Published online Dec 7, 2013. doi: 10.3748/wjg.v19.i45.8357
Revised: August 6, 2013
Accepted: September 16, 2013
Published online: December 7, 2013
Processing time: 211 Days and 10.4 Hours
AIM: To determine the added value of hepatobiliary phase (HBP) gadoxetic acid-enhanced magnetic resonance imaging (MRI) in evaluating hepatic nodules in high-risk patients.
METHODS: The institutional review board approved this retrospective study and waived the requirement for informed consent. This study included 100 patients at high risk for hepatocellular carcinoma (HCC) and 105 hepatic nodules that were larger than 1 cm. A blind review of two MR image sets was performed in a random order: set 1, unenhanced (T1- and T2-weighted) and dynamic images; and set 2, unenhanced, dynamic 20-min and HBP images. The diagnostic accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were compared for the two image sets. Univariate and multivariate analyses were performed on the MR characteristics utilized to diagnose HCC.
RESULTS: A total of 105 hepatic nodules were identified in 100 patients. Fifty-nine nodules were confirmed to be HCC. The diameter of the 59 HCCs ranged from 1 to 12 cm (mean: 1.9 cm). The remaining 46 nodules were benign (28 were of hepatocyte origin, nine were hepatic cysts, seven were hemangiomas, one was chronic inflammation, and one was focal fat infiltration). The diagnostic accuracy significantly increased with the addition of HBP images, from 88.7% in set 1 to 95.5% in set 2 (P = 0.002). In set 1 vs set 2, the sensitivity and NPV increased from 79.7% to 93.2% and from 78.9% to 91.8%, respectively, whereas the specificity and PPV were not significantly different. The hypointensity on the HBP images was the most sensitive (93.2%), and typical arterial enhancement followed by washout was the most specific (97.8%). The multivariate analysis revealed that typical arterial enhancement followed by washout, hyperintensity on T2-weighted images, and hypointensity on HBP images were statistically significant MRI findings that could diagnose HCC (P < 0.05).
CONCLUSION: The addition of HBP gadoxetic acid-enhanced MRI statistically improved the diagnostic accuracy in HCCs larger than 1 cm. Typical arterial enhancement followed by washout and hypointensity on HBP images are useful for diagnosing HCC.
Core tip: This study demonstrated the added value of hepatobiliary phase gadoxetic acid-enhanced magnetic resonance imaging (MRI) for diagnosing hepatocellular carcinoma, based on the changes in contrast uptake on hepatobiliary phase images during hepatocarcinogenesis. The pitfalls of interpreting hepatobiliary phase MRI are important to recognize in obtaining the most accurate results.
- Citation: Phongkitkarun S, Limsamutpetch K, Tannaphai P, Jatchavala J. Added value of hepatobiliary phase gadoxetic acid-enhanced MRI for diagnosing hepatocellular carcinoma in high-risk patients. World J Gastroenterol 2013; 19(45): 8357-8365
- URL: https://www.wjgnet.com/1007-9327/full/v19/i45/8357.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i45.8357
Liver cancer is the second most frequent cause of death in men and the sixth leading cause of death in women worldwide. Southeast Asia is one of the regions with the greatest incidences of liver cancer, and hepatocellular carcinoma (HCC), associated with the hepatitis B virus, accounts for the majority of the cases[1,2].
The therapeutic approach for HCC depends on the accurate diagnosis and identification of HCC lesions, i.e., the number, size, and location of the lesions. In practice, the high prevalence of benign lesions in cirrhotic livers and the variability in HCC imaging characteristics, which depend on differentiation of the state of the disease, render the detection of HCC difficult. Magnetic resonance imaging (MRI), particularly contrast-enhanced dynamic MRI, plays an important role in accurately diagnosing HCC[3]. A study by Forner et al[4] reported that the sensitivity and specificity were as high as 62% and 97%, respectively, for detecting HCC with conventional contrast-enhanced dynamic MRI. Nevertheless, undetectable HCC lesions pose a serious issue.
A recent molecular study reported that organic anion transporting polypeptide 8 (OATP8) is expressed in hepatocytes and that OATP8 expression significantly decreases during the multistep process of hepatocarcinogenesis[5]. A newly developed liver-specific contrast agent, gadoxetic acid, is taken up by hepatocytes via OATP8 in an in vitro study[6]. Therefore, the use of liver-specific contrast-enhanced MRI might provide superior detection and characterization of HCC[7-9].
Gadoxetic acid, or gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA), has the properties of being both an extracellular gadolinium chelate and a hepatobiliary agent, thus enabling dynamic perfusion imaging, delayed hepatocyte uptake, and biliary excretion. A recent study demonstrated that, compared with conventional contrast-enhanced dynamic and T2-weighted MRI, Gd-EOB-DTPA-enhanced hepatobiliary phase MRI improved HCC detection[3]. However, there are no supportive data for such a benefit in the Thai population, which has a high prevalence of HCC with different risk factors. Because of differences in the population and the nature of the disease, we performed a study to determine the added value of Gd-EOB-DTPA-enhanced hepatobiliary phase MRI for diagnosing HCC in high-risk patients.
This retrospective study was approved by our institutional review board, which waived the informed consent requirement. Between January 2010 and July 2010, 100 consecutive patients (mean age: 59.5 years old; range: 27-89 years), consisting of 71 men (mean age: 59.4 years; range: 27-89 years) and 29 women (mean age: 59.6 years old; range: 36-81 years), were registered as high-risk patients with HCC and underwent Gd-EOB-DTPA-enhanced MRI. The high-risk patients included those with chronic viral hepatitis infections (HBV or HCV infection), liver cirrhosis, or elevated serum alpha-fetoprotein (AFP) levels. Among the 100 patients, 76 had liver cirrhosis. Forty-five patients had hepatitis B-related cirrhosis, 16 had hepatitis C-related cirrhosis, nine had alcoholic cirrhosis, six had nonalcoholic steatohepatitis, 22 had a chronic hepatitis infection (20 with chronic hepatitis B and two with chronic hepatitis C), and the remaining two patients only presented with abnormal serum AFP. The Child-Pugh score was calculated for all of the patients within 60 d of the MRI scan (Table 1).
| Variables | Results |
| Age (mean ± SD; range, yr) | 59.5 ± 11.4 (range 27-89) |
| Sex (male:female) | 71:29 |
| Risk factors for HCC | |
| Chronic HBV infection | 20 (20) |
| Chronic HCV infection | 2 (2) |
| Hepatitis B-related cirrhosis | 45 (45) |
| Hepatitis C-related cirrhosis | 16 (16) |
| Alcoholic cirrhosis | 9 (9) |
| Nonalcoholic steatohepatitis | 6 (6) |
| Only high serum AFP levels | 2 (2) |
| Child-Pugh score | |
| A | 94 (94) |
| B | 5 (5) |
| C | 1 (1) |
| Alpha-fetoprotein (mean ± SD; range, ng/mL) | 111.6 ± 935.1 (range 0.98-8973) |
| < 20 | 84 (84) |
| 20-200 | 5 (5) |
| > 200 | 3 (3) |
| Total bilirubin (mean ± SD; range, mg/dL) | 1.43 ± 2.5 (range 0.2-17.1) |
The reference standard for diagnosing HCC was classified as follows: based on pathologically proven HCC (by surgical specimen or percutaneous biopsy); by lipiodol staining after transhepatic arterial chemoembolization (TACE); or on the basis of progression of the disease on follow-up computed tomography (CT) or MRI performed at least 6 mo after the initial imaging.
All of the MRI studies were performed with a 1.5-T system (Signa HDxt; GE Healthcare, Milwaukee, Wisconsin, United States) and a 3.0-T system (Intera Achieva; Philips Medical Systems, Best, The Netherlands) with phased-array coils. All of the images were obtained on the axial plane. The field of view (32-40 cm × 32-40 cm) was adjusted for each patient. The MR protocol consisted of a double-echo T1-weighted gradient-echo sequence (in-phase and opposed-phase), a respiratory-triggered fast-spin T2-weighted sequence, and a contrast-enhanced dynamic sequence. The parameters for all of the sequences are presented in Table 2. For the contrast-enhanced dynamic MR images, Gd-EOB-DTPA was administered at 0.025 mmol per kilogram of body weight at 2 mL per second, followed by a 10-mL saline flush. After administering the contrast, early arterial phase (25-30 s), late arterial phase (45-50 s), portal venous phase (65-70 s), equilibrium phase (5 min), and additional hepatobiliary phase (after 20 min) images were obtained.
| Parameters | Double-echo T1-weighted gradient echo | Respiratory-triggered fast-spin T2-weighted | T1-weighted Gd-EOB-DTPA-enhanced | |||
| 1.5-T | 3.0-T | 1.5-T | 3.0-T | 1.5-T | 3.0-T | |
| Matrix | 256 × 192 | 228 × 136 | 320 × 224 | 320 × 188 | 256 × 192 | 180 × 163 |
| Section thickness (mm) | 6-8 | 6 | 4 | 6 | 2-3 | 4 |
| Intersection gap (mm) | 2 | 2 | 1 | 2 | - | - |
| TR (ms) | 180-220 | 150-250 | 2000-4000 | 2500-3500 | 4.2 | 2.8 |
| TE (ms) | 2.3-4.6 | 1.15-2.3 | 60 | 65-85 | 2 | 1.4 |
| Flip angle (degrees) | 80 | 70 | 90 | 120 | 12 | 10 |
| Reduction factor | 2 | 2 | 2 | 2 | 2 | 2 |
Two sets of MR images were reviewed by an experienced gastrointestinal radiologist (S.P.) in a random order. The observer was blinded to the patient history, the findings from other imaging modalities, treatments, outcomes, and the final diagnoses. Set 1 of the MR images contained unenhanced (pre-contrast T1- and T2-weighted images) and Gd-EOB-DTPA-enhanced dynamic images (arterial, portal venous, and 5-min equilibrium phases); set 2 consisted of unenhanced, Gd-EOB-DTPA-enhanced dynamic, and 20-min hepatobiliary phase images. The MR images in set 2 were obtained at least 1 mo after finishing the reviews of all of the MR images in set 1. A diagnosis of HCC met at least one of the following criteria: (1) a liver nodule 1 cm or larger, with increased enhancement on arterial phase images and washout on portal venous or equilibrium phase images[10]; or (2) a hypervascular liver nodule 1 cm or larger, with no uptake on 20-min hepatobiliary phase Gd-EOB-DTPA-enhanced MR images[11].
The observer recorded the possibility of HCC for each dominant lesion (defined as 1 cm or larger) using a four-point confidence rating scale: (1) unlikely to be HCC; (2) a concerning nodule; (3) probably HCC; and (4) definitely HCC. A score of 0 was recorded when the observer did not find any dominant lesions. Lesions that received a score of 3 or 4 were classified as positive for HCC in later analyses. The size and location of each lesion were clearly documented using liver segmental anatomy, defined by Couinaud classification and MR characteristics (in-phase, opposed-phase, T2-weighted, arterial phase, portal venous phase, 5-min equilibrium phase, and 20-min hepatobiliary phase images, which were only obtained in set 2). All of the MR images were reviewed with Picture Archiving and Communications System (PACS) and Digital Imaging and Communications in Medicine Conformance (Synapse, version 3.2.0, FUJIFILM Medical Systems United Stated’s Synapse® PACS system, Stamford, Connecticut, United States).
Statistical analyses were performed using statistical software (SPSS, version 17.0.1, SPSS, Chicago, Illinois, United States). Descriptive statistics were calculated for all of the clinical variables and for those evaluated on the MR images. For continuous data (age, AFP level, total bilirubin level, and lesion size), the means, SDs, and ranges were calculated. Descriptive statistics, such as raw numbers and percentages, were determined for categorical data. An alternative free-response receiver operating characteristic analysis was performed on the confidence rating scale of the observer. The area under the alternative free-response receiver operating characteristic curve (Az) was utilized to determine the diagnostic accuracy of detecting HCC in each image set and according to lesion size (1-2 cm or larger than 2 cm). Sensitivity, specificity, positive predictive values (PPVs), and negative predictive values (NPVs) were calculated for the two MR image sets.
The relationships between each MRI variable and the presence of an HCC lesion were tested via univariate analysis. Significant variables identified by the univariate analysis were integrated into the logistic regression model for the multivariate analysis. The MRI variables with significant associations in the multivariate analysis were regarded as significant predictors of the presence of HCC. All of the statistical tests were two-tailed, and a P value of < 0.05 indicated statistical significance.
A total of 105 hepatic nodules were identified in 100 patients. Fifty-nine nodules were confirmed to be HCC by pathologic examination of surgical resection or percutaneous biopsy specimens (n = 23), by lipiodol staining after TACE (n = 30), or on the basis of disease progression on follow-up CT or MRI at least 6 mo after the initial imaging (n = 6; mean: 11.9 mo). The diameter of the 59 HCCs ranged from 1 to 12 cm (mean: 1.9 cm). The remaining 46 nodules were benign (28 were of hepatocyte origin, nine were hepatic cysts, seven were hemangiomas, one was chronic inflammation, and one was focal fat infiltration). Four of the 46 nodules were pathologically confirmed as benign lesions (two were regenerative nodules, one was a cirrhotic nodule, and one was a hemangioma), and 42 nodules were considered benign because they decreased or remained unchanged in size on the last follow-up CT, or MRI (mean: 22.1 mo).
The diagnostic accuracy of diagnosing HCC in the two image sets is presented in Table 3. The diagnostic accuracy of the combination of unenhanced, Gd-EOB-DTPA-enhanced dynamic, and 20-min hepatobiliary phase images (set 2, 95.5%) was significantly greater than that of the unenhanced and Gd-EOB-DTPA-enhanced dynamic images (set 1, 88.7%) (P = 0.002; Figure 1). For 1- to 2-cm lesions, the diagnostic accuracy significantly improved from 86.7% in set 1 to 94.0% in set 2 (P = 0.008), whereas there was no statistical improvement for lesions > 2 cm (94.4% in set 1 and 100% in set 2; P = 0.145).
| Conventional dynamic MRI | Conventional dynamic MRI+HBP | P value | |
| All lesions | 88.7% | 95.5% | 0.002 |
| Lesions 1-2 cm | 86.7% | 94.0% | 0.008 |
| Lesions > 2 cm | 94.4% | 100% | 0.145 |
The sensitivity, specificity, PPVs, and NPVs for the two image sets are presented in Table 4. The addition of 20-min hepatobiliary phase images increased the sensitivity from 79.7% (95%CI: 67.2-89) to 93.2% (95%CI: 83.5-98.1). The specificity of both sets was 97.8% (95%CI: 85.5-99.9). The addition of 20-min hepatobiliary phase images increased the positive and NPVs from 97.9% (95%CI: 88.9-99.9) to 98.2% (95%CI: 90.4-100) and from 78.9% (95%CI: 66.1-88.6) to 91.8% (95%CI: 80.4-97.7), respectively.
| Conventional dynamic MRI | 95%CI | Conventional dynamic MRI+HBP | 95%CI | |
| Sensitivity | 79.7% | 67.2-89.0 | 93.2% | 83.5-98.1 |
| Specificity | 97.8% | 85.5-99.9 | 97.8% | 88.5-99.9 |
| Positive predictive value | 97.9% | 88.9-99.9 | 98.2% | 90.4-100.0 |
| Negative predictive value | 78.9% | 66.1-88.6 | 91.8% | 80.4-97.7 |
The sensitivity, specificity, PPVs, and NPVs of each MRI characteristic utilized to diagnose HCC were as follows: (1) fat metamorphosis: 16.9% (95%CI: 8.4-29), 95.7% (95%CI: 85.2-99.5), 83.3% (95%CI: 51.6-97.9), and 47.3% (95%CI: 36.9-57.9); (2) hyperintensity on T2-weighted images: 66.1% (52.6-77.9), 60.9% (45.4-74.9), 68.4% (54.8-80.1), and 58.3% (43.2-72.4); (3) arterial enhancement: 88.1% (77.1-95.1), 67.4% (52.0-80.5), 77.6% (65.8-86.9), and 81.6% (65.7-92.3); (4) arterial enhancement and washout on venous or equilibrium phase images: 79.7% (67.2-89.0), 97.8% (88.5-99.9), 97.9% (88.9-99.9), and 78.9% (66.1-88.6); (5) hypointensity on 20-min hepatobiliary phase images: 93.2% (83.5-98.1), 34.8% (21.4-50.2), 64.7% (53.6-74.8), and 80% (56.3-94.3); and (6) arterial enhancement and no or partial uptake on 20-min hepatobiliary phase images: 83.1% (71.0-91.6), 82.6% (68.6-92.2), 86% (74.2-93.7), and 79.2% (65.0-89.5). The MRI finding with the greatest sensitivity (93.2%) for detecting HCC was hypointensity on the 20-min hepatobiliary phase images, and the greatest specificity (97.8%) for detecting HCC occurred with typical arterial enhancement and washout on venous or equilibrium phase images. Excluding cysts and hemangiomas, the specificity of diagnosing HCC was greater for hyperintense T2-weighted images and for hypointensities on hepatobiliary phase images (Table 5).
| MRI findings | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
| Fat metamorphosis | 16.9 (8.4-29.0) | 93.3 (77.9-99.2) | 83.3 (51.6-97.9) | 36.4 (25.7-48.1) |
| Hyperintensity on T2-weighted images | 66.1 (52.6-77.9) | 93.3 (77.9-99.2) | 95.1 (83.5-99.4) | 58.3 (43.2-72.4) |
| Arterial enhancement | 88.1 (77.1-95.1) | 70.0 (50.6-85.3) | 85.2 (73.8-93.0) | 75.0 (55.1-89.3) |
| Arterial enhancement and washout on venous or equilibrium phase | 79.7 (67.2-89.0) | 96.7 (82.8-99.9) | 97.9 (88.9-99.9) | 70.7 (54.5-83.9) |
| Hypointensity on 20-min hepatobiliary phase | 93.2 (83.5-98.1) | 53.3 (34.3-71.7) | 79.7 (68.3-88.4) | 80.0 (56.3-94.3) |
| Arterial enhancement and no/partial uptake on 20-min hepatobiliary phase | 83.1 (71.0-91.6) | 93.3 (77.9-99.2) | 96.1 (86.5-99.5) | 73.7 (56.9-86.6) |
Table 6 summarizes the results of the univariate analysis of the ability of the MRI findings in the two groups to diagnose HCC, as well as the results after excluding cysts and hemangiomas. Although almost all of the MRI features were statistically significant in the univariate analysis, the multivariate analysis revealed that only the arterial enhancement and washout on venous or equilibrium phase images were statistically significant MRI findings (P < 0.001; OR = 102.0; 95%CI: 10.3-1012.4; Table 7). Furthermore, typical arterial enhancement followed by washout on venous or equilibrium phases, hyperintensity on T2-weighted images, and hypointensity on 20-min hepatobiliary phase images were statistically significant MRI findings, after excluding cysts and hemangiomas.
| MRI findings | All lesions (n = 105) | Lesions excluding cysts and hemangiomas (n = 89) | ||||
| HCC (n = 59) | Not HCC (n = 46) | P value | HCC (n = 59) | Not HCC (n = 30) | P value | |
| Fat metamorphosis | 10 (16.9) | 2 (4.3) | 0.061 | 10 (16.9) | 2 (6.7) | 0.190 |
| Hyperintensity on T2-weighted images | 39 (66.1) | 18 (39.1) | 0.007 | 39 (66.1) | 2 (6.7) | < 0.001 |
| Arterial enhancement | 52 (88.1) | 15 (32.6) | < 0.001 | 52 (88.1) | 9 (30) | < 0.001 |
| Arterial enhancement and washout on venous or equilibrium phase images | 47 (79.7) | 1 (2.2) | < 0.001 | 47 (79.7) | 1 (3.3) | < 0.001 |
| Hypointensity on 20-min hepatobiliary phase images | 55 (93.2) | 30 (65.2) | 0.001 | 55 (93.2) | 14 (46.7) | < 0.001 |
| Arterial enhancement and no/partial uptake on 20-min hepatobiliary phase images | 49 (83.1) | 8 (17.4) | < 0.001 | 49 (83.1) | 2 (6.7) | < 0.001 |
| MRI findings | All lesions (n = 105) | Lesions excluding cysts and hemangiomas (n = 89) | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Hyperintensity on T2-weighted images | 1.1 | 0.3-4.3 | 0.86 | 12.6 | 1.8-87.8 | 0.01 |
| Arterial enhancement | 1.8 | 0.4-7.0 | 0.42 | 3.5 | 0.5-22.8 | 0.18 |
| Arterial enhancement and washout on venous or equilibrium phase images | 102.0 | 10.3-1012.4 | < 0.001 | 16.9 | 1.3-221.1 | 0.03 |
| Hypointensity on 20-min hepatobiliary phase images | 1.8 | 0.4-8.6 | 0.48 | 6.7 | 1.1-41.9 | 0.04 |
Our study demonstrated that adding hepatobiliary phase Gd-EOB-DTPA-enhanced MRI significantly improved the accuracy of diagnosing HCC. The significant improvement was particularly apparent for small lesions (1-2 cm in diameter). No significant differences were observed for lesions larger than 2 cm in diameter. This finding could be explained by most large HCCs being unequivocally diagnosed based on clearly enhanced characteristics using conventional dynamic MRI. Smaller HCCs sometimes exhibit atypical enhancement characteristics. Therefore, the inclusion of the hepatobiliary phase provided more information for including or excluding the diagnosis of HCC for small nodules. However, other important information for diagnosing HCC was still based on conventional dynamic MRI.
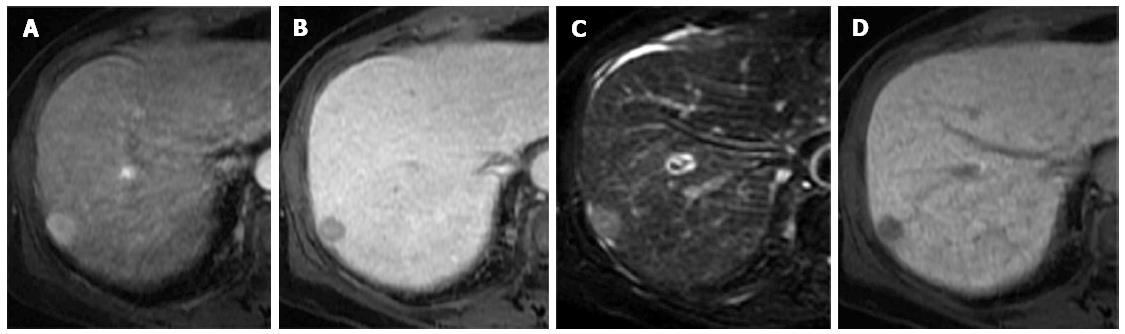
Sensitivity increased with the addition of hepatobiliary phase images, whereas specificity did not change. The inclusion of hepatobiliary phase images allowed subtle abnormalities present in other sequences to be visualized more clearly. The results of our study agreed with those of recent reports in terms of the improvement of diagnostic performance, the characterization of HCC, and the dismissal of pseudolesions[3,10,12-14]. In our study, adding the hepatobiliary phase images increased lesion detection by eight HCCs (13.5%), compared with conventional dynamic MRI (Figure 2).
Our study also highlighted other characteristic MRI findings that were involved in diagnosing HCC. Fat metamorphosis, which can be seen with chemical shift GRE imaging, was present in 10 of 59 HCCs (16.9%) with high specificity (93.3%). Two of the 46 benign lesions (4.3%) with fat components were histologically confirmed to be regenerative nodules in the background of cirrhosis and focal fat infiltration in normal livers. The results were similar to those in the study by Martín et al[15]. The presence of various fat alterations might be a significant morphological marker for malignant transformation from adenomatous hyperplasia to HCC, although it occurs rarely and can be present in benign hepatic nodules in cirrhotic patients[15,16].
A recent study demonstrated that T2-weighted images did not provide additional diagnostic value in detecting and characterizing focal lesions. The heterogeneity and hyperintense fibrotic septa and bridges in cirrhotic liver parenchyma can obscure moderately hyperintense HCCs on T2-weighted images, and 42%-53% of HCCs can also be isointense to hypointense on T2-weighted images[17]. In this study, however, the specificity improved with the addition of T2-weighted images after excluding cysts and hemangiomas, and T2-weighted images had the greatest sensitivity among all of the tests, with an odds ratio statistically different from 1 for differentiating HCC from benign lesions larger than 1 cm; these results were similar to those of previous reports[18,19].
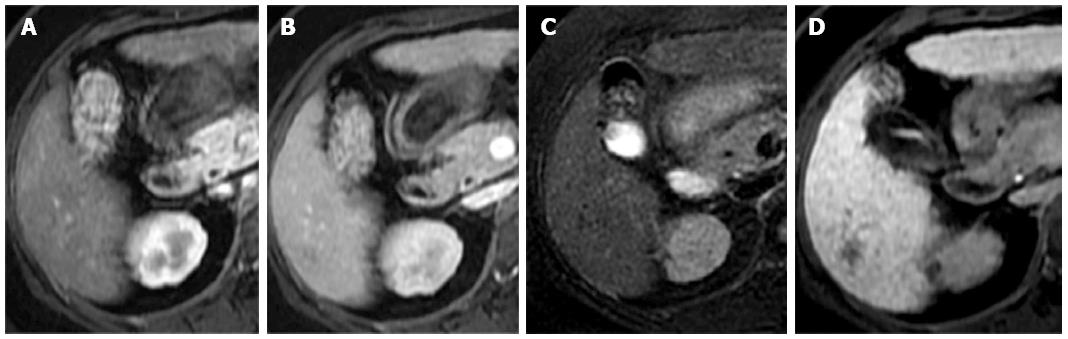
In 2010, the American Association for the Study of Liver Diseases guidelines for 1- to 2-cm HCCs were changed to require the visualization of a typical enhancement pattern using only one contrast-enhanced imaging technique[10]. In our series, a typical enhancement pattern was observed in 47 of 59 HCC lesions (79.7%) and in one of 30 benign lesions (3.3%); this benign lesion was determined by tissue biopsy to be a regenerating nodule. In our study, 12 of 59 HCC lesions (20.3%) had no typical arterial enhancement or washout on portal venous or equilibrium phase images, and these lesions were classified as well-differentiated HCCs (Figure 3); one had a motion artifact that resulted in a technical error. A recent study by Witjes et al[20] demonstrated a strong association between the presence of washout on dynamic MRI and moderately to poorly differentiated HCC.
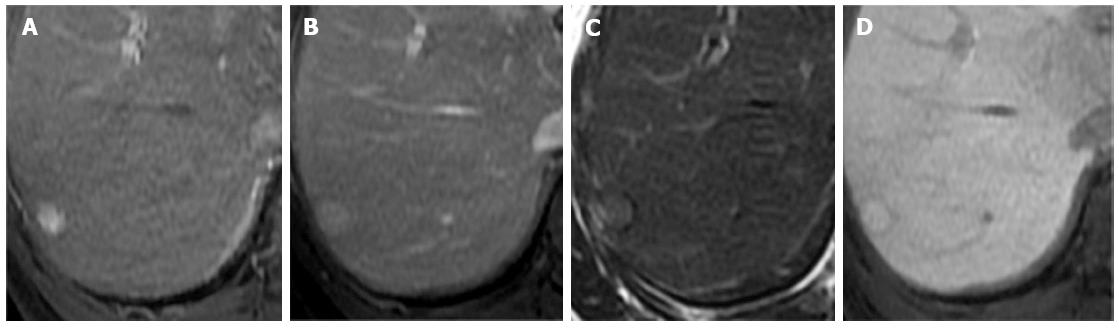
The majority of the HCCs in our study (55 lesions, 93.2%) had no uptake or partial uptake on the hepatobiliary phase images. Only four HCC lesions (6.8%) were iso- or hyperintense on the hepatobiliary phase images. Fortunately, three-quarters were scored as probably HCC based on the presence of a hypointense capsule, even without the typical enhancement pattern (Figure 3). Two lesions were histologically confirmed to be well-differentiated HCCs. The diminished obviousness of these well-differentiated lesions on hepatobiliary phase images might have been related to residual hepatocyte activity within the lesions, enabling the delayed uptake of the contrast material[3]. Another possible cause of diminished obviousness on the hepatobiliary phase images was impaired hepatic function or hyperbilirubinemia, which can affect the hepatocyte uptake of the contrast agent in the liver parenchyma[21]. However, all of the false-negative cases in our study that demonstrated iso- or hyperintensity on the hepatobiliary phase images had normal bilirubin levels. A recent study by Kim et al[22] reported that 10% of HCCs were hyperintense on hepatobiliary phase images, and most of the hyperintense HCCs were either well differentiated or moderately differentiated.
A variety of benign hepatic lesions can appear hypointense on hepatobiliary-phase MRI because of the following causes: (1) a lack of functional hepatocytes in the lesion; (2) damage to the functional hepatocytes from infection or inflammation; and (3) impairment of the biliary function of the lesion[23]. In this study, hepatic cysts and hemangiomas accounted for most of the benign hypointense lesions on hepatobiliary phase images. To reduce false-positive findings, hepatobiliary phase imaging should be considered an adjunct method, rather than the sole method, for diagnosing HCC. By excluding benign hepatic cysts and hemangiomas, the arterial enhancement with hypointensity on hepatobiliary phase images had high diagnostic accuracy, comparable to the typical enhancement pattern for HCC (sensitivity: 83.1% vs 79.7%; specificity: 93.3% vs 96.7%). However, false-positive cases accounted for one (3.3%) typical enhancement pattern and two (6.7%) arterial enhancement patterns with hypointensity on hepatobiliary phase images. These cases were hypothesized to be dysplastic nodules that had stable appearances on imaging.
Several limitations of this study should be addressed. First, the retrospective nature introduced the possibility of selection bias, although we attempted to avoid bias by recruiting consecutively registered patients who met the inclusion criteria. Second, not all of the lesions were confirmed pathologically, which could have introduced verification bias. Histopathology was obtained for 23 of 59 (39%) HCC lesions and four of 46 (8.7%) benign lesions. However, we attempted to avoid this factor by extending the follow-up period to confirm the benign diagnoses (mean: 22.2 mo). Additionally, lesions smaller than 1 cm in diameter were not included in the study. Third, because we had only one observer scoring the images, testing inter-observer reliability was not feasible, and observer bias could have occurred. Finally, our findings might not reflect a direct comparison between imaging with an extracellular contrast agent and a liver-specific hepatobiliary agent. Hepatobiliary enhancement can begin as early as the first pass (the portal venous phase); therefore, it can be difficult to define clearly which phase is the most important for diagnosing HCC: the contrast-enhanced dynamic images or the hepatobiliary phase images.
In a conclusions, The addition of hepatobiliary phase imaging after the intravenous injection of Gd-EOB-DTPA significantly improved diagnostic accuracy in detecting HCC in high-risk patients. Typical arterial enhancement followed by washout, hyperintensity on T2-weighted images, and hypointensity on 20-min hepatobiliary phase images were useful for diagnosing HCCs larger than 1 cm.
Dynamic imaging plays an important role in diagnosis of hepatocellular carcinoma (HCC), which is based on typical enhancement characteristics. For years, rapid arterial enhancement and rapid washout pattern in dynamic contrast study is accepted as one of diagnostic criteria without tissue pathology. However, there are still limitations in term of sensitivity and specificity, particular in small lesions or during early phase of hepatocarcinogenesis. The newly developed hepatocyte-specific contrast [gadolinium ethoxybenzyl diethylenetriaminepentaacetic (Gd-EOB-DTPA)] that has ability to evaluate both enhancement characteristics and hepatocyte function could have a potential in improving diagnostic accuracy.
The change of organic anion transporting polypeptide 8 expression in hepatocytes during the multistep process of hepatocarcinogenesis could be imaged by a newly developed hepatocyte-specific contrast agent, gadoxetic acid, on dynamic magnetic resonance imaging (MRI). The use of hepatocyte-specific contrast-enhanced MRI provides superior detection and characterization of HCC.
Adding hepatobiliary phase Gd-EOB-DTPA-enhanced MRI significantly improved the accuracy of diagnosing HCC. The significant improvement is apparent for small lesions (1-2 cm in diameter), for including or excluding the diagnosis of HCC, particular when the nodules exhibit atypical enhancement characteristics. The specificity can be improved with the addition of T2-weighted images after excluding cysts and hemangiomas during interpretation.
A typical enhancement pattern on dynamic imaging for diagnosing HCC has limited sensitivity, particular for small lesions. Therefore, the addition of hepatobiliary phase imaging after the intravenous injection of Gd-EOB-DTPA will improve diagnostic accuracy in detecting HCC. However, a variety of benign hepatic lesions can appear hypointense on hepatobiliary-phase MRI. To get the best diagnostic accuracy, the hepatobiliary phase imaging should be interpreted in together with early dynamic phases and T2-weighted images.
Gadoxetic acid (Gd-EOB-DTPA)-enhanced MRI of the liver has certain advantages over other imaging modalities in the detection and characterization of HCC in the high-risk liver. Hepatobiliary phase images obtained after Gd-EOB-DTPA-enhanced MRI imaging may improve diagnosis of HCC and assist in surgical planning. This study aims to the added value of hepatobiliary phase Gadoxetic acid-enhanced MRI for diagnosis of HCC by the fact that there would be the changes of hepatocyte-specific contrast uptake on hepatobiliary phase during hepatocarcinogenesis and the interpretation’s pitfalls of hepatobiliary phase MRI is also important to know for getting the most accurate result.
P- Reviewers: Liu B, Niu ZS, Xie F S- Editor: Wen LL L- Editor: A E- Editor: Ma S
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25540] [Article Influence: 1824.3] [Reference Citation Analysis (7)] |
| 2. | Vatanasapt V, Sriamporn S, Vatanasapt P. Cancer control in Thailand. Jpn J Clin Oncol. 2002;32 Suppl:S82-S91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Ahn SS, Kim MJ, Lim JS, Hong HS, Chung YE, Choi JY. Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology. 2010;255:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 282] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 4. | Forner A, Vilana R, Ayuso C, Bianchi L, Solé M, Ayuso JR, Boix L, Sala M, Varela M, Llovet JM. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 727] [Article Influence: 42.8] [Reference Citation Analysis (1)] |
| 5. | Kitao A, Matsui O, Yoneda N, Kozaka K, Shinmura R, Koda W, Kobayashi S, Gabata T, Zen Y, Yamashita T. The uptake transporter OATP8 expression decreases during multistep hepatocarcinogenesis: correlation with gadoxetic acid enhanced MR imaging. Eur Radiol. 2011;21:2056-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 6. | Leonhardt M, Keiser M, Oswald S, Kühn J, Jia J, Grube M, Kroemer HK, Siegmund W, Weitschies W. Hepatic uptake of the magnetic resonance imaging contrast agent Gd-EOB-DTPA: role of human organic anion transporters. Drug Metab Dispos. 2010;38:1024-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Halavaara J, Breuer J, Ayuso C, Balzer T, Bellin MF, Blomqvist L, Carter R, Grazioli L, Hammerstingl R, Huppertz A. Liver tumor characterization: comparison between liver-specific gadoxetic acid disodium-enhanced MRI and biphasic CT--a multicenter trial. J Comput Assist Tomogr. 2006;30:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Hammerstingl R, Huppertz A, Breuer J, Balzer T, Blakeborough A, Carter R, Fusté LC, Heinz-Peer G, Judmaier W, Laniado M. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol. 2008;18:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 313] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 9. | Kim SH, Kim SH, Lee J, Kim MJ, Jeon YH, Park Y, Choi D, Lee WJ, Lim HK. Gadoxetic acid-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR Am J Roentgenol. 2009;192:1675-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 10. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6572] [Article Influence: 469.4] [Reference Citation Analysis (1)] |
| 11. | Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 664] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 12. | Golfieri R, Renzulli M, Lucidi V, Corcioni B, Trevisani F, Bolondi L. Contribution of the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI to Dynamic MRI in the detection of hypovascular small (≤ 2 cm) HCC in cirrhosis. Eur Radiol. 2011;21:1233-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Huppertz A, Balzer T, Blakeborough A, Breuer J, Giovagnoni A, Heinz-Peer G, Laniado M, Manfredi RM, Mathieu DG, Mueller D. Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology. 2004;230:266-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 323] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 14. | Chou CT, Chen YL, Su WW, Wu HK, Chen RC. Characterization of cirrhotic nodules with gadoxetic acid-enhanced magnetic resonance imaging: the efficacy of hepatocyte-phase imaging. J Magn Reson Imaging. 2010;32:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Martín J, Sentís M, Zidan A, Donoso L, Puig J, Falcó J, Bella R. Fatty metamorphosis of hepatocellular carcinoma: detection with chemical shift gradient-echo MR imaging. Radiology. 1995;195:125-130. [PubMed] |
| 16. | Eguchi A, Nakashima O, Okudaira S, Sugihara S, Kojiro M. Adenomatous hyperplasia in the vicinity of small hepatocellular carcinoma. Hepatology. 1992;15:843-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 94] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Hussain HK, Syed I, Nghiem HV, Johnson TD, Carlos RC, Weadock WJ, Francis IR. T2-weighted MR imaging in the assessment of cirrhotic liver. Radiology. 2004;230:637-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Bashir MR, Gupta RT, Davenport MS, Allen BC, Jaffe TA, Ho LM, Boll DT, Merkle EM. Hepatocellular carcinoma in a North American population: does hepatobiliary MR imaging with Gd-EOB-DTPA improve sensitivity and confidence for diagnosis? J Magn Reson Imaging. 2013;37:398-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Kim JE, Kim SH, Lee SJ, Rhim H. Hypervascular hepatocellular carcinoma 1 cm or smaller in patients with chronic liver disease: characterization with gadoxetic acid-enhanced MRI that includes diffusion-weighted imaging. AJR Am J Roentgenol. 2011;196:W758-W765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Witjes CD, Willemssen FE, Verheij J, van der Veer SJ, Hansen BE, Verhoef C, de Man RA, Ijzermans JN. Histological differentiation grade and microvascular invasion of hepatocellular carcinoma predicted by dynamic contrast-enhanced MRI. J Magn Reson Imaging. 2012;36:641-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Murakami T, Kim T, Gotoh M, Hasuike Y, Kato N, Miyazawa T, Monden M, Nakamura H. Experimental hepatic dysfunction: evaluation by MR imaging with Gd-EOB-DTPA. Acad Radiol. 1998;5 Suppl 1:S80-S82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Kim MJ, Rhee HJ, Jeong HT. Hyperintense lesions on gadoxetate disodium-enhanced hepatobiliary phase imaging. AJR Am J Roentgenol. 2012;199:W575-W586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Hwang HS, Kim SH, Jeon TY, Choi D, Lee WJ, Lim HK. Hypointense hepatic lesions depicted on gadobenate dimeglumine-enhanced three-hour delayed hepatobiliary-phase MR imaging: differentiation between benignancy and malignancy. Korean J Radiol. 2009;10:294-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |