano H, Niveloni S, Kogan Z, Valero J, Boerr L, Bai JC. Value of endoscopic markers in celiac disease. Dig Dis Sci. 1993;38:2028-2033. [PubMed] |
| 15. | Brocchi E, Tomassetti P, Misitano B, Epifanio G, Corinaldesi R, Bonvicini F, Gasbarrini G, Corazza G. Endoscopic markers in adult coeliac disease. Dig Liver Dis. 2002;34:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Cammarota G, Fedeli P, Gasbarrini A. Emerging technologies in upper gastrointestinal endoscopy and celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2009;6:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Smith AD, Graham I, Rose JD. A prospective endoscopic study of scalloped folds and grooves in the mucosa of the duodenum as signs of villous atrophy. Gastrointest Endosc. 1998;47:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Magazzu G, Bottari M, Tuccari G, Arco A, Pallio S, Lucanto C, Tortora A, Barresi G. Upper gastrointestinal endoscopy can be a reliable screening tool for celiac sprue in adults. J Clin Gastroenterol. 1994;19:255-257; discussion 257-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Dickey W, Hughes D. Prevalence of celiac disease and its endoscopic markers among patients having routine upper gastrointestinal endoscopy. Am J Gastroenterol. 1999;94:2182-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Mauriño E, Bai JC. Endoscopic markers of celiac disease. Am J Gastroenterol. 2002;97:760-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Oxentenko AS, Grisolano SW, Murray JA, Burgart LJ, Dierkhising RA, Alexander JA. The insensitivity of endoscopic markers in celiac disease. Am J Gastroenterol. 2002;97:933-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Ravelli AM, Tobanelli P, Minelli L, Villanacci V, Cestari R. Endoscopic features of celiac disease in children. Gastrointest Endosc. 2001;54:736-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Corazza GR, Caletti GC, Lazzari R, Collina A, Brocchi E, Di Sario A, Ferrari A, Gasbarrini G. Scalloped duodenal folds in childhood celiac disease. Gastrointest Endosc. 1993;39:543-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Lecleire S, Di Fiore F, Antonietti M, Savoye G, Lemoine F, Le Pessot F, Lerebours E, Ducrotté P. Endoscopic markers of villous atrophy are not useful for the detection of celiac disease in patients with dyspeptic symptoms. Endoscopy. 2006;38:696-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Bardella MT, Minoli G, Radaelli F, Quatrini M, Bianchi PA, Conte D. Reevaluation of duodenal endoscopic markers in the diagnosis of celiac disease. Gastrointest Endosc. 2000;51:714-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Brandimarte G, Tursi A. Endoscopic treatment of benign anastomotic esophageal stenosis with electrocautery. Endoscopy. 2002;34:399-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Shah VH, Rotterdam H, Kotler DP, Fasano A, Green PH. All that scallops is not celiac disease. Gastrointest Endosc. 2000;51:717-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Gasbarrini A, Ojetti V, Cuoco L, Cammarota G, Migneco A, Armuzzi A, Pola P, Gasbarrini G. Lack of endoscopic visualization of intestinal villi with the “immersion technique” in overt atrophic celiac disease. Gastrointest Endosc. 2003;57:348-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Cammarota G, Pirozzi GA, Martino A, Zuccalà G, Cianci R, Cuoco L, Ojetti V, Landriscina M, Montalto M, Vecchio FM. Reliability of the “immersion technique” during routine upper endoscopy for detection of abnormalities of duodenal villi in patients with dyspepsia. Gastrointest Endosc. 2004;60:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Cammarota G, Cuoco L, Cesaro P, Santoro L, Cazzato A, Montalto M, La Mura R, Larocca LM, Vecchio FM, Gasbarrini A. A highly accurate method for monitoring histological recovery in patients with celiac disease on a gluten-free diet using an endoscopic approach that avoids the need for biopsy: a double-center study. Endoscopy. 2007;39:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Cammarota G, Cazzato A, Genovese O, Pantanella A, Ianiro G, Giorgio V, Montalto M, Vecchio FM, Larocca LM, Gasbarrini G. Water-immersion technique during standard upper endoscopy may be useful to drive the biopsy sampling of duodenal mucosa in children with celiac disease. J Pediatr Gastroenterol Nutr. 2009;49:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Cammarota G, Cesaro P, Martino A, Zuccalà G, Cianci R, Nista E, Larocca LM, Vecchio FM, Gasbarrini A, Gasbarrini G. High accuracy and cost-effectiveness of a biopsy-avoiding endoscopic approach in diagnosing coeliac disease. Aliment Pharmacol Ther. 2006;23:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Lee SK, Green PH. Endoscopy in celiac disease. Curr Opin Gastroenterol. 2005;21:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Wong Kee Song LM, Adler DG, Chand B, Conway JD, Croffie JM, Disario JA, Mishkin DS, Shah RJ, Somogyi L, Tierney WM. Chromoendoscopy. Gastrointest Endosc. 2007;66:639-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 35. | Stevens FM, McCarthy CF. The endoscopic demonstration of coeliac disease. Endoscopy. 1976;8:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Niveloni S, Fiorini A, Dezi R, Pedreira S, Smecuol E, Vazquez H, Cabanne A, Boerr LA, Valero J, Kogan Z. Usefulness of videoduodenoscopy and vital dye staining as indicators of mucosal atrophy of celiac disease: assessment of interobserver agreement. Gastrointest Endosc. 1998;47:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Rubio-Tapia A, Murray JA. Novel endoscopic methods for the evaluation of the small-bowel mucosa. Gastrointest Endosc. 2007;66:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Bruno MJ. Magnification endoscopy, high resolution endoscopy, and chromoscopy; towards a better optical diagnosis. Gut. 2003;52 Suppl 4:iv7-i11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Siegel LM, Stevens PD, Lightdale CJ, Green PH, Goodman S, Garcia-Carrasquillo RJ, Rotterdam H. Combined magnification endoscopy with chromoendoscopy in the evaluation of patients with suspected malabsorption. Gastrointest Endosc. 1997;46:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 69] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Kiesslich R, Mergener K, Naumann C, Hahn M, Jung M, Koehler HH, Nafe B, Kanzler S, Galle PR. Value of chromoendoscopy and magnification endoscopy in the evaluation of duodenal abnormalities: a prospective, randomized comparison. Endoscopy. 2003;35:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Badreldin R, Barrett P, Wooff DA, Mansfield J, Yiannakou Y. How good is zoom endoscopy for assessment of villous atrophy in coeliac disease? Endoscopy. 2005;37:994-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Cammarota G, Martino A, Pirozzi GA, Cianci R, Cremonini F, Zuccalà G, Cuoco L, Ojetti V, Montalto M, Vecchio FM. Direct visualization of intestinal villi by high-resolution magnifying upper endoscopy: a validation study. Gastrointest Endosc. 2004;60:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Cammarota G, Martino A, Di Caro S, Cianci R, Lecca PG, Vecchio FM, Gasbarrini G. High-resolution magnifying upper endoscopy in a patient with patchy celiac disease. Dig Dis Sci. 2005;50:601-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Banerjee R, Shekharan A, Ramji C, Puli SR, Kalapala R, Ramachandani M, Gupta R, Lakhtakia S, Tandan M, Rao GV. Role of magnification endoscopy in the diagnosis and evaluation of suspected celiac disease: correlation with histology. Indian J Gastroenterol. 2007;26:67-69. [PubMed] |
| 45. | Lo A, Guelrud M, Essenfeld H, Bonis P. Classification of villous atrophy with enhanced magnification endoscopy in patients with celiac disease and tropical sprue. Gastrointest Endosc. 2007;66:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Pohl J, May A, Rabenstein T, Pech O, Ell C. Computed virtual chromoendoscopy: a new tool for enhancing tissue surface structures. Endoscopy. 2007;39:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 47. | Pohl J, May A, Rabenstein T, Pech O, Nguyen-Tat M, Fissler-Eckhoff A, Ell C. Comparison of computed virtual chromoendoscopy and conventional chromoendoscopy with acetic acid for detection of neoplasia in Barrett’s esophagus. Endoscopy. 2007;39:594-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 48. | Osawa H, Yoshizawa M, Yamamoto H, Kita H, Satoh K, Ohnishi H, Nakano H, Wada M, Arashiro M, Tsukui M. Optimal band imaging system can facilitate detection of changes in depressed-type early gastric cancer. Gastrointest Endosc. 2008;67:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Pohl J, Nguyen-Tat M, Pech O, May A, Rabenstein T, Ell C. Computed virtual chromoendoscopy for classification of small colorectal lesions: a prospective comparative study. Am J Gastroenterol. 2008;103:562-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 50. | Cammarota G, Cesaro P, Cazzato A, Fedeli P, Sparano L, Vecchio FM, Larocca LM, Gasbarrini G. Optimal band imaging system: a new tool for enhancing the duodenal villous pattern in celiac disease. Gastrointest Endosc. 2008;68:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | van den Broek FJ, Fockens P, Dekker E. Review article: New developments in colonic imaging. Aliment Pharmacol Ther. 2007;26 Suppl 2:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Dekker E, Fockens P. New imaging techniques at colonoscopy: tissue spectroscopy and narrow band imaging. Gastrointest Endosc Clin N Am. 2005;15:703-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Banerjee R, Reddy DN. High-resolution narrow-band imaging can identify patchy atrophy in celiac disease: targeted biopsy can increase diagnostic yield. Gastrointest Endosc. 2009;69:984-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Singh R, Nind G, Tucker G, Nguyen N, Holloway R, Bate J, Shetti M, George B, Tam W. Narrow-band imaging in the evaluation of villous morphology: a feasibility study assessing a simplified classification and observer agreement. Endoscopy. 2010;42:889-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA. Optical coherence tomography. Science. 1991;254:1178-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9722] [Cited by in RCA: 7025] [Article Influence: 206.6] [Reference Citation Analysis (0)] |
| 56. | Tearney GJ, Brezinski ME, Bouma BE, Boppart SA, Pitris C, Southern JF, Fujimoto JG. In vivo endoscopic optical biopsy with optical coherence tomography. Science. 1997;276:2037-2039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1019] [Cited by in RCA: 688] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 57. | Brand S, Poneros JM, Bouma BE, Tearney GJ, Compton CC, Nishioka NS. Optical coherence tomography in the gastrointestinal tract. Endoscopy. 2000;32:796-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Hsiung PL, Pantanowitz L, Aguirre AD, Chen Y, Phatak D, Ko TH, Bourquin S, Schnitt SJ, Raza S, Connolly JL. Ultrahigh-resolution and 3-dimensional optical coherence tomography ex vivo imaging of the large and small intestines. Gastrointest Endosc. 2005;62:561-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Hatta W, Uno K, Koike T, Yokosawa S, Iijima K, Imatani A, Shimosegawa T. Optical coherence tomography for the staging of tumor infiltration in superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2010;71:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Hariri LP, Tumlinson AR, Besselsen DG, Utzinger U, Gerner EW, Barton JK. Endoscopic optical coherence tomography and laser-induced fluorescence spectroscopy in a murine colon cancer model. Lasers Surg Med. 2006;38:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Chen Y, Aguirre AD, Hsiung PL, Desai S, Herz PR, Pedrosa M, Huang Q, Figueiredo M, Huang SW, Koski A. Ultrahigh resolution optical coherence tomography of Barrett’s esophagus: preliminary descriptive clinical study correlating images with histology. Endoscopy. 2007;39:599-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 62. | Evans JA, Poneros JM, Bouma BE, Bressner J, Halpern EF, Shishkov M, Lauwers GY, Mino-Kenudson M, Nishioka NS, Tearney GJ. Optical coherence tomography to identify intramucosal carcinoma and high-grade dysplasia in Barrett’s esophagus. Clin Gastroenterol Hepatol. 2006;4:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 63. | Isenberg G, Sivak MV, Chak A, Wong RC, Willis JE, Wolf B, Rowland DY, Das A, Rollins A. Accuracy of endoscopic optical coherence tomography in the detection of dysplasia in Barrett’s esophagus: a prospective, double-blinded study. Gastrointest Endosc. 2005;62:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 64. | Jäckle S, Gladkova N, Feldchtein F, Terentieva A, Brand B, Gelikonov G, Gelikonov V, Sergeev A, Fritscher-Ravens A, Freund J. In vivo endoscopic optical coherence tomography of esophagitis, Barrett’s esophagus, and adenocarcinoma of the esophagus. Endoscopy. 2000;32:750-755. [PubMed] |
| 65. | Li XD, Boppart SA, Van Dam J, Mashimo H, Mutinga M, Drexler W, Klein M, Pitris C, Krinsky ML, Brezinski ME. Optical coherence tomography: advanced technology for the endoscopic imaging of Barrett’s esophagus. Endoscopy. 2000;32:921-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 147] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 66. | Cobb MJ, Hwang JH, Upton MP, Chen Y, Oelschlager BK, Wood DE, Kimmey MB, Li X. Imaging of subsquamous Barrett’s epithelium with ultrahigh-resolution optical coherence tomography: a histologic correlation study. Gastrointest Endosc. 2010;71:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 67. | Adler DC, Zhou C, Tsai TH, Lee HC, Becker L, Schmitt JM, Huang Q, Fujimoto JG, Mashimo H. Three-dimensional optical coherence tomography of Barrett’s esophagus and buried glands beneath neosquamous epithelium following radiofrequency ablation. Endoscopy. 2009;41:773-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 68. | Seitz U, Freund J, Jaeckle S, Feldchtein F, Bohnacker S, Thonke F, Gladkova N, Brand B, Schröder S, Soehendra N. First in vivo optical coherence tomography in the human bile duct. Endoscopy. 2001;33:1018-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Testoni PA, Mariani A, Mangiavillano B, Arcidiacono PG, Di Pietro S, Masci E. Intraductal optical coherence tomography for investigating main pancreatic duct strictures. Am J Gastroenterol. 2007;102:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Masci E, Mangiavillano B, Albarello L, Mariani A, Doglioni C, Testoni PA. Pilot study on the correlation of optical coherence tomography with histology in celiac disease and normal subjects. J Gastroenterol Hepatol. 2007;22:2256-2260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 71. | Masci E, Mangiavillano B, Albarello L, Mariani A, Doglioni C, Testoni PA. Optical coherence tomography in the diagnosis of coeliac disease: a preliminary report. Gut. 2006;55:579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 72. | Masci E, Mangiavillano B, Barera G, Parma B, Albarello L, Mariani A, Doglioni C, Testoni PA. Optical coherence tomography in pediatric patients: a feasible technique for diagnosing celiac disease in children with villous atrophy. Dig Liver Dis. 2009;41:639-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Odagi I, Kato T, Imazu H, Kaise M, Omar S, Tajiri H. Examination of normal intestine using confocal endomicroscopy. J Gastroenterol Hepatol. 2007;22:658-662. [PubMed] |
| 74. | Goetz M, Kiesslich R. Advances of endomicroscopy for gastrointestinal physiology and diseases. Am J Physiol Gastrointest Liver Physiol. 2010;298:G797-G806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest Endosc. 2005;62:686-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 267] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 76. | Zambelli A, Villanacci V, Buscarini E, Lupinacci G, De Grazia F, Brambilla G, Menozzi F, La Mantia L, Bassotti G. Confocal laser endomicroscopy in celiac disease: description of findings in two cases. Endoscopy. 2007;39:1018-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Trovato C, Sonzogni A, Ravizza D, Fiori G, Rossi M, Tamayo D, Miller MJ, Bardella MT, Crosta C. Celiac disease: in vivo diagnosis by confocal endomicroscopy. Gastrointest Endosc. 2007;65:1096-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Spada C, Hassan C, Galmiche JP, Neuhaus H, Dumonceau JM, Adler S, Epstein O, Gay G, Pennazio M, Rex DK. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2012;44:527-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 79. | Tennyson CA, Green PH. The role of capsule endoscopy in patients with nonresponsive celiac disease. Gastrointest Endosc. 2011;74:1323-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 80. | Spada C, Riccioni ME, Urgesi R, Costamagna G. Capsule endoscopy in celiac disease. World J Gastroenterol. 2008;14:4146-4151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 81. | Petroniene R, Dubcenco E, Baker JP, Warren RE, Streutker CJ, Gardiner GW, Jeejeebhoy KN. Given capsule endoscopy in celiac disease. Gastrointest Endosc Clin N Am. 2004;14:115-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 82. | Petroniene R, Dubcenco E, Baker JP, Ottaway CA, Tang SJ, Zanati SA, Streutker CJ, Gardiner GW, Warren RE, Jeejeebhoy KN. Given capsule endoscopy in celiac disease: evaluation of diagnostic accuracy and interobserver agreement. Am J Gastroenterol. 2005;100:685-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 83. | Hopper AD, Sidhu R, Hurlstone DP, McAlindon ME, Sanders DS. Capsule endoscopy: an alternative to duodenal biopsy for the recognition of villous atrophy in coeliac disease? Dig Liver Dis. 2007;39:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Rondonotti E, de Franchis R. Diagnosing coeliac disease: is the videocapsule a suitable tool? Dig Liver Dis. 2007;39:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Rondonotti E, Spada C, Cave D, Pennazio M, Riccioni ME, De Vitis I, Schneider D, Sprujevnik T, Villa F, Langelier J. Video capsule enteroscopy in the diagnosis of celiac disease: a multicenter study. Am J Gastroenterol. 2007;102:1624-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 86. | Kesari A, Bobba RK, Arsura EL. Video capsule endoscopy and celiac disease. Gastrointest Endosc. 2005;62:796-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 87. | Toth E, Ohlsson B, Ljungberg O, Thorlacius H. Celiac disease diagnosed using video capsule endoscopy in a patient with Crohn’s disease. Endoscopy. 2006;38:548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 88. | Biagi F, Rondonotti E, Campanella J, Villa F, Bianchi PI, Klersy C, De Franchis R, Corazza GR. Video capsule endoscopy and histology for small-bowel mucosa evaluation: a comparison performed by blinded observers. Clin Gastroenterol Hepatol. 2006;4:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 89. | Cellier C, Green PH, Collin P, Murray J. ICCE consensus for celiac disease. Endoscopy. 2005;37:1055-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 90. | Lidums I, Cummins AG, Teo E. The role of capsule endoscopy in suspected celiac disease patients with positive celiac serology. Dig Dis Sci. 2011;56:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 91. | Daum S, Wahnschaffe U, Glasenapp R, Borchert M, Ullrich R, Zeitz M, Faiss S. Capsule endoscopy in refractory celiac disease. Endoscopy. 2007;39:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 92. | Kodashima S, Fujishiro M. Novel image-enhanced endoscopy with i-scan technology. World J Gastroenterol. 2010;16:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 130] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 93. | Testoni PA, Notaristefano C, Vailati C, Di Leo M, Viale E. High-definition colonoscopy with i-Scan: better diagnosis for small polyps and flat adenomas. World J Gastroenterol. 2012;18:5231-5239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 94. | Pigò F, Bertani H, Manno M, Mirante V, Caruso A, Barbera C, Manta R, Bassotti G, Olivetti G, Conigliaro RL. i-Scan high-definition white light endoscopy and colorectal polyps: prediction of histology, interobserver and intraobserver agreement. Int J Colorectal Dis. 2013;28:399-406. [PubMed] |
| 95. | Lee CK, Lee SH, Hwangbo Y. Narrow-band imaging versus I-Scan for the real-time histological prediction of diminutive colonic polyps: a prospective comparative study by using the simple unified endoscopic classification. Gastrointest Endosc. 2011;74:603-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 96. | Hoffman A, Sar F, Goetz M, Tresch A, Mudter J, Biesterfeld S, Galle PR, Neurath MF, Kiesslich R. High definition colonoscopy combined with i-Scan is superior in the detection of colorectal neoplasias compared with standard video colonoscopy: a prospective randomized controlled trial. Endoscopy. 2010;42:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 97. | Hoffman A, Kagel C, Goetz M, Tresch A, Mudter J, Biesterfeld S, Galle PR, Neurath MF, Kiesslich R. Recognition and characterization of small colonic neoplasia with high-definition colonoscopy using i-Scan is as precise as chromoendoscopy. Dig Liver Dis. 2010;42:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 98. | Neumann H, Neufert C, Vieth M, Siebler J, Mönkemüller K, Neurath MF. High-definition endoscopy with i-scan enables diagnosis of characteristic mucosal lesions in Whipple‘s disease. Endoscopy. 2012;44 Suppl 2 UCTN:E217-E218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 99. | Kang HS, Hong SN, Kim YS, Park HS, Kim BK, Lee JH, Kim SI, Lee TY, Kim JH, Lee SY. The efficacy of i-SCAN for detecting reflux esophagitis: a prospective randomized controlled trial. Dis Esophagus. 2013;26:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 100. | Kim MS, Choi SR, Roh MH, Lee JH, Jang JS, Kim BG, Kim SO, Han JS, Hsing CT. Efficacy of I-scan endoscopy in the diagnosis of gastroesophageal reflux disease with minimal change. Clin Endosc. 2011;44:27-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 101. | Hoffman A, Basting N, Goetz M, Tresch A, Mudter J, Biesterfeld S, Galle PR, Neurath MF, Kiesslich R. High-definition endoscopy with i-Scan and Lugol’s solution for more precise detection of mucosal breaks in patients with reflux symptoms. Endoscopy. 2009;41:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 102. | Hoffman A, Korczynski O, Rahman F, Hansen T, Goetz M, Galle PR, Kiesslich R. S1586: I-Scan Is As Effective As Acetic Acid Guided Chromoendoscopy and Superior to Random Biopsies Diagnosing Barrett's Epithelium - A Prospective Randomized Trial. Gastrointest Endosc. 2010;71:AB201. [DOI] [Full Text] |
| 103. | Cammarota G, Ianiro G, Sparano L, La Mura R, Ricci R, Larocca LM, Landolfi R, Gasbarrini A. Image-enhanced endoscopy with I-scan technology for the evaluation of duodenal villous patterns. Dig Dis Sci. 2013;58:1287-1292. [PubMed] |