Published online Dec 7, 2013. doi: 10.3748/wjg.v19.i45.8326
Revised: August 9, 2013
Accepted: September 16, 2013
Published online: December 7, 2013
Processing time: 202 Days and 16 Hours
AIM: To assess whether the use of porcine models is useful for learning endoscopic submucosal dissection (ESD), thus contributing to its subsequent application in human patients.
METHODS: This study/learning process was carried out in 3 phases: Phase I: Ex vivo animal; Phase II: In vivo animal; Phase III: Humans. One endoscopist performed 30 gastric ESDs in porcine models, and later 5 gastric ESDs in 5 patients. The ESD was done following the method practiced at the National Cancer Center in Tokyo, Japan. Technical aspects, size, time and speed of ESD, as well as complications were registered. In patients, their clinical, endoscopic and histologic evolution was additionally added.
RESULTS: Thirty en bloc ESDs were carried out in animal models. The mean ± SD size of the pieces was of 28.4 ± 1.2 mm, and the time of ESD was 41.7 ± 2.4 min. The time of ESD in the first 15 procedures was 43.0 ± 3.0 min whereas in the next 15 procedures, the time was 40.3 ± 3.9 min, P = 0.588. The speed in the first 15 ESDs was 1.25 ± 0.11 cm2/min vs 2.12 ± 0.36 cm2/min in the remaining 15, P = 0.028. There were no complications. In patients, 5 lesions were resected en bloc. The size of the pieces was 25.2 ± 5.1 mm and the time was 85.0 ± 25.6 min. Endoscopic and histological controls did not show evidence of residual neoplastic tissue.
CONCLUSION: A sequential ESD training program of a unique endoscopist, based on the practice in porcine models, contributed to learning ESD for its subsequent application in humans, yielding good results in efficacy and safety.
Core tip: This study was conducted with the purpose of determining in a prospective manner the results, the efficacy and safety of learning endoscopic submucosal dissection (ESD), in porcine models and assessing whether this practice contributes to subsequent application of this technique in patients. The present study shows interesting findings in this regards. The authors have demonstrated the value of a sequential ESD program and believe that it can contribute to disseminate this technique and encourage its learning mostly in western countries where it is still not a common practice for different reasons.
- Citation: González N, Parra-Blanco A, Villa-Gómez M, Gamba A, Taullard A, Silveira A, Sanguinetti A, Olano C, Cohen H. Gastric endoscopic submucosal dissection: From animal model to patient. World J Gastroenterol 2013; 19(45): 8326-8334
- URL: https://www.wjgnet.com/1007-9327/full/v19/i45/8326.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i45.8326
Endoscopic submucosal dissection (ESD) is a technique for resection of early neoplastic gastric lesions. It is applied to extirpate large lesions (greater than 15 mm), removing in one bloc more healthy adjacent tissue (horizontal oncologic border) and more submucosal tissue (vertical oncologic border), thus obtaining more complete resections than with the standard mucosectomy and decreasing the probability of relapses[1-5]. The ESD was developed in 1999 in Japan, initially for resection of gastric tumors, and later of esophageal, colonic and rectal lesions[1,2]. Although it is an effective, safe and widely accepted[3,4] technique in experienced hands, its application in Western countries has not yet been extensive due to several reasons. One of them is that the main indication of ESD in Japan is early gastric cancer, which has a high prevalence in that country but is less frequent in the Western world, which makes learning this technique a difficult task[5].
To learn the ESD technique, Japanese authors have suggested a structured and progressive training program, with the recommendation of being initially applied in porcine models. First ex vivo and then in vivo in order to acquire the necessary technical skill and experience before moving on to practice ESD in patients, mainly when it cannot be performed under the direct supervision of experts[6].
Despite these recommendations, there are not many publications on gastric ESD results in porcine models as part of the training for the practice of this technique[7-10].
To prospectively determine the results, efficacy and safety of ESD in porcine models during the training period. To assess whether the practice in porcine models contributes to its subsequent application in patients.
One endoscopist with experience in ESD (Parra-Blanco A) trained a younger one (Gonzalez N), in that technique. This one had previous experience in diagnostic and emergency gastroscopy and therapeutic colonoscopy and in performing endoscopic mucosal resection. During this training period, González N observed 6 ESD procedures, of which 5 were conducted in ex vivo models and one in the stomach of a live porcine. He additionally performed two gastric dissections in cadaveric models and one dissection of a gastric lesion in an in vivo model.
The training was further supplemented with the reading of articles related to ESD, in order to internalize theoretical concepts along with the visualization of videos showing how this technique was applied by Japanese experts.
He finally carried out a study/learning process in 3 phases: Phase I: Ex vivo animals; Phase II: In vivo animals; Phase III: Humans.
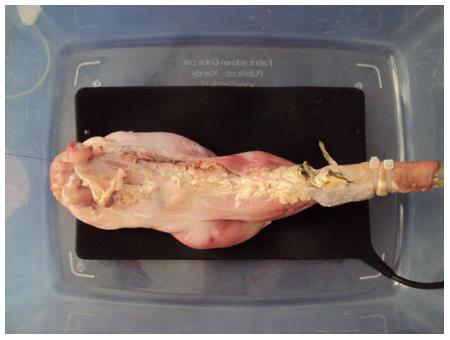
Ex vivo model: For the cadaveric stomach models a plastic box (45 cm × 30 cm × 20 cm) that had a plastic tube (overtube) to which the esophagus was connected, was designed (Figure 1). On some occasions, a cut-out 10 mL plastic syringe was used as a connecting device with a 14 mm diameter to connect the esophaguses of small pigs.
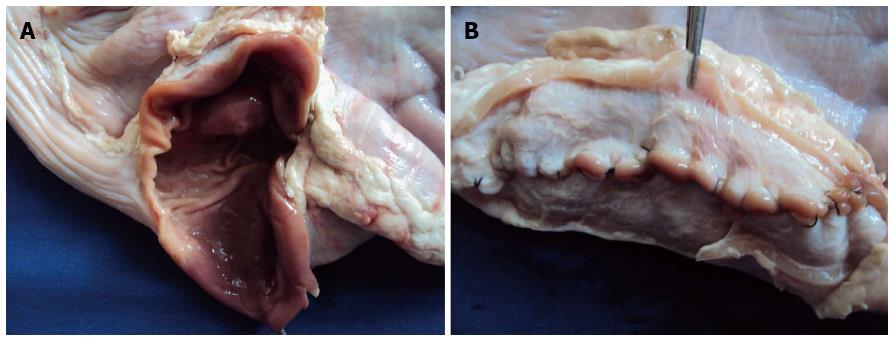
Twenty cadaveric stomachs of pigs ranging between 20 and 150 kg were used. In some stomachs 2 dissections were performed. The stomachs, which were stored in the freezer at a temperature of -16 degrees Celsius, were collected 12 h prior to the procedure and were left at room temperature for their subsequent use, in order to preserve the wall elasticity at the moment of performing the procedure. In most cases, the stomachs were opened on the greater curvature so that profuse washing with water and soap could be carried out. This allowed us to perform a thorough cleaning of the mucosa with a brush and later drying it with gauze, thus achieving adequate cleaning without the inconvenience of persistent mucus which is hard to eliminate. Later the stomachs were sutured with coated vicryl suture or prolene suture 3-0 without any insufflation inconvenience whatsoever in any of the cases (Figure 2).
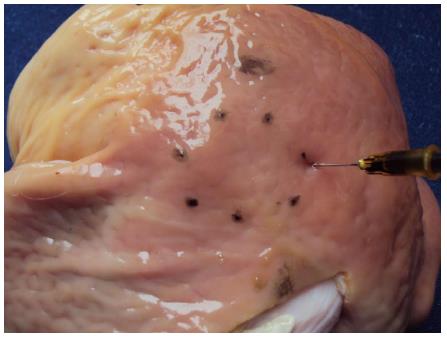
In those cases were the stomachs were closed, a 10 cm long incision was performed with a scalpel on the greater curvature. Another advantage that an open stomach offers is the possibility of carrying out one or more artificial lesions of different diameters and in different topographies through tattooing with Chinese ink or methylene blue (Figure 3).
To adhere the esophagus to the overtube, plastic clamps were used to prevent the air from coming out and slipping with the movements of the endoscope.
To conduct the study, an Olympus gastroscope (GIF-CV-160: 9.2 mm, diameter/2.8 mm, therapeutic channel) was used and then intended for use in models.
In vivo model: Two domestic female pigs (Sus scrofa domesticus) of about 30-40 kg underwent ESD. For 48 h they were given a liquid diet and experienced an 8 h-long total fast prior to the procedure. The general anesthesia was administered and controlled by 2 expert veterinarians, who were in charge of monitoring the vital signs and control to prevent complications.
As premedication, 15 mg/kg + 2 ketamine and 1.1 mg/kg intramuscular xylazine were used. For anesthetic induction 5 mg/kg + 0.5 mg/kg (iv) midazolan was administered, and for maintenance sedation a dilution of similar amounts of 5% ketamine and 5% midazolan (iv), administering approximately 2 mL every 10 min as needed and according to breathing frequency.
Once the procedures were over, a liquid diet was administered for 24 h and then a hypercaloric diet. Omeprazole 20 mg was administered orally every 12 h until the pigs were sacrificed by veterinarians as regulations indicate, following quality standards and required controls in this type of study. Animal handling was carried out by 2 veterinarians in compliance with the current regulations[11].
After performing 30 ESDs, number recommended by Japanese experts[11], 28 in the cadaveric animal model and 2 ESDs in in vivo animal models, 5 gastric dissection procedures were carried out in 5 patients, previous consultation of each case with one of the authors (Parra-Blanco A). The patients were told to discontinue NSAIDs and/or anticoagulants 15 d prior to the procedure, having to present at the moment of the appointment the coagulation test done the day before. The studies were done with an Olympus gastroscope (GIF-CV-160).
The first two cases were carried out in a surgical room and were treated under general anesthesia. A surgical team was present at the operating room in the event that some complications might occur. The other 3 cases were performed in a therapeutic endoscopy room with general anaesthesia. The hospital emergency surgery team was well aware of the procedure.
All the patients were informed of the benefits and of the possible serious complications that might arise with the use of this technique, and signed an informed consent.
An endoscopic control was carried out monthly until the fourth month, with conventional endoscopic vision, chromoendoscopy with FICE using filter number 4 and magnification. A photographic register was made and biopsies were taken of the scar area and of suspicious areas 2 or 3 mo after the procedures.
The ESD was done in a similar way than the one generally carried out at the National Cancer Center in Japan[7]: (1) artificial lesion marking using a coagulation current with needle knife (NK: Needle Knife, Boston, Scientifics, Natick, Massachusetts) or dual knife (Olympus KD-650L); (2) a mixture solution (including 100 mL of normal saline, 1 mL of methylene blue, 1 mL of epinephrine or hyaluronic acid alone), was injected into the submucosa; (3) circumferential incision of 5 mm was made outside of the artificial lesion. The incision was performed with NK, dual knife or insulated tip needle knife (IT Knife 2 o, Olympus KD-611L) as single instruments or combined (NK-IT Knife 2 or dual Knife-IT Knife 2). In cases where IT Knife 2 was used, 2 cuts were made with needle knife (hours 6 and 12) to facilitate the incision; on some occasions, it was necessary to perform 1 or 2 more incisions (hours 11 and 2); and (4) the submucosal dissection was performed in all the cases with IT Knife 2, and at times with NK. In the majority of the cases, a transparent cap (Olympus D-201-12704, Tokyo, Japan) was attached to the tip of the gastroscope to provide direct views of the submucosal layer.
For the purpose of facilitating dissection through a better visualization of the plane separation between the submucosa and the muscular layer, in 3 cases ESD was carried out with the clip technique (Resolution Clip. Boston Scientifics R) and an elastic band[12].
The Erbotom ICC 200 ERBE system was used (ERBE, Elektromedizin GmbH, Tübingen, Germany), effect 3, endocut mode with a potence of 120-60 W was used for the circumferential incision; 80-40 W for the submucosal incision, and soft coagulation (40 W) for the haemostasis.
For bleeding control, a coagulation tweezer (Coagrasper, FD-41OLR) with soft coagulation was used. All the resected pieces were stacked on cork with pins and their measurements were calculated in millimeters and photographically registered. All the procedures were recorded on DVD.
For the prospective analysis of the results obtained, the following variables were registered: (1) lesion topography; (2) size of the resected lesion; (3) total dissection time (circumferential cut and submucosal dissection ); (4) surgical instruments and technique used; (5) type of resection (en bloc resection or fragmented resection); (6) complications (perforation, bleeding); (7) weight of pig whose stomach was used; and (8) condition of the stomach; fresh (cadaveric but not frozen), thawed (after preservation in freezer) or in vivo porcine. In the case of the patients the following variables were registered: whether the ESD was complete (resection with a tumor-free margin in which both the lateral and basal margins were free of tumor cells) or partial (resection in which the tumor extended into the lateral or basal margin, or the margins were indeterminate because of artificial burn effects) and the clinical, endoscopic and histologic evolution.
For the statistical analysis, the description with mean values (standard deviation) for quantitative variables and frequencies for qualitative variables is presented. The Student’s t test was used (statistical significance threshold of α = 0.05) after assumption of normal distribution, to verify differences in terms of qualitative variables. The statistical software used was SPSS 17.0.
In a period of 5 mo and 13 d, 30 ESDs were performed with an average of 1.4 ESD per week (Table 1). Eighty percent of these ESDs (n = 24) were done in frozen stomachs, 13.3% (n = 4) in fresh stomachs, and 6.7% (2) in in vivo stomachs. Concerning the topography, 90% (n = 27) of ESDs were performed in the gastric body, 6.7% (2) in antrum and 3.3% (1), in cardias. In the two in vivo models, the regions were antrum and body. In the four fresh stomachs, 3 were in the body and 1 in the antrum. In the 24 thawed stomachs, 23 were in the body and the remaining one in the cardias. The mean ± SD size of the pieces was of 28.4 ± 1.2 mm (range: 20-45 mm).
| Cases | Topography | Size (mm) | Instrument used | Time dissection (min) | En bloc/pieces | Complications (perforation, bleeding) | Condition of stomach | Weight of the pig (kg) |
| 1 | ANTRUM | 22 | IT,NK | 40 | BLOC | NO | F | 70 |
| 2 | BODY | 24 | IT,NK | 50 | BLOC | NO | F | 70 |
| 3 | BODY | 30 | IT,NK | 43 | BLOC | NO | D | 150 |
| 4 | BODY | 30 | IT (CLIPS) | 45 | BLOC | NO | D | 150 |
| 5 | BODY | 20 | IT,NK, | 30 | BLOC | NO | D | 70 |
| 6 | BODY | 20 | IT,NK | 50 | BLOC | NO | D | 70 |
| 7 | BODY | 25 | IT,NK | 45 | BLOC | NO | D | 50 |
| 8 | BODY | 42 | IT,NK | 70 | BLOC | NO | D | 50 |
| 9 | BODY | 21 | IT,NK | 35 | BLOC | NO | D | 20 |
| 10 | BODY | 25 | IT, NK | 35 | BLOC | NO | D | 20 |
| 11 | BODY | 30 | IT,NK | 55 | BLOC | NO | D | 20 |
| 12 | BODY | 25 | IT,NK (CLIP) | 50 | BLOC | NO | D | 20 |
| 13 | BODY | 26 | IT, DK | 40 | BLOC | NO | F | 20 |
| 14 | BODY | 26 | IT,DK | 35 | BLOC | NO | F | 20 |
| 15 | BODY | 25 | IT, DK | 22 | BLOC | NO | D | 20 |
| 16 | BODY | 27 | IT, DK | 27 | BLOC | NO | D | 20 |
| 17 | BODY | 30 | IT, DK | 35 | BLOC | NO | D | 20 |
| 18 | ANTRUM | 35 | IT,DK (CLIP) | 50 | BLOC | NO | IN VIVO | 30 |
| 19 | CARDIAS | 25 | IT,DK | 55 | BLOC | NO | D | 30 |
| 20 | BODY | 45 | IT,DK | 70 | BLOC | NO | D | 30 |
| 21 | BODY | 30 | IT,DK | 40 | BLOC | NO | D | 30 |
| 22 | BODY | 35 | IT,NK | 45 | BLOC | NO | D | 30 |
| 23 | BODY | 30 | IT,DK | 45 | BLOC | NO | D | 30 |
| 24 | BODY | 35 | IT,DK | 50 | BLOC | NO | D | 30 |
| 25 | BODY | 30 | IT,NK | 55 | BLOC | NO | IN VIVO | 30 |
| 26 | BODY | 20 | IT,NK | 40 | BLOC | NO | D | 30 |
| 27 | BODY | 25 | IT,NK | 36 | BLOC | NO | D | 30 |
| 28 | BODY | 25 | IT,NK | 18 | BLOC | NO | D | 30 |
| 29 | BODY | 30 | IT,NK | 20 | BLOC | NO | D | 30 |
| 30 | BODY | 40 | IT,NK | 19 | BLOC | NO | D | 30 |
Keeping in mind the condition of the stomach, the mean ± SD size for the thawed stomachs was of 28.8 ± 1.4 mm (range: 22.0-45.0 mm). For the fresh stomachs, the mean ± SD size was of 24.5 ± 0.9 mm (range: 22.0-26.0 mm). In the cases of in vivo stomachs, the mean ± SD size was of 32.5.6 ± 2.5 mm (range: 30-35 mm).
According to the topography where the procedure was performed, the following was observed in relation to the size: for the region of the antrum the mean ± SD size was of 28.5 ± 6.5 mm (range: 22-35 mm). For the region of the body, the mean ± SD size was of 28.6 ± 1.2 mm (range: 20.0-45.0 mm). In the case of the cardias, the size was of 25.0 mm.
The total mean ± SD time of the procedure was 41.7 ± 2.4 min (range: 18-70 min). If a cutoff value of 30 mm for the size of the resected piece is established, the dissection time for those lesser than 30 mm was of 38.0 ± 2.3 min, whereas for sizes greater or equal than 30 mm, was 45.9 ± 4.0 min. The difference in dissection time was of 8 min between both size categories with a cutoff value of 30 mm difference which is not significant, P = 0.104. The mean ± SD weight of the pigs where the pieces were obtained from was 41.7 ± 6.1 kg (range: 20-150 kg).
The total mean ± SD time of ESD (circumferential cut and submucosal dissection) in the first 15 procedures was 43.0 ± 3.0 min (range: 22-70 min). In the next 15 procedures, the mean ± SD time dropped to 40.3 ± 3.9 min (range: 18-70 min), having found no significant differences for the dissection time between the first and the second 15 procedures (P = 0.588). The mean ± SD size of the pieces in the first 15 procedures was of 26.1 ± 1.4 mm and for the second 15 procedures the mean ± SD size increased significantly to 30 ± 1.7 mm (P = 0.039). In order to assess if there was any progress in learning, the ESD speed in the second 15 procedures was also calculated and this was compared to the ESD speed in the second 15 procedures. For this purpose, the area of the resected pieces was calculated by using the formula for calculating the area of a circle: (Area = Pi × radius2) ESD speed was calculated by dividing the area of the resected piece in relation to the total dissection time (speed = area/time).
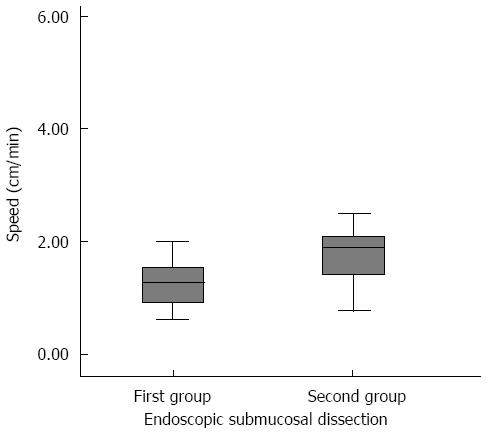
In the first 15 dissections, the mean ± SD speed was 1.25 ± 0.11 cm2/min (95%CI: 1.02-1.48). In the case of the second 15 dissections, the mean ± SD speed was of 2.12 ± 0.36 cm2/min (95%CI: 1.35-2.90) (Figure 4). Through a Student’s t test for independent samples, it can be shown that there are statistically significant differences in the speed of both dissection groups (P = 0.028).
Due to a possible variation related to the state of the stomach (thawed, fresh, in vivo), additionally, only the time of the resections carried out in pre-frozen stomachs in the first 15 ESDs and in the group of the second 15 were analyzed. In the first 15 ESD, 11 resections were performed in thawed stomachs with a mean dissection time of 43.6 min vs 13 dissections in thawed stomachs in the second group with a mean time of 38.4 min, these differences were not significant (P = 0.386).
In the 30 dissection cases the ESDs were completed achieving the resection of the pieces in one single bloc on all occasions. There were no complications (perforation) in this group, as there was no bleeding in the in vivo models.
Five surgical procedures in 5 patients with neoplastic lesions (intramucosal carcinoma, 2 adenomatous lesions with low grade dysplasia, and 2 adenomatous lesions with high grade dysplasia) were performed in a period of 6 mo. The endoscopic (Paris classification[13]), and histologic characteristics and results are shown in Table 2. The mean ± SD size of the lesions was of 25.2 ± 5.1 mm (range: 18.0-45.0 mm).
| Topography | Size (mm) | Lesion type1 | Total time ESD (min) | En bloc resection | Complications | Histology | Complete ESD2 |
| Incisura angularis | 25 | 0-IIa | 65 | Yes | No | Adenoma LGD | Yes |
| Antrum | 20 | 0-IIa | 60 | Yes | No | Intramucosal carcinoma | Yes |
| Antrum | 18 | 0-IIa | 30 | Yes | No | Adenoma HGD | Yes |
| Body | 18 | 0-IIa | 90 | Yes | No | Adenoma LGD | Yes |
| Subcarinal | 45 | 0-IIa | 180 | Yes | Bleeding | Adenoma HGD | Yes |
In all the cases, the medical instrument used for the dissection was IT-Knife 2. The mean ± SD dissection time was 85.0 ± 25.6 min (range: 30-180 min).
In 3 of the procedures in the incisura angularis and in the antrum, the dissection time did not exceed 65 min, reaching 90 min in the high body region and 180 min in the subcarinal area. The piece of lesser size (18.0 mm) was done in 30 min, while the larger lesion (45.0 mm.) lasted 180 min. In one of the procedures some bleeding occurred, which was controlled satisfactorily with a coagulation tweezer.
The five lesions were resected en bloc. The pathology study of the pieces reported that the dissections were complete and curative, since no submucosal invasion or vascular or lymphatic involvement was found, nor were detected cell nests of the undifferentiated type in none of the cases (en bloc R0 resection).
After the procedures were completed, all the patients went into a surgical room; per oral was discontinued for 24 h; 1000cc iv glucophysiological serum was administered plus 2 g of potassium chloride every 12 h, and Omeprazole 40 mg iv every 12 h for 72 h. When discharged from hospital, they all received proton pump inhibitors in simple dosage every 12 h until complete scar formation was confirmed by endoscopy.
All of the patients had a good short (immediate post-procedure) and long-term (4 mo post-procedure) clinical evolution without abdominal pain or evidence of digestive bleeding. In the endoscopic controls conducted with conventional vision, intelligent chromoendoscopy with FICE (Filter 4) and magnification, there was no evidence of residual neoplastic tissue. The biopsies conducted in the scar areas, taken 2 or 3 mo after each procedure tested negative for residual neoplastic tissue.
The endoscopic submucosal dissection is a complex and difficult procedure to learn, being conducted mostly in Asian countries, where a large number of superficial gastrointestinal lesions are diagnosed and treated by this technique.
The difficulties inherent to this procedure, the long process of training required to perform it, and the low frequency of superficial gastric lesions diagnosed in Western countries are some of the causes that explain why very few medical centers outside Japan perform this procedure. Furthermore, access to learning and application of this technique is limited due to lack of Japanese experts in most Western countries. Although outstanding scientific evidence has been published on its applications and results, the publications related to its teaching are scarce, not existing to date a universally accepted standardized learning program on porcine models for subsequent application of this technique in patients.
Gotoda et al[6], have developed training programs on ESD in porcine models. They suggest that these models are a way of rapid rise into the learning curve of this technique in a relatively short period of time and thus may favor its learning in Western countries. They also state that at least 30 submucosal dissections of gastric lesions should be performed to achieve a certain degree of mastery, since at the beginning of the learning curve it is estimated that the perforation rate may reach 20%[6].
In Uruguay, the standardization of ESD teaching does not yet exist and the lack of experts on this technique hampers its dissemination. Due to this inconvenience, as it was formerly pointed out, the practice of ESD in institutions where experts are not available may be developed through the combination of training in animal models before performing endoscopic procedures of this nature in humans. Some authors have described their teaching experience in the acquisition of technical skills using porcine models for the training of ESD for gastric lesions.
Vázquez-Sequeiros et al[8], have described a sequential training program on ESD with the aim of identifying a cheap, safe, efficient and reproducible method for the learning and dissemination of this technique in Spain. According to these authors, such training can be conducted in 4 phases: (1) theoretical phase based on the acquisition of basic knowledge on ESD and in the review of scientific literature; (2) training in an ex vivo animal model; (3) training in an in vivo animal supervised by an expert; and (4) application of the ESD technique in patients. In this study, 4 endoscopists performed a total of 12 gastric ESDs in porcine models (6 in ex vivo model and 6 in in vivo model) and later a gastric ESD supervised by an expert in one patient.
Tanimoto et al[14] assessed the usefulness of an in vivo canine model for ESD practice. They performed 5 esophageal dissections and 5 stomach dissections, completing all the procedures without any complications. Even though the number and size of the resected pieces were small, the authors recommend the use of this model, which being an in vivo model, has the advantage of serving for a more real context and closer to dissections conducted in animals.
Parra-Blanco et al[7], have proposed an ESD learning strategy based on an ex vivo gastric porcine model and in an in vivo porcine model. After an initial ESD learning period conducted in isolated animal stomachs and supervised by an expert, one endoscopist performed a training procedure in esophagus and stomach in an in vivo porcine model, dividing the learning period in 2 phases, conducting in each phase 11 ESDs. As the learning process progressed, it was noticed that without existing differences in the size of the resected pieces, only if the gastric resections were considered, the time spent on the dissection phase and the total time were lesser in the second phase as compared to the first one, which suggested the acquisition of a certain skill in ESD performance. However according to the authors, one of the restrictions of this study was the fact that there was no assessment of the impact of previous training on ex vivo animal model under supervision, which does not prove if this initial step is necessary to undertake self-learning in animal model. The subsequent impact of animal training in the ESD performance in humans was not evaluated either.
In the present study, the impact of training on the animal model was evaluated early in human patients; therefore, there is a description of the results when the same endoscopist applies the technique in patients after the training program in animal models, thus yielding good results in terms of efficacy and safety.
In relation to the state of the stomachs at the moment of performing the procedures, in most cases, ESDs were performed in pre-frozen porcine stomachs because of its usefulness. However, the authors recommend, if possible, using fresh stomachs due to the fact that the walls are frequently more rigid in pre-frozen organs and, especially, submucosal injection may prove more difficult.
Another important element to be keep in mind is the right size of the organs. Large pigs (greater than 70 kg) have larger stomachs which allow for the performance of multiple resections in different places. In this study, porcine organs ranging from 20 to 150 kg were used. The organs of smaller animals have thinner walls with a greater risk of perforation, and the movements in the stomach are limited due to their reduced size. The use of small stomachs might have the potential benefit of requiring greater skill and therefore the endoscopist will have a greater degree of difficulty. Consequently, the authors recommend the use of small stomachs after an initial practice in large organs in order to acquire greater skill.
Concerning the type of animal model, the in vivo porcine model has proven to be more adequate for endoscopic formation in the upper digestive tract[15-17]. It is important and recommendable to perform ESD in in vivo porcine model as a prior step to the application of this technique in humans, since this type of model offers several advantages. The principal advantage of the in vivo model, as compared to the ex vivo model is that the former is more realistic, with presence of peristaltic, intraluminal secretions, and hemorrhage is a possible complication. However, in an initial stage, the basic movements and the ESD strategy may and should be first learned in ex vivo models, and probably there is no justifications for using in vivo pigs initially, which are more expensive, require more preparation and technical assistance, and it would not be ethically correct[18].
As an objective variable for measuring skill acquisition and learning progress, the following markers of technical skill in ESD have been proposed: decrease in time employed in the procedure, greater speed in the dissection process and/or achieving a greater complete resection rate without need of help from the supervising endoscopist.
Based on the above mentioned, the results of this study suggest that there was progress in the learning curve in animal models. As it can be seen in the results, the time required for the dissection was reduced in the second 15 procedures performed in porcine models. Even though this difference was not significant when compared to the first 15 procedures in animal model, this could be due to the fact that the size of the pieces in the second 15 procedures increased in a significant way. Likewise, it can also be noticed that there was an increase in the dissection speed in the second ESDs, this being the sign of greater acquisition of skill in the technique and therefore progress in its learning.
Distension and elasticity of the stomach walls in different samples (fresh, thawed or in vivo) may vary and thus interfere in the dissection time. As it is increased in the frozen models because of lesser distension and elasticity of the tissues, only the times of the dissections performed in frozen stomachs in the first 15 and in the second 15 procedures were compared. In the analysis of the results it was observed that in the second group the procedures were also performed in shorter time, although this difference was not significant. Therefore, the reduction in time cannot be attributed to the state of the stomachs.
One of the restrictions of this study is the fact that very few cases were performed in human patients. Furthermore, it is the experience of just one center and one single endoscopist. The ability and experience of each endoscopist is different, not homogeneous, so this result does not assure its reproducibility.
With respect to the pattern of lesions, usually, the pattern of early gastric cancer is detected as 0-I, II-C or 0-III types in Japan and most of the world. However, in the present study, the most common early gastric cancer was the type IIa.
In this article we have discussed the learning curve for ESD, but it is very important to mention that these techniques are useful only after acquiring proficiency in an accurate diagnosis of the early gastrointestinal cancer lesions with the adequate technology and knowledge of early gastrointestinal cancer classifications (i.e., Vienna and Paris Classifications; Chromoendoscopy and Magnification).
Therefore, before starting the practice of this technique, it is necessary and more important that the endoscopist knows how to thoroughly examine the neoplastic lesion to achieve the identification of endoscopic characteristics that predict the existence of deep invasion, an increased risk of lymph node metastasis and to demarcate the line between the area with cancer and without cancer. This step, prior to the ESD, is very important, because it may avoid unnecessary surgical resection or endoscopy. Furthermore, it is important that before starting to develop this technique, the endoscopist acquires ample experience in therapeutic endoscopy, since he must be able to deal with and solve possible complications during the ESD process, such as bleeding o perforation.
It is worth mentioning that even though the training in ESD in porcine models is safe and accessible, it is recommendable to carry out all of the steps suggested before performing this technique in humans. Besides, it is important to highlight that to define the learning curve only in terms of time, dissection speed or number of procedures conducted is not appropriate since this may vary according to the conditions of each center, and mostly according to the skills of each endoscopist. This is the reason why it is very important to be self-critical, know each one’s limitations and to receive permanent feedback, so that the ESD practice can be carried out in a safe and efficient way. When the time comes to move on to performing ESD in humans, it is recommendable that this practice be progressive, starting with resection of lesions in areas with lesser technical difficulty (antrum, incisura angularis) and later ESD in lesions with greater difficulty (lesser curvature, subcarinal region).
Due to the scarce number of patients that can be benefited of the application of ESD in Western countries (mostly due to the low prevalence of early gastric neoplasia), the interval between dissection procedures in humans can be too long. This can be the reason why in this period the technical skills and acquired confidence can decrease. To overcome this situation, the authors recommend that before the performance of this technique in humans, it is necessary to previously perform it in animal model.
In Latin America, the standardization of ESD teaching does not exist and there is shortage of experts in this technique, thus hampering its dissemination. For this reason, it is important to train endoscopy teams to perform ESD so that they can share the knowledge developed on the basis of the experience acquired during the learning curve and to form multicenter endoscopy teams who can do research work, develop this technique in-depth and design structured training programs suited to the needs and resources available.
In conclusion, according to the analysis of the results obtained, the authors conclude that in Uruguay an ESD sequential training program of a unique endoscopist, initially based on practice in porcine model (performing the number of dissections recommended by Japanese experts), conducted in the absence of experts to supervise the procedures, contributed to the learning of the ESD technique, and yielded promising results in efficacy and safety for its subsequent application in humans, even though the sample is small.
The authors wish to express their sincere thanks to veterinary doctors, Dr. Diego Fernández and Dr. Lorena López for their invaluable cooperation in the preparation, handling and administration of the anaesthesia in the in vivo animal models, Mrs. Elizabeth Silva and Mr. Evaristo González for supplying the cadaveric stomachs and the in vivo models. We would also like to extend our appreciation to Assistant Endoscopists, Sandra Mellían, Adrián Duarte, Dardo Debenedetti, to Administrative Staff Member, Karina Balmelly, and to Mr. Juan Carlos Márquez, for their support and participation during the training in models. Our sincere gratitude also goes to the surgical team (Quirúrgica “B”, Professor Dr. César Canessa) for his support in the event complications might arise during the procedures in humans.
Endoscopic submucosal dissection (ESD) is a technique for resection of early neoplastic lesions of the gastrointestinal tract, thus obtaining more complete resections than with the standard mucosectomy and decreasing the probability of relapses. However, it is associated with a high rate of complications, but it is an effective and safe technique in experienced hands, and widely accepted. In the absence of experts in western countries, practicing with animal models may be helpful to overcome the learning curve.
ESD is an effective and safe technique in experienced hands, and widely accepted. In the present study, the authors demonstrated the feasibility of using porcine models as a learning resource to teach the ESD technique. The results of multicenter studies of ESD in humans after previous training with animal models have to be explored further.
In Latin American countries there are few training centers at which an endoscopy fellow can be trained in the ESD technique. More ESD training centers in Latin America are needed to offer our patients this technique with good results.
ESD has been considered technically difficult. The potential implications of starting such a complex technique in an animal model rather than in human cases are evident. A formal training program is still necessary to teach this technique in western countries and Latin America. For this reason, animal models are an invaluable learning resource. It would be justified to start training with animal models instead of with real patients, if experts are not available to supervise the procedures.
ESD is an endoscopic method for complete en bloc resection of early gastrointestinal neoplasms with minimal risk of deeper wall-layer involvement or lymph node metastases. In selected cases, ESD may replace surgery and provide clean margins for accurate histological diagnosis of the lesion borders and complete curative treatment.
This is an interesting investigation that presents the feasibility of using porcine models for ESD training. It provides an alternative way to learn ESD in Western countries.
P- Reviewers: Kita H, Kawahara Y, Lin JK, Naito Y S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 459] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 2. | Yamamoto H. Endoscopic submucosal dissection of early cancers and large flat adenomas. Clin Gastroenterol Hepatol. 2005;3:S74-S76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1148] [Article Influence: 47.8] [Reference Citation Analysis (4)] |
| 4. | Muto M, Miyamoto S, Hosokawa A, Doi T, Ohtsu A, Yoshida S, Endo Y, Hosokawa K, Saito D, Shim CS. Endoscopic mucosal resection in the stomach using the insulated-tip needle-knife. Endoscopy. 2005;37:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Gotoda T. Endoscopic resection for premalignant and malignant lesions of the gastrointestinal tract from the esophagus to the colon. Gastrointest Endosc Clin N Am. 2008;18:435-450, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Gotoda T, Friedland S, Hamanaka H, Soetikno R. A learning curve for advanced endoscopic resection. Gastrointest Endosc. 2005;62:866-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Parra-Blanco A, Arnau MR, Nicolás-Pérez D, Gimeno-García AZ, González N, Díaz-Acosta JA, Jiménez A, Quintero E. Endoscopic submucosal dissection training with pig models in a Western country. World J Gastroenterol. 2010;16:2895-2900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Vázquez-Sequeiros E, de Miquel DB, Olcina JR, Martín JA, García M, Lucas DJ, Garrido E, González C, Blanco AP, Arnau MR. Training model for teaching endoscopic submucosal dissection of gastric tumors. Rev Esp Enferm Dig. 2009;101:546-552. [PubMed] |
| 9. | Berr F, Ponchon T, Neureiter D, Kiesslich T, Haringsma J, Kaehler GF, Schmoll F, Messmann H, Yahagi N, Oyama T. Experimental endoscopic submucosal dissection training in a porcine model: learning experience of skilled Western endoscopists. Dig Endosc. 2011;23:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Balogh G, Dubravcsik Z, Szepes A, Madácsy L. [Endoscopic submucosal dissection in our practice -- new possibilities in the endoscopic treatment of neoplastic changes in the alimentary canal]. Orv Hetil. 2012;153:824-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Russell WMS, Burch RL. The Principles of Humane Experimental Technique. Reprinted by UFAW, 1992: 8 Hamilton Close, South Mimms, Potters Bar, Herts EN6 3QD England. London: Methuen 1959; . |
| 12. | Parra-Blanco A, Nicolas D, Arnau MR, Gimeno-Garcia AZ, Rodrigo L, Quintero E. Gastric endoscopic submucosal dissection assisted by a new traction method: the clip-band technique. A feasibility study in a porcine model (with video). Gastrointest Endosc. 2011;74:1137-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Tanimoto MA, Torres-Villalobos G, Fujita R, Santillan-Doherty P, Albores-Saavedra J, Gutierrez G, Martin-del-Campo LA, Bravo-Reyna C, Villanueva O, Villalobos JJ. Endoscopic submucosal dissection in dogs in a World Gastroenterology Organisation training center. World J Gastroenterol. 2010;16:1759-1764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Parra-Blanco A, Saito Y, Yahagi N, Antillon M, Deprez P, Fukunaga S, Hayashi T, Hotta K, Nakajima T, Toyonaga T. Recommendations about training for colorectal endoscopic submucosal dissection in the Western world: results of a survey to experts. Gastrointest Endosc. 2011;73:AB419-AB420. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Deprez PH, Bergman JJ, Meisner S, Ponchon T, Repici A, Dinis-Ribeiro M, Haringsma J. Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy. 2010;42:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Parra-Blanco A, González N, González R, Ortiz-Fernández-Sordo J, Ordieres C. Animal models for endoscopic training: do we really need them? Endoscopy. 2013;45:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Parra-Blanco A, Gonzalez N, Arnau MR. Ex vivo and in vivo models for endoscopic submucosal dissection training. Clin Endosc. 2012;45:350-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |