Published online Dec 7, 2013. doi: 10.3748/wjg.v19.i45.8292
Revised: October 4, 2013
Accepted: October 13, 2013
Published online: December 7, 2013
Processing time: 113 Days and 10.3 Hours
AIM: To investigate the inhibitory effects of sinomenine (SIN) combined with 5-fluorouracil (5-FU) on esophageal carcinoma in vitro and in vivo.
METHODS: Esophageal carcinoma (Eca-109) cells were cultured in DMEM. The single or combined growth inhibition effects of SIN and 5-FU on the Eca-109 cells were examined by measuring the absorbance of CCK-8 dye in living cells. Hoechst 33258 staining and an Annexin V/PI apoptosis kit were used to detect the percentage of cells undergoing apoptosis. Western blotting was used to investigate the essential mechanism underlying SIN and 5-FU-induced apoptosis. SIN at 25 mg/kg and 5-FU at 12 mg/kg every 3 d, either combined or alone, was injected into nude mice and tumor growth inhibition and side effects of the drug treatment were observed.
RESULTS: SIN and 5-FU, both in combination and individually, significantly inhibited the proliferation of Eca-109 cells and induced obvious apoptosis. Furthermore, the combined effects were greater than those of the individual agents (P < 0.05). Annexin V/PI staining and Hoechst 33258 staining both indicated that the percentage of apoptotic cells induced by SIN and 5-FU combined or alone were significantly different from the control (P < 0.05). The up-regulation of Bax and down-regulation of Bcl-2 showed that the essential mechanism of apoptosis induced by SIN and 5-FU occurs via the mitochondrial pathway. SIN and 5-FU alone significantly inhibited the growth of tumor xenografts in vivo, and the combined inhibition rate was even higher (P < 0.05). During the course of chemotherapy, no obvious side effects were observed in the liver or kidneys.
CONCLUSION: The combined effects of SIN and 5-FU on esophageal carcinoma were superior to those of the individual compounds, and the drug combination did not increase the side effects of chemotherapy.
Core tip: The cooperative inhibitory effects of sinomenine (SIN) and 5-fluorouracil (5-FU) on esophageal carcinoma in vitro and in vivo were investigated. SIN and 5-FU alone or in combination significantly inhibited the proliferation and induced apoptosis of esophageal carcinoma cells in a dose-dependent manner. The essential mechanism underlying SIN and 5-FU-induced apoptosis, as investigated by Western blotting, involved the mitochondrial pathway. No obvious side effects were observed in the liver and kidneys of nude mice. These results indicated that the combined effects of SIN and 5-FU on esophageal carcinoma were superior to the effects of the individual compounds, without an increase in side effects.
- Citation: Wang J, Yang ZR, Dong WG, Zhang JX, Guo XF, Song J, Qiu S. Cooperative inhibitory effect of sinomenine combined with 5-fluorouracil on esophageal carcinoma. World J Gastroenterol 2013; 19(45): 8292-8300
- URL: https://www.wjgnet.com/1007-9327/full/v19/i45/8292.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i45.8292
Esophageal carcinoma is one of the most refractory and common malignant diseases worldwide and is associated with poor disease outcomes[1,2]. An estimated 482300 new esophageal cancer cases and 406800 deaths occurred globally in 2008[2]. Generally, the primary tumor of most patients can be cured with surgical resection, however, due to early distant metastases, the remainder of patients will eventually succumb to the disease. Despite the advances in surgical methods combined with perioperative treatment that have led to improved prognoses, the overall mortality rate remains low due to early distant metastases[3-5]. Systemic chemotherapy is regarded as one of the most effective treatments to improve survival. Although chemotherapy forms an important part of the multidisciplinary treatment approach for metastatic disease, the toxicity of the agents to normal tissues has been the main obstacle to successful treatment. Therefore, to enhance efficacy and reduce toxicity, combined treatments of several chemotherapy regimens are often used.
5-Fluorouracil (5-FU) is universally used as an anti-cancer agent in esophageal carcinoma. Cisplatin and fluorouracil combination therapy has been a standard choice for treating esophageal cancer, for which the median survival time is 9.2 mo for responders and 5.3 mo for non-responders, with a resulting 1-year survival rate of approximately 27.8%-37.6%[6-8]. To improve the prognosis of patients with esophageal cancer, more effective novel regimens with high therapeutic effect are urgently needed.
Sinomenine (SIN) is an immunosuppressive compound extracted from the Chinese medicinal plant Sinomenium acutum which has been successfully used in Chinese folk medicine to treat various autoimmune diseases for centuries[9]. Previous studies have indicated that SIN exhibits a wide range of significant pharmacological activities, including anti-inflammatory, anti-rheumatic, anti-arrhythmic, anti-angiogenesis, analgesic and immunosuppressive effects[10-12]. Lu et al[13] observed that SIN can promote cell cycle arrest in the G1 phase, which was associated with increased p21/WAP/Cip expression. Additionally, SIN induces caspase-dependent apoptosis, which is involved in the disruption of mitochondrial membrane potential. SIN can also significantly inhibit basic fibroblast growth factor (bFGF)-induced angiogenesis, an effect that may render the compound a prime candidate for possible anti-cancer agents[12].
The aim of this study was to evaluate the effects of SIN combined with 5-FU in terms of their activity against esophageal carcinoma in vitro and in vivo.
SIN (C19H23NO4·0.3 CHCl3) and 5-FU (C4H3FN2O2) were purchased from Sigma (Sigma-Aldrich, St. Louis, MO, United States) and dissolved at 100 mmol/L in dimethylsulfoxide (DMSO) for storage at -20 °C. The esophageal carcinoma cell line Eca-109 was obtained from China Center for Type Culture Collection. The growth medium consisted of DMEM (Gibco BRL Gaithersburg, MD, United States) containing 10% fetal bovine serum. Cell cultures were maintained at 37 °C in a 95% humidified atmosphere of 5% CO2 in air.
Cell proliferation in vitro was determined using the WST-8 Cell Counting Kit-8 (Beyotime Biotechnology, Jiangsu, China) as previously described[14]. Generally, cells were seeded in 96-well micro plates at a density of 5 × 103/well in 0.1 mL growth medium. After exposure to different concentrations of SIN (40, 80, 160, 320, 640 μmol/L), 5-FU (40, 80, 160, 320, 640 μmol/L) or SIN + 5-FU for 48 h, 10 μL CCK-8 solution was added to each well. The plates were then incubated for an additional 2 h at 37 °C. The optical density at 450 nm was measured by a microplate reader (BIO-RAD iMark). The procedure was performed in triplicate.
The amount of phosphotidylserine exposed on the extracellular membrane of the apoptotic cells was quantified by the Annexin V-FITC kit. Following incubation with SIN (160 μmol/L) and 5-FU (160 μmol/L) alone or combined for 48 h, adherent cells were harvested by mild trypsinization, washed twice with cold PBS and resuspended in 500 μL binding buffer. After adding 5 μL Annexin V-FITC conjugate and 10 μL Propidium Iodide (PI), the cells were incubated for 15 min at room temperature in the dark. Flow cytometric analysis was performed immediately with FACSCalibur using the CellQuest software.
The morphological features of apoptotic cells were detected by Hoechst 33258 staining following the manufacturer’s protocol (Beyotime Biotechnology, Jiangsu, China). Cells were seeded on sterile cover glasses placed in the 6-well plates. After exposure to SIN (160 μmol/L), 5-FU (160 μmol/L) alone or combined for 48 h, the cells were fixed, washed and stained with 0.5 mL Hoechst 33258 staining solution for 5 min at room temperature in the dark. Then washed twice with PBS and stained nuclei were scored and categorized according to the condensation and staining characteristics of chromatin under a fluorescence microscope (Olympus, Shinjuku-ku, Tokyo, Japan). Ten random fields per dish were observed and counted.
Whole protein extracts were prepared and analyzed by western blotting using a wet transfer system (Bio-Rad, Hercules, CA, United States). Protein samples (20 μg) were resolved by electrophoresis of SDS-polyacrylamide gels and electro-transferred to nitrocellulose membranes. Transfer efficiency and homogeneous loading was assessed by Ponceau stain. Membranes were blocked for 1 h in 10% non-fat milk, incubated with titrated primary antibody overnight at 4 °C and then labeled with the horseradish peroxidase conjugated secondary antibody for 2 h at ambient temperature. The following antibodies were used: Bax, Bcl-2, GAPDH (Santa Cruz Biotechnology, CA, United States) 1:1000. Antibody reaction was revealed using an enhanced chemiluminescence system (Millipore, Bedford, MA, United States) and then exposed with Kodak X-ray film. Protein band intensity was determined using the CMIASWIN video imaging system (Bio-Rad, Hercules, CA, United States).
Animal experiments, which complied with the NIH Guide for the Care and Use of Laboratory Animals, were approved by the committee on Animal Experiment of Wuhan University. Esophageal carcinoma tumors were established in male BALB/c nude mice (4-6 wk, Laboratory Animal Central of Wuhan University) by serial subcutaneous transplantation of Eca-109 cells (1 × 107 cells/mouse). Eighteen mice bearing xenografted tumors were obtained and a further six mice inoculated with saline acted as controls. When the tumors reached approximately 100 mm3 in size, the mice were divided into four groups (P < 0.05): SIN group (25 mg/kg), 5-FU group (12 mg/kg), combination group, and saline control group. Treatment was administered via intratumoral injection every 3 d. Tumor size was measured 2-3 times per week in two dimensions by calipers, and the volumes were calculated using the following formula: volume (mm3) = [length × (width)2×π/6].
For histologic analysis, tumor tissues were prepared according to a standard protocol, and the paraffin-embedded tumor tissue sections were subsequently stained with hematoxylin and eosin (HE). For TUNEL assay, an in situ apoptosis detection kit (Roche, Branchburg, NJ, United States) was used to detect apoptotic cells in tumor tissue sections. The apoptotic cells were identified, counted (nine random fields per slide), and analyzed under light microscopy (Olympus, Japan).
Blood samples were collected by cardiac puncture. The biomarkers of liver and renal injury, such as the activity of alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN) and serum creatinine (Cr), were detected using an OLYMPUS AU5400 analyzer (OLYMPUS, Japan).
All data were expressed as the mean ± SE of the mean, and then subjected to the unpaired Student’s t test. Statistical significance was defined as a value of P < 0.05.
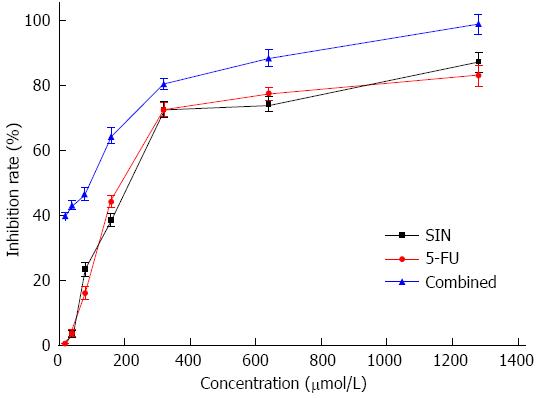
The inhibition of Eca-109 cell proliferation by SIN and 5-FU was assessed by the WSK-8 cell viability assay after 48 h of drug exposure, following 24 h culture in a drug-free medium. As shown in Figure 1, the growth of Eca-109 cells was significantly inhibited in a dose-dependent manner (P < 0.05). Significant differences were observed for each concentration of the combined treatment (SIN:5-FU ratio was 1:1) compared to SIN or 5-FU given individually (P < 0.05), especially at lower concentrations.
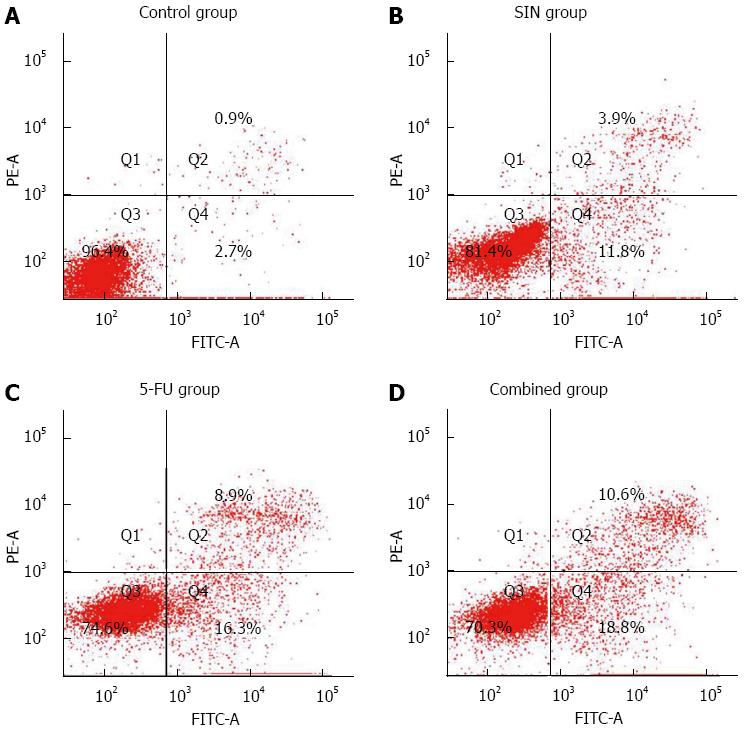
Apoptosis induced by SIN and 5-FU was confirmed by Annexin V/PI staining to quantify the amount of phosphotidylserine exposed on the apoptotic cellular membrane. As shown in Figure 2, the percentage of Annexin V-positive/PI-negative cells increased progressively in Eca-109 cells incubated at low concentrations of SIN (160 μmol/L) and 5-FU (160 μmol/L) for 48 h. SIN and 5-FU alone significantly induced apoptosis, as compared with the control group (P < 0.05); furthermore, the combined treatment effect was stronger than that of SIN and 5-FU given individually (P < 0.05, Figure 2).
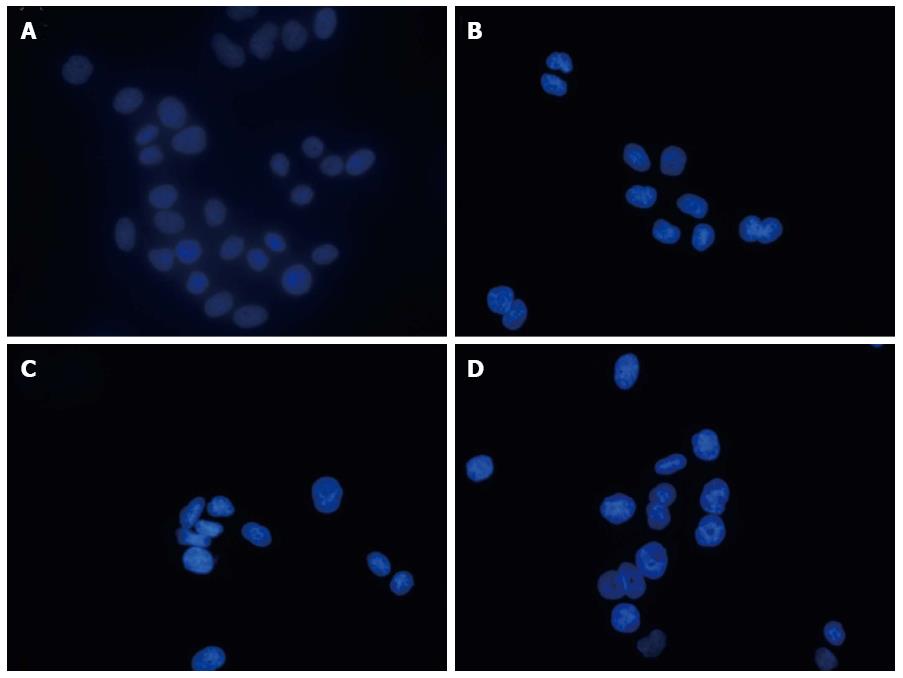
The morphological features of apoptotic cells were detected by Hoechst 33258 staining. The apoptotic nuclei were assessed by changes revealed by Hoechst staining and were identified by condensed chromatin as well as nuclear fragmentation with formation of apoptotic bodies. Ten random fields per dish were observed and counted under a fluorescence microscope. The mean values are expressed as the percentage of apoptotic nuclei per field. Significant changes were detected in the number of apoptotic cells, and the percentage of apoptotic cells induced by SIN and 5-FU combined or alone were significant when compared with the group control (P < 0.05, Figure 3); furthermore, the apoptotic rate of the combined treatment was greater than those of the individual treatments (P < 0.05, Figure 3).
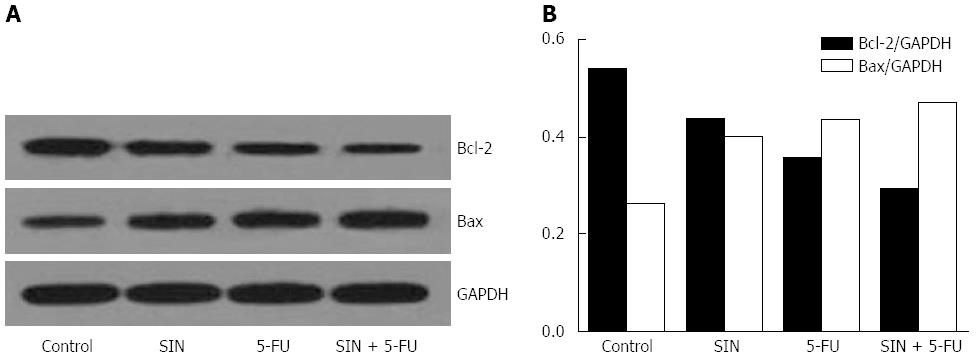
To further investigate the essential mechanism underlying SIN- and 5-FU-induced apoptosis, their effects on the mitochondrial pathway were examined. As shown in Figure 4, SIN (160 μmol/L) and 5-FU (160 μmol/L) treatment combined or alone caused an increase in Bax/GAPDH protein levels and a decrease in Bcl-2/GAPDH levels in Eca-109 cells, which led to a decrease in the antiapoptotic/proapoptotic (Bcl-2/Bax) protein ratio. The apoptotic effect in the combined treatment group was significantly greater than that of the individual treatment groups.
The effects of SIN and 5-FU on the growth of primary tumor xenografts in nude mice were examined. None of the mice died during the course of the experiment, and all 24 mice successfully grew tumor xenografts. After 14 d growth, the tumor xenografts reached a mean size of 100 mm3. The mice were then randomly divided into four treatment groups, and no significant differences were observed in tumor size in the different groups at the start of the study. The results demonstrated that the tumor volumes and weights in the treatment groups were both significantly reduced compared with the saline control group (P < 0.05, Tables 1 and 2), while the extent of tumor reduction differed for each group. The combination treatment induced greater tumor growth suppression than did SIN or 5-FU alone (P < 0.05, Table 1), and a difference in tumor weight was also observed between groups (P < 0.05, Table 2). When compared with the control group, the tumor volume inhibition rate for the combination group was 91.22%, whereas the inhibition rates for the SIN and 5-FU groups were 64.68% and 71.68%, respectively (Table 1). When compared with the control group, the tumor weight inhibition rate for the combination group was 83.38%, whereas the inhibition rates for the SIN and 5-FU groups were 63.10% and 61.97%, respectively (Table 2). The results suggest that SIN combined with 5-FU exhibits significant anti-tumor potential in vivo.
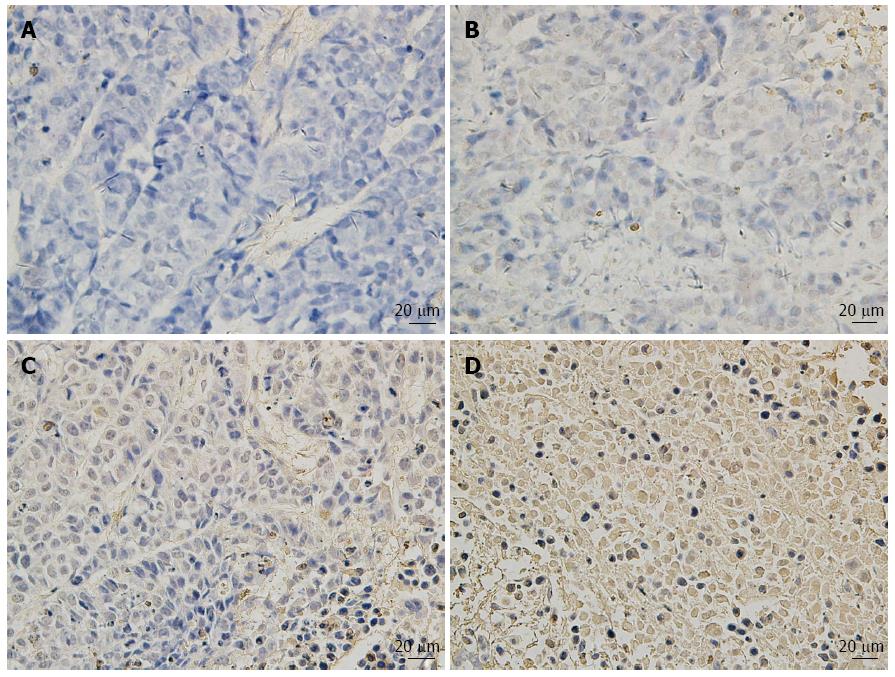
HE staining and TUNEL analysis of the subcutaneous primary tumor sections indicated that SIN and 5-FU individually or in combination induced significant apoptosis of Eca-109 cells in vivo, as compared with the saline control group (P < 0.05, Figure 5), although the degree of apoptosis differed for each group; the apoptotic rate in the combination treatment group was greater than that in the SIN alone and 5-FU alone groups.
The above data indicated that the effects of SIN combined with 5-FU against esophageal carcinoma in vivo were superior to that of SIN and 5-FU used individually.
At the end of the experiment, the nude mice were sacrificed and necropsied. No obvious metastasis, hemorrhage, or injury of the liver and kidneys was observed by visual examination.
Blood samples collected by cardiac puncture were used to monitor hepatic and renal toxicity. As biomarkers of liver and renal injury, the activity of ALT and AST, as well as the urea and Cr values, were determined to evaluate potential toxicity. The hepatic and renal toxicity induced by SIN and 5-FU alone or in combination is shown in Table 3. Compared to the control group, there were no significant increases in the values of ALT, AST, urea, and Cr (P > 0.05, Table 3), and no significant differences were observed between the SIN alone, 5-FU alone and combination treatment groups (P > 0.05, Table 3). At necropsy, the livers and kidneys of the mice from the different treatment groups appeared smooth and normal in color. There was no significant difference in the liver and renal volume or weight between the treated groups and the control group, and the histological pathology examination showed no obvious lesions.
| Group | ALT (U/L) | AST (U/L) | Urea (μmol/L) | Cr (μmol/L) |
| SIN | 45.50 (7.37) | 143.67 (28.82) | 8.18 (2.04) | 16.63 (3.41) |
| 5-FU | 45.33 (6.02) | 145.33 (18.53) | 8.04 (1.20) | 15.73 (4.30) |
| SIN + 5-FU | 46.17 (7.17) | 146.83 (19.55) | 8.28 (1.44) | 16.68 (2.68) |
| Tumor control | 45.83 (3.76) | 144.67 (17.32) | 7.98 (0.70) | 16.74 (4.46) |
| Normal control | 46.00 (5.00) | 145.17 (11.27) | 8.09 (1.03) | 16.29 (4.51) |
Esophageal carcinoma is one of the most aggressive malignancies and remains a major public health threat globally. In 2008, the number of new esophageal cancer cases was estimated to be 482300 which accounts for 3.8% of all cancers, while the number of deaths was 406800 which accounts for approximately 5.4% of global cancer mortalities[2]. More effective treatments, as well as methods for earlier diagnosis, have led to improved survival over recent decades. However, patients with esophageal cancer still exhibit rapid progression and poor prognosis, with a natural disease history of 6-8 mo and a 5-year survival rate of 5%-7%[15], owing to extensive local invasion, lymph node involvement and distant metastases at the time of diagnosis[16]. Consequently, more effective chemotherapies have become an important means of extending the survival of esophageal cancer patients.
5-FU is widely used in chemotherapeutic regimens, including those for cancers of the gastrointestinal tract and breast. Although 5-FU-based chemotherapy improves the overall survival of patients with esophageal carcinoma, the response rate is extremely low, and even the combination of 5-FU with newer chemotherapies such as cisplatin and doxorubicin only generates response rates of 25%-35%, with 1-year survival rates of 27%-37%[6,7]. To improve the prognosis of patients with esophageal cancer, researchers have undertaken the investigation of novel drugs and combinations of chemotherapeutics.
Over the last few years, interest in exploring the use of traditional medicines for the prevention or treatment of tumors has increased. SIN is an alkaloid derived from the stem of the Chinese medical plant Sinomenium acutum, which has been used in traditional Chinese medicine for over 2000 years to treat various rheumatoid diseases with minimal side effects[17,18]. The chemical structure of SIN has been clarified, and its potential value in treating rheumatoid arthritis has been recognized by Western medicine. Many studies have indicated that SIN has a wide range of significant pharmacological actions, such as anti-inflammatory, anti-arrhythmic, anti-angiogenesis and immunosuppressive effects. Previous studies have demonstrated that inflammation can affect the angiogenesis and invasion of various types of tumors[19,20]. Kok et al[12] found out that SIN possesses anti-angiogenic activity in several systems both in vitro and in vivo. Similarly, in a recent study, SIN was demonstrated to induce apoptosis by modulating NF-κB expression and activity[21], and was able to significantly inhibit NF-κB activity even at > 10 ng/mL[13]. Based on these results, SIN was hypothesized to enhance the sensitivity of various cancers to anti-cancer drugs.
In the present study, we investigated the inhibitory effects of SIN combined with 5-FU treatment on esophageal carcinoma in vitro and in vivo. We found that SIN and 5-FU alone can significantly inhibit the proliferation of Eca-109 cells in a dose-dependent manner (P < 0.05). In addition, the combined effect of SIN and 5-FU on the proliferation of human esophageal carcinoma was superior to that of SIN or 5-FU alone in vitro and in vivo. We also examined the apoptotic effect induced by SIN combined with 5-FU or administered individually. The results indicated that SIN and 5-FU alone significantly induced apoptosis compared with the control, and that the combined treatment effects were stronger than the individual effects of SIN and 5-FU.
SIN, as a typical anti-arrhythmic drug, has also been reported to inhibit the proliferation and induce apoptosis in various tumors[22]. However, the exact mechanisms underlying this apoptotic effect are poorly understood. Apoptosis is a tightly controlled type of cell death, with characteristic effects such as cell shrinkage, membrane blebbing and DNA fragmentation. The signals for apoptosis can be either initiated extrinsically through the death receptor pathway or intrinsically through the mitochondrial pathway[23,24]. Mitochondria are considered to play a pivotal role in apoptosis. The mitochondrial pathway has been shown to be an important signaling pathway for apoptosis, and SIN has been proven to utilize this pathway to induce the apoptosis of cancer cells[25-27]. 5-FU is well known to inhibit the thymic pyrimidine nucleotidase of tumor cells and affect DNA stability[28]. Moreover, 5-FU has been demonstrated to induce the apoptosis of various cancer cells such as breast and colon cancer, with resulting changes in p53[29], caspase-3[30] and caspase-8[31]. In this study, we confirm that SIN combined with 5-FU treatment can induce apoptosis through the mitochondrial pathway.
A mouse tumor xenograft model was established, and the animals were administered chemotherapy consisting of SIN (25 mg/kg) and 5-FU (12 mg/kg) every 3 d. Similar effects on the proliferation and apoptosis of esophageal cancer cells induced by SIN and 5-FU were also observed in vivo. No adverse effects such as gastroenterol disturbance, hemorrhage or kidney dysfunction were observed. The model was also used to evaluate the potential histological pathology and hepatic and renal toxicity resulting from treatment, with no significant differences found between the treatment and control groups. Thus, we conclude that SIN combined with 5-FU demonstrates an anti-cancer effect with no increase in side effects in vivo.
In conclusion, SIN combined with 5-FU exhibited a significantly superior anti-cancer effect in comparison to SIN or 5-FU alone. In addition, this study demonstrated that SIN and 5-FU did not increase toxicity in vivo when used in combination. Above all, the present study indicates the potential for the combined use of SIN and 5-FU in clinical treatment.
We are very grateful to Mr. Hong Xia from the Key Laboratory of Hubei Province for Digestive System Disease for assistance in data collection.
Esophageal carcinoma is one of the most refractory and common malignant diseases and is associated with poor outcome. To enhance the effect of systemic chemotherapy and reduce the toxicity, combined treatments with several regimens are often used. 5-Fluorouracil (5-FU) is universally used as an anti-cancer agent in esophageal carcinoma. Sinomenine (SIN) is an immunosuppressive compound extracted from the Chinese medicinal plant Sinomenium acutum; this compound has a wide range of significant pharmacological actions and has been hypothesized to enhance the sensitivity of various cancers to anti-cancer drugs.
As an alkaloid derived from a medical plant, SIN has been used in traditional Chinese medicine for over 2000 years. In the area of SIN treatment for cancer, the crucial areas of research are to confirm its effects, investigate the agent’s mechanism of action and identify combined systemic chemotherapy regimens to prevent the growth of cancer.
Few studies have described the anti-inflammatory, anti-rheumatic, anti-cancer, and anti-angiogenesis effects of SIN. The results of this study suggest that the combined effects of SIN and 5-FU on the growth of esophageal carcinoma are superior, with no observed increase in side effects. The essential mechanism underlying SIN- and 5-FU-induced apoptosis, as investigated by Western blotting, involved the mitochondrial pathway.
In this study, the combined effects of SIN and 5-FU, as well as their essential mechanism were investigated in esophageal carcinoma. The combined use of these two medicines generated a superior anti-cancer effect without increased side effects. This finding may help to provide novel combined systemic chemotherapy in the treatment of esophageal carcinoma.
SIN: SIN is an alkaloid derived from the stem of the Chinese medical plant Sinomenium acutum, which has been used in traditional Chinese medicine for over 2000 years to treat various rheumatoid diseases. The chemical structure of SIN has been clarified, and its potential value in the treatment of immunological and other diseases is recognized by Western medicine.
This is a valuable original study in which the authors examine the inhibitory effects of SIN combined with 5-FU on esophageal carcinoma Eca-109 cells in vitro and in vivo. The results are exciting and suggest that the combined use of SIN and 5-FU is superior to the effects of the individual agents. This study is important for enhancing the efficacy and reducing the toxicity of chemotherapy regimens for patients with esophageal cancer.
P- Reviewers: Alshehabi Z, Ma JY, Yokoyama Y S- Editor: Cui XM L- Editor: Webster JR E- Editor: Ma S
| 1. | Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol. 2009;24:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 271] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25538] [Article Influence: 1824.1] [Reference Citation Analysis (7)] |
| 3. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2219] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 4. | Ozawa S, Tachimori Y, Baba H, Fujishiro M, Matsubara H, Numasaki H, Oyama T, Shinoda M, Takeuchi H, Tanaka O. Comprehensive Registry of Esophageal Cancer in Japan, 2003. Esophagus. 2011;8:9-29. [RCA] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Shimizu H, Shiozaki A, Fujiwara H, Komatsu S, Ichikawa D, Okamoto K, Murayama Y, Kuriu Y, Ikoma H, Nakanishi M. Predictive factors for early recurrence in patients with esophageal squamous cell carcinoma after curative esophagectomy. Esophagus. 2012;9:17-24. [DOI] [Full Text] |
| 6. | Honda M, Miura A, Izumi Y, Kato T, Ryotokuji T, Monma K, Fujiwara J, Egashira H, Nemoto T. Doxorubicin, cisplatin, and fluorouracil combination therapy for metastatic esophageal squamous cell carcinoma. Dis Esophagus. 2010;23:641-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Hwang-Bo J, Yoo KH, Park JH, Jeong HS, Chung IS. Recombinant canstatin inhibits angiopoietin-1-induced angiogenesis and lymphangiogenesis. Int J Cancer. 2012;131:298-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Tanaka Y, Yoshida K, Sanada Y, Osada S, Yamaguchi K, Takahashi T. Biweekly docetaxel, cisplatin, and 5-fluorouracil (DCF) chemotherapy for advanced esophageal squamous cell carcinoma: a phase I dose-escalation study. Cancer Chemother Pharmacol. 2010;66:1159-1165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Park C, Jung JH, Kim ND, Choi YH. Inhibition of cyclooxygenase-2 and telomerase activities in human leukemia cells by dideoxypetrosynol A, a polyacetylene from the marine sponge Petrosia sp. Int J Oncol. 2007;30:291-298. [PubMed] |
| 10. | Qian L, Xu Z, Zhang W, Wilson B, Hong JS, Flood PM. Sinomenine, a natural dextrorotatory morphinan analog, is anti-inflammatory and neuroprotective through inhibition of microglial NADPH oxidase. J Neuroinflammation. 2007;4:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Li L, Gao XL, Ding BX. [Inhibitory effect of sinomenine on H2O2-induced apoptosis in neonatal rat cardiomyocytes]. Zhongguo Zhongyao Zazhi. 2008;33:939-941, 961. [PubMed] |
| 12. | Kok TW, Yue PY, Mak NK, Fan TP, Liu L, Wong RN. The anti-angiogenic effect of sinomenine. Angiogenesis. 2005;8:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Lu XL, Zeng J, Chen YL, He PM, Wen MX, Ren MD, Hu YN, Lu GF, He SΧ. Sinomenine hydrochloride inhibits human hepatocellular carcinoma cell growth in vitro and in vivo: involvement of cell cycle arrest and apoptosis induction. Int J Oncol. 2013;42:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Zhang HY, Sun H. Up-regulation of Foxp3 inhibits cell proliferation, migration and invasion in epithelial ovarian cancer. Cancer Lett. 2010;287:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Anderson SE, Minsky BD, Bains M, Kelsen DP, Ilson DH. Combined modality therapy in esophageal cancer: the Memorial experience. Semin Surg Oncol. 2003;21:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Koyama H, Iwata H, Kuwabara Y, Iwase H, Kobayashi S, Fujii Y. Gelatinolytic activity of matrix metalloproteinase-2 and -9 in oesophageal carcinoma; a study using in situ zymography. Eur J Cancer. 2000;36:2164-2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Shu L, Yin W, Zhang J, Tang B, Kang YX, Ding F, Hua ZC. Sinomenine inhibits primary CD4+ T-cell proliferation via apoptosis. Cell Biol Int. 2007;31:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Nishida S, Satoh H. In vitro pharmacological actions of sinomenine on the smooth muscle and the endothelial cell activity in rat aorta. Life Sci. 2006;79:1203-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Peters WH, te Morsche RH, Roelofs HM, Mathus-Vliegen EM, Berkhout M, Nagengast FM. COX-2 polymorphisms in patients with familial adenomatous polyposis. Oncol Res. 2009;17:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Mantovani A, Garlanda C, Allavena P. Molecular pathways and targets in cancer-related inflammation. Ann Med. 2010;42:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Yuan HD, Chung SH. Protective effects of fermented ginseng on streptozotocin-induced pancreatic beta-cell damage through inhibition of NF-kappaB. Int J Mol Med. 2010;25:53-58. [PubMed] |
| 22. | Jiang T, Zhou L, Zhang W, Qu D, Xu X, Yang Y, Li S. Effects of sinomenine on proliferation and apoptosis in human lung cancer cell line NCI-H460 in vitro. Mol Med Rep. 2010;3:51-56. [PubMed] |
| 23. | Chen M, Wang J. Initiator caspases in apoptosis signaling pathways. Apoptosis. 2002;7:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 342] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 24. | Green DR. Apoptotic pathways: ten minutes to dead. Cell. 2005;121:671-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 596] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 25. | Waxman DJ, Schwartz PS. Harnessing apoptosis for improved anticancer gene therapy. Cancer Res. 2003;63:8563-8572. [PubMed] |
| 26. | Hsu HF, Houng JY, Kuo CF, Tsao N, Wu YC. Glossogin, a novel phenylpropanoid from Glossogyne tenuifolia, induced apoptosis in A549 lung cancer cells. Food Chem Toxicol. 2008;46:3785-3791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Magesh V, Lee JC, Ahn KS, Lee HJ, Lee HJ, Lee EO, Shim BS, Jung HJ, Kim JS, Kim DK. Ocimum sanctum induces apoptosis in A549 lung cancer cells and suppresses the in vivo growth of Lewis lung carcinoma cells. Phytother Res. 2009;23:1385-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Yeh KH, Yeh SH, Hsu CH, Wang TM, Ma IF, Cheng AL. Prolonged and enhanced suppression of thymidylate synthase by weekly 24-h infusion of high-dose 5-fluorouracil. Br J Cancer. 2000;83:1510-1515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Matsuhashi N, Saio M, Matsuo A, Sugiyama Y, Saji S. The evaluation of gastric cancer sensitivity to 5-FU/CDDP in terms of induction of apoptosis: time- and p53 expression-dependency of anti-cancer drugs. Oncol Rep. 2005;14:609-615. [PubMed] |
| 30. | Wu XX, Kakehi Y, Mizutani Y, Lu J, Terachi T, Ogawa O. Activation of caspase-3 in renal cell carcinoma cells by anthracyclines or 5-fluorouracil. Int J Oncol. 2001;19:19-24. [PubMed] |
| 31. | Adachi Y, Taketani S, Oyaizu H, Ikebukuro K, Tokunaga R, Ikehara S. Apoptosis of colorectal adenocarcinoma induced by 5-FU and/or IFN-gamma through caspase 3 and caspase 8. Int J Oncol. 1999;15:1191-1196. [PubMed] |