Published online Dec 7, 2013. doi: 10.3748/wjg.v19.i45.8335
Revised: September 13, 2013
Accepted: September 16, 2013
Published online: December 7, 2013
Processing time: 134 Days and 20.7 Hours
AIM: To investigate the association of procalcitonin (PCT) with ulcerative colitis (UC) activity.
METHODS: Serum PCT levels, C-reactive protein (CRP) levels, the erythrocyte sedimentation rate, and the white blood cell count were analyzed in 18 patients with UC and 11 healthy volunteers. Serum PCT levels were analyzed by an electrochemiluminescence immunoassay. Severity assessments were based on Truelove and Witts’ severity index. Correlation of serum PCT and CRP levels with UC activity was examined. Moreover, we assessed serum PCT and CRP levels in patients with a Mayo endoscopic subscore.
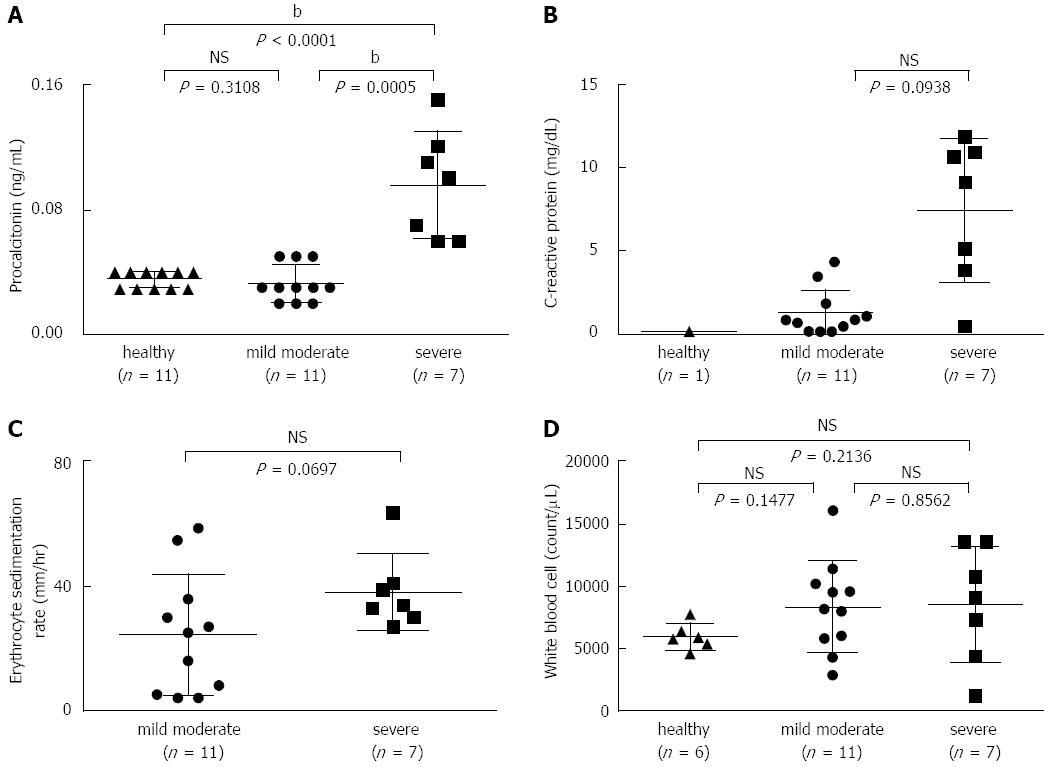
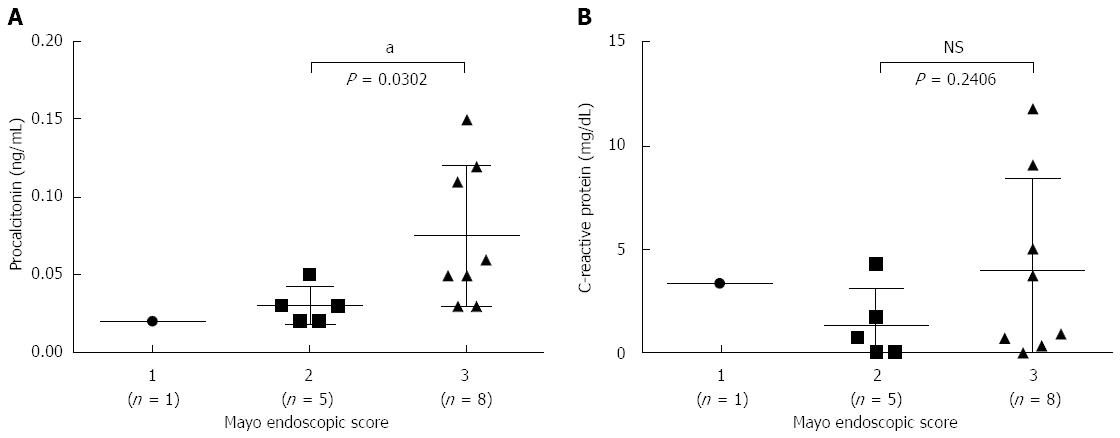
RESULTS: Serum PCT levels in severe UC patients (n = 7) (0.096 ± 0.034 ng/mL) were significantly higher than in mild-to-moderate UC patients (n = 11) (0.033 ± 0.012 ng/mL) and healthy volunteers (n = 11) (0.035 ± 0.005 ng/mL) (P = 0.0005 and P < 0.0001, respectively). In addition, there was no difference in serum PCT levels between mild-to-moderate UC patients and healthy volunteers. Interestingly, patients with a Mayo endoscopic subscore of 3 points displayed significantly increased levels of serum PCT (0.075 ± 0.043 ng/mL) compared with patients with a subscore of 2 points (0.03 ± 0.011 ng/mL) (P = 0.0302). Moreover, CRP levels in patients with severe UC or a Mayo endoscopic subscore of 3 points were not significantly higher than in patients with mild-to-moderate UC or a Mayo endoscopic subscore of 3 points.
CONCLUSION: Serum PCT levels were significantly correlated with UC activity.
Core tip: To investigate the association of procalcitonin (PCT) with ulcerative colitis (UC) activity, we analyzed 18 UC patients (7 with severe and 11 with mild-to-moderate UC) and 11 healthy volunteers. Serum PCT levels in severe UC patients were significantly higher than in mild-to-moderate UC patients and healthy volunteers. Moreover, patients with a Mayo endoscopic subscore of 3 points displayed significantly increased serum PCT levels compared with patients with a subscore of 2 points. However, serum C-reactive protein was not associated with disease activity or the Mayo endoscopic score. Thus, serum PCT may be a good biomarker for assessing UC activity.
- Citation: Koido S, Ohkusa T, Takakura K, Odahara S, Tsukinaga S, Yukawa T, Mitobe J, Kajihara M, Uchiyama K, Arakawa H, Tajiri H. Clinical significance of serum procalcitonin in patients with ulcerative colitis. World J Gastroenterol 2013; 19(45): 8335-8341
- URL: https://www.wjgnet.com/1007-9327/full/v19/i45/8335.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i45.8335
Ulcerative colitis (UC) and Crohn’s disease (CD) are idiopathic inflammatory bowel diseases that are generally complicated by systemic or local infection. Clinical, endoscopic, histological, and radiological investigations are typically necessary to make an accurate diagnosis and assessment of disease activity[1]; however, disease activity in UC is difficult to assess objectively because clinical disease activity indices depend on several subjective components. Biological markers have also been investigated in the United States for diagnostic purposes, for the assessment of disease activity and the prediction of relapse, and for monitoring the effect of therapy[2]. However, traditional markers, such as C-reactive protein (CRP), the erythrocyte sedimentation rate (ESR), and the white blood cell (WBC) count, are still the most common markers used in clinical practice[3]. Among these biological markers, CRP is a protein produced by the liver in response to various acute and chronic inflammatory conditions and has been shown to be a good marker for predicting the course and outcome of several diseases[4]. Importantly, heterogeneity exists among individuals’ immune responses, and elevations in CRP levels are more common in CD than in UC due to differences in acute-phase responses in CD and UC[5]. Indeed, only 51% of UC patients with active disease based on colonoscopy have elevated CRP levels[6]. This finding suggests that CRP levels add to clinical assessment’s ability to predict active mucosal inflammation in UC patients. Thus, the UC biomarkers currently used in clinical practice are of low sensitivity and often do not reliably assess clinical activity[2].
Procalcitonin (PCT), a prohormone composed of 116 amino acids, is the precursor of the calcium homoeostasis hormone calcitonin, which is found in thyroid C cells and pulmonary endocrine cells[7]. Clinically relevant levels of PCT influence the immunologic responses that contribute to systemic inflammatory responses and septic shock[8]. Many studies have indicated that PCT is an excellent marker of bacterial infection in patients with sepsis and its related conditions[9,10]. As a marker of disease activity, PCT has also been evaluated in chronic inflammatory and autoimmune conditions, such as pulmonary Wegener’s granulomatosis[11] and systemic lupus erythematosus/systemic antineutrophil cytoplasmic antibody-associated vasculitis[12]; therefore, serum PCT levels may be helpful for predicting the disease activity of UC.
This prospective study aimed to assess the correlation of serum PCT levels with UC activity. Moreover, we compared PCT levels, CRP levels, the ESR, and the WBC count and evaluated the additional diagnostic benefit of measuring serum PCT levels along with CRP levels in the assessment of disease activity in UC.
This study was conducted prospectively from March 2011 to May 2013 in a single-center cohort. The study protocol was reviewed and approved by the ethics committee of the Jikei Institutional Review Board, Jikei University School of Medicine, and the clinical study committee of Jikei University Kashiwa Hospital. All of the subjects provided written informed consent. The diagnosis of UC was confirmed by a typical history combined with appropriate endoscopic, histopathological, and radiologic findings. The eligibility criteria for study participation were mild-to-severe UC[13] with an endoscopy score of at least 1 (erythema, a decreased vascular pattern, and mild friability) on a scale of 0 (normal or inactive) to 3 (spontaneous bleeding and ulceration)[14] and/or watery diarrhea at least 5 times/d with visible blood in the stools. Severity assessments were based on Truelove and Witts’ severity index[13]. After total colonoscopy, the endoscopic findings were evaluated according to the Mayo system, with scores of 0-3[14]. Patients with toxic megacolon or concomitant diseases, including diabetes, hematological disorders, any malignancies, obvious infection or sepsis, chronic liver disorder, or any liver or renal diseases were excluded. Subjects who were recruited had venous blood samples drawn to assess PCT levels, CRP levels, the WBC count, and the ESR. Where clinically indicated, stool cultures were performed to exclude infective etiology for diarrhea symptoms.
When patients had an established diagnosis of UC, serum PCT levels, CRP levels, the WBC count, and the ESR were examined. Serum PCT levels were analyzed by an electrochemiluminescence immunoassay (ECLIA) at the SRL (Tokyo, Japan), with a lower detection limit of 0.02 ng/mL. The normal value for PCT levels was defined as < 0.05 ng/mL, with a cut-off of ≥ 0.5 ng/mL being indicative of bacterial sepsis at the SRL. Based on our laboratory reference, the normal limits for the other inflammatory variables were a CRP level of 0.0-0.3 mg/dL, a WBC count of 3300-8600 count/μL, and an ESR of 2-19 mm/h.
Values are expressed as the mean ± SD. The statistical analysis was performed using the Mann-Whitney test and the Kruskal-Wallis one-way analysis of variance on ranks. Proportions were compared using Fisher’s exact test and Cramer’s V post-test. The statistical analysis was performed with STAT VIEW software, version J 5.1 (SAS Institute, Inc., Cary, NC, United States), which was used for all analyses. P < 0.05 was considered significant.
Eighteen UC patients (mean age: 39.6 ± 15.2 years; 13 male, 5 female) were included in the study. The distribution of age and sex was not significantly different between mild-to-moderate UC (mean age: 39.3 ± 12.5 years; 6 male, 5 female), severe UC (mean age; 40.0 ± 19.7 years; 7 male, 0 female), and the control healthy volunteers (mean age 40.2 ± 9.92 years, 10 male, 1 female) (Table 1). In patients with UC, mild-to-moderate activity was observed in 11 patients (61.1%), and severe activity was observed in 7 (38.9%). Moreover, there was no difference in the extent of disease and concomitant medication use between mild-to-moderate and severe UC (Table 1).
| Mild-to-moderate1 | Severe | P value | |
| Age upon entry, yr (mean ± SD) | 39.3 ± 12.5 | 40.0 ± 19.7 | > 0.2 |
| Gender, male/female | 6/5 | 7/0 | > 0.2 |
| Extent of disease | |||
| Number of patients | > 0.2 | ||
| Extensive | 7 | 7 | |
| Left-side | 4 | 0 | |
| Proctitis | 0 | 0 | |
| Concomitant medication use | |||
| Number of patients | > 0.2 | ||
| Sulfasalazine | 3 | 0 | |
| 5-ASA | 9 | 7 | |
| Glucocorticoid | 6 | 2 | |
| Immunomodulator | 1 | 0 | |
In this study, serum PCT levels were analyzed by ECLIA at the SRL, with a lower detection limit of 0.02 ng/mL. The normal value for PCT levels has been defined as < 0.05 ng/mL, with a cut-off of ≥ 0.5 ng/mL being indicative of bacterial sepsis at the SRL. There was no significant difference in serum PCT levels between patients with mild-to-moderate UC (0.033 ± 0.012 ng/mL) and healthy volunteers (0.035 ± 0.005 ng/mL) (P = 0.3108) (Figure 1A). Interestingly, patients with severe UC displayed significantly increased levels of serum PCT (0.096 ± 0.034 ng/mL) compared with patients with mild-to-moderate UC and healthy volunteers (P = 0.0005 and P < 0.0001, respectively, Figure 1A). Moreover, CRP levels, the ESR, and the WBC count in severe UC were not significantly higher than in mild-to-moderate UC (P = 0.0938, 0.0697, and 0.8562, respectively) (Figure 1B-D).
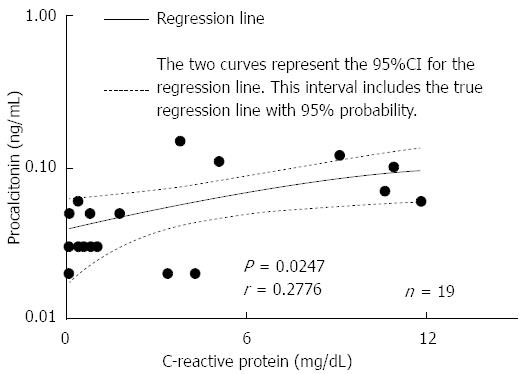
Among mild-to-moderate UC patients, 3 displayed 0.05 ng/mL serum PCT, which was above normal; however, another 8 exhibited normal levels. Interestingly, serum PCT levels were > 0.06 ng/mL in all 7 patients with severe UC. In addition, the levels in all healthy volunteers were < 0.04 ng/mL, which was defined as normal at the SRL. A PCT cut-off value of 0.055 ng/mL had good diagnostic accuracy for detecting severe UC, as demonstrated by the sensitivity (100%; 95%CI: 59.04-100.0) and specificity (100%; 95%CI: 71.51-100.0) (Table 2). Moreover, at a cut-off level of CRP > 3.6 mg/dL, the sensitivity for detecting severe UC was 85.71% (95%CI: 42.13-99.64), and the specificity was 90.91% (95%CI: 58.72-99.77) (Table 2). In addition, there was a significant correlation between PCT and CRP levels (r = 0.2776, P = 0.0247) (Figure 2). Taken together, these results suggest that serum PCT is superior as a detection marker of severe UC compared with CRP.
| Marker | Sensitivity | 95%CI | Specificity | 95%CI |
| Procalcitonin (ng/mL) | ||||
| > 0.0250 | 100% | 59.04-100.0 | 27.27% | 6.022-60.97 |
| > 0.0400 | 100% | 59.04-100.0 | 72.73% | 39.03-93.98 |
| > 0.0550 | 100% | 59.04-100.0 | 100% | 71.51-100.0 |
| > 0.0650 | 71.43% | 29.04-96.33 | 100% | 71.51-100.0 |
| > 0.0850 | 57.14% | 18.41-90.10 | 100% | 71.51-100.0 |
| > 0.1050 | 42.86% | 9.899-81.59 | 100% | 71.51-100.0 |
| > 0.1150 | 28.57% | 3.669-70.96 | 100% | 71.51-100.0 |
| > 0.1350 | 14.29% | 0.3610-57.87 | 100% | 71.51-100.0 |
| C-reactive protein (mg/dL) | ||||
| > 0.2500 | 100% | 59.04-100.0 | 27.27% | 6.022-60.97 |
| > 0.5000 | 85.71% | 42.13-99.64 | 36.36% | 10.93-69.21 |
| > 0.7000 | 85.71% | 42.13-99.64 | 45.45% | 16.75-76.62 |
| > 0.9000 | 85.71% | 42.13-99.64 | 63.64% | 30.79-89.07 |
| > 1.400 | 85.71% | 42.13-99.64 | 72.73% | 39.03-93.98 |
| > 2.600 | 85.71% | 42.13-99.64 | 81.82% | 48.22-97.72 |
| > 3.600 | 85.71% | 42.13-99.64 | 90.91% | 58.72-99.77 |
| > 4.050 | 71.43% | 29.04-96.33 | 90.91% | 58.72-99.77 |
| > 4.700 | 71.43% | 29.04-96.33 | 100% | 71.51-100.0 |
| > 7.100 | 57.14% | 18.41-90.10 | 100% | 71.51-100.0 |
| > 9.850 | 42.86% | 9.899-81.59 | 100% | 71.51-100.0 |
| > 10.75 | 28.57% | 3.669-70.96 | 100% | 71.51-100.0 |
| > 11.35 | 14.29% | 0.3610-57.87 | 100% | 71.51-100.0 |
| > 11.35 | 14.29% | 0.3610-57.87 | 100% | 76.84-100.0 |
Regarding Truelove and Witts’ scoring, it could be difficult to use a scale of 1-3. Therefore, 14 patients with UC were also examined for their Mayo endoscopic score and serum PCT and CRP levels after total colonoscopy in this study. As only 1 patient had an endoscopic subscore of 1 point, we assessed serum PCT and CRP levels in patients with a subscore of 2 (n = 5) or 3 (n = 8) points. There was no significant difference in serum CRP levels between patients with a subscore of 2 or 3 points (P = 0.2406), whereas patients with a subscore of 3 points displayed significantly increased levels of serum PCT (0.075 ± 0.043 ng/mL) compared with patients with a subscore of 2 points (0.03 ± 0.011 ng/mL) (P = 0.0302) (Figure 3).
This study demonstrates that serum PCT levels are correlated with clinical, biological, or endoscopic disease activity in patients with UC. Moreover, serum PCT levels have superior diagnostic accuracy for detecting severe UC in comparison with CRP levels, the ESR, and the WBC count.
The assessment of disease activity in patients with UC has been performed using clinical and endoscopic indices. Biological markers are a noninvasive way of objectively measuring inflammation and can play an adjunctive or primary role in the assessment of disease activity. The currently used UC biomarkers, such as CRP, the ESR, and WBC and platelet counts, have low sensitivity and often do not reliably assess clinical activity[2,15]. It has been reported that although both PCT and CRP are acute-phase reactant proteins, PCT is a more specific marker of bacterial infections[16]. Moreover, PCT is an excellent marker of bacterial infection in patients with sepsis and its related conditions[9,10]. There is growing evidence that colonic luminal bacteria contribute to clinical activity in UC[17], suggesting that serum PCT levels may be an important biomarker for detecting active UC; however, little is known about whether serum PCT is a reliable marker for the diagnosis of active UC. To date, four studies have evaluated PCT levels in adult patients with UC[18-21]. Among the four studies, only one[21] evaluated the correlation between serum PCT levels and Truelove and Witts’ severity index or a simple clinical colitis activity index (SCCAI) > 5[22]; however, this report demonstrated conflicting results when evaluating serum PCT as a biological marker of UC activity. Our present study is the first to show that serum PCT levels in patients with UC are significantly correlated with Truelove and Witts’ severity index and a Mayo endoscopic subscore. Importantly, serum PCT levels in patients with a Mayo endoscopic subscore of 3 points are significantly higher than in patients with a subscore of 2 points. PCT levels have been evaluated in chronic inflammatory and autoimmune conditions, such as pulmonary Wegener’s granulomatosis[11] and systemic lupus erythematosus/systemic antineutrophil cytoplasmic antibody-associated vasculitis[12]. Moreover, PCT levels have been associated with bacterial infection[9,10]. Severe UC patients are characterized by bacterial invasion due to a defensive deficiency of the mucosa; thus, it is possible that PCT levels are correlated with disease activity in UC. In our study, serum PCT levels were analyzed by ECLIA, which showed good reproducibility, linearity, and functional sensitivity compared with previously reported immunofluorescent assays[23]. The superior detection of serum PCT in our study may be explained, at least in part, by differences in methodology. In contrast, serum CRP levels in severe UC and in patients with a Mayo endoscopic subscore of 3 points were not significantly higher than in mild-to-moderate UC or in patients with a subscore of 2 points, respectively. It has been reported that local and systemic administration of steroids has the potential to decrease CRP levels[24-27], whereas PCT levels appear to be unaltered[24,25]. In the current study, there was no significant difference in concomitant medication use, such as steroid use, between mild-to-moderate and severe UC. Therefore, it is suggested that PCT levels are correlated with disease activity in UC.
In conclusion, our results suggest that serum PCT levels are useful in clinical practice to assess disease activity in patients with UC. The limitation of our study is the relatively small sample size that was evaluated. Thus, further studies are needed to evaluate the clinical significance of serum PCT in a large sample of UC patients. Moreover, a definitive approach is needed to assess serum PCT levels as a marker for managing severe UC patients after treatment.
Biological markers have been investigated in the United States for the assessment of disease activity. Among traditional biological markers, C-reactive protein (CRP) has been shown to be a good marker for predicting the course and outcome of several diseases. However, only 51% of ulcerative colitis (UC) patients with active disease based on colonoscopy have elevated CRP levels, suggesting that CRP levels add to clinical assessment’s ability to predict active mucosal inflammation in UC patients.
Traditional biological markers, such as CRP, often do not reliably assess clinical activity in UC patients. In the assessment of clinical activity in UC, the research hotspot is the identification of excellent biomarkers.
It has been reported that although procalcitonin (PCT) is an excellent specific marker of bacterial infections in patients with sepsis and its related conditions. Moreover, colonic luminal bacteria contribute to clinical activity in UC. Thus, serum PCT levels may be an important biomarker for detecting active UC. This prospective study aimed to assess the correlation of serum PCT levels with UC activity. Authors showed that serum PCT levels in patients with UC were significantly correlated with Truelove and Witts’ severity index and the Mayo endoscopic subscore.
The study’s results suggest that serum PCT is an excellent biological marker for assessing the activity of UC.
UC and Crohn’s disease are idiopathic inflammatory bowel diseases that are generally complicated by systemic or local infection. Clinical UC activity is difficult to assess objectively because of several subjective components. PCT is the precursor of the calcium homoeostasis hormone calcitonin, which is found in thyroid C cells and pulmonary endocrine cells.
The strength of this study is a well-executed study. The statistics are appropriate, and the manuscript is well written. This study demonstrates that serum PCT levels are useful in clinical practice to assess disease activity in patients with UC. However, the limitation of the study is the relatively small sample size evaluated. The study recommend a future study on the results of treatment based on PCT levels in a large sample.
P- Reviewers: Afzal NA, Hokama A, Kim YJ S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Bruining DH, Loftus EV. Evolving diagnostic strategies for inflammatory bowel disease. Curr Gastroenterol Rep. 2006;8:478-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 633] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 3. | D’Haens G, Sandborn WJ, Feagan BG, Geboes K, Hanauer SB, Irvine EJ, Lémann M, Marteau P, Rutgeerts P, Schölmerich J. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 790] [Article Influence: 43.9] [Reference Citation Analysis (1)] |
| 4. | Tillett WS, Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J Exp Med. 1930;52:561-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 899] [Cited by in RCA: 891] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 5. | Saverymuttu SH, Hodgson HJ, Chadwick VS, Pepys MB. Differing acute phase responses in Crohn’s disease and ulcerative colitis. Gut. 1986;27:809-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Solem CA, Loftus EV, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 404] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 7. | Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | Liappis AP, Gibbs KW, Nylen ES, Yoon B, Snider RH, Gao B, Becker KL. Exogenous procalcitonin evokes a pro-inflammatory cytokine response. Inflamm Res. 2011;60:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Whicher J, Bienvenu J, Monneret G. Procalcitonin as an acute phase marker. Ann Clin Biochem. 2001;38:483-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Le Moullec JM, Jullienne A, Chenais J, Lasmoles F, Guliana JM, Milhaud G, Moukhtar MS. The complete sequence of human preprocalcitonin. FEBS Lett. 1984;167:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 167] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Zycinska K, Wardyn KA, Zielonka TM, Tyszko P, Straburzynski M. Procalcitonin as an indicator of systemic response to infection in active pulmonary Wegener’s granulomacytosis. J Physiol Pharmacol. 2008;59 Suppl 6:839-844. [PubMed] |
| 12. | Eberhard OK, Haubitz M, Brunkhorst FM, Kliem V, Koch KM, Brunkhorst R. Usefulness of procalcitonin for differentiation between activity of systemic autoimmune disease (systemic lupus erythematosus/systemic antineutrophil cytoplasmic antibody-associated vasculitis) and invasive bacterial infection. Arthritis Rheum. 1997;40:1250-1256. [PubMed] |
| 13. | Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1832] [Cited by in RCA: 1863] [Article Influence: 26.6] [Reference Citation Analysis (1)] |
| 14. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1958] [Cited by in RCA: 2244] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 15. | Linskens RK, van Bodegraven AA, Schoorl M, Tuynman HA, Bartels P. Predictive value of inflammatory and coagulation parameters in the course of severe ulcerative colitis. Dig Dis Sci. 2001;46:644-648. [PubMed] |
| 16. | Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39:206-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1067] [Cited by in RCA: 1128] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 17. | Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1071] [Cited by in RCA: 920] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 18. | Herrlinger KR, Dittmann R, Weitz G, Wehkamp J, Ludwig D, Schwab M, Stange EF, Fellermann K. Serum procalcitonin differentiates inflammatory bowel disease and self-limited colitis. Inflamm Bowel Dis. 2004;10:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Thia KT, Chan ES, Ling KL, Ng WY, Jacob E, Ooi CJ. Role of procalcitonin in infectious gastroenteritis and inflammatory bowel disease. Dig Dis Sci. 2008;53:2960-2968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Oruç N, Ozütemiz O, Osmanoğlu N, Ilter T. Diagnostic value of serum procalcitonin in determining the activity of inflammatory bowel disease. Turk J Gastroenterol. 2009;20:9-12. [PubMed] |
| 21. | Oussalah A, Laurent V, Bruot O, Guéant JL, Régent D, Bigard MA, Peyrin-Biroulet L. Additional benefit of procalcitonin to C-reactive protein to assess disease activity and severity in Crohn’s disease. Aliment Pharmacol Ther. 2010;32:1135-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1033] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 23. | Prieto B, Miguel D, Costa M, Coto D, Alvarez FV. New quantitative electrochemiluminescence method (ECLIA) for interleukin-6 (IL-6) measurement. Clin Chem Lab Med. 2010;48:835-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Müller B, Peri G, Doni A, Perruchoud AP, Landmann R, Pasqualini F, Mantovani A. High circulating levels of the IL-1 type II decoy receptor in critically ill patients with sepsis: association of high decoy receptor levels with glucocorticoid administration. J Leukoc Biol. 2002;72:643-649. [PubMed] |
| 25. | de Kruif MD, Lemaire LC, Giebelen IA, Struck J, Morgenthaler NG, Papassotiriou J, Elliott PJ, van der Poll T. The influence of corticosteroids on the release of novel biomarkers in human endotoxemia. Intensive Care Med. 2008;34:518-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Rinaldi S, Adembri C, Grechi S, De Gaudio AR. Low-dose hydrocortisone during severe sepsis: effects on microalbuminuria. Crit Care Med. 2006;34:2334-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, Della Porta R, Giorgio C, Blasi F, Umberger R. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 494] [Article Influence: 24.7] [Reference Citation Analysis (0)] |