INTRODUCTION
Severe acute pancreatitis (SAP) is a serious medical condition with a high mortality rate. Intestinal barrier dysfunction in SAP patients leads to endotoxemia and multiple organ failure, and contributes to disease progression[1-3]. It is helpful to elucidate the pathogenetic mechanisms underlying intestinal barrier damage in SAP for successful treatment of patients who are critically ill in the great majority of cases.
Intestinal mucosal barrier is composed of a layer of crosslinked, highly differentiated epithelial cells located at the apical junction complex (AJC). A tight AJC maintains epithelial cell polarity, reduces the gap between epithelial cells, and decreases epithelial permeability. Therefore, the AJC can prevent intestinal toxins, pathogens and other exogenous substances from entering into the submucosal tissues and the circulation through the epithelial layer. The AJC is believed to be the mainstay structure that maintains the intestinal barrier and prevents enteric endotoxemia, and damage can lead to intestinal barrier dysfunction[4-8].
The mechanisms underlying AJC damage in SAP remain elusive. The structure and function of AJC depend on the regulation of its component proteins. miRNA is a newly established protein regulator[9-11] and is critically involved in maintaining epithelial junctions and polarity, as well as cytoskeletal remodeling in tumors[12-14]. Tang et al[15] reported that the expression of zonula occludens (ZO)-1 protein, a major AJC component, was downregulated by miRNA (miR)-212 in Caco-2 cells and patients with alcoholic live disease and intestinal barrier dysfunction. Ye et al[16] reported that downregulated expression of occludin, the component protein of AJC, was regulated by miR-122 in mouse and Caco-2 cells and resulted in intestinal barrier dysfunction. Aside from their critical role in maintaining intestinal epithelial junctions and regulating AJC proteins, miRNAs, especially miR-155, are also implicated in inflammatory processes[17,18], which normally occur at the early stage of SAP. It has been also reported that miR-155 is a major inflammatory mediator[17-21] and regulates the inflammatory response and carcinogenesis[22]. However, it is yet to be investigated how miR-155 regulates AJC protein expression in intestinal epithelial cells.
The objectives of this study were to investigate how miR-155 regulates AJC protein expression, to predict target genes for miR-155, and to identify the possible miR-155 regulatory pathways in SAP.
MATERIALS AND METHODS
Laboratory animals and experimental protocols
The animal experimentation protocol was approved by the Animal Research Committee at First People’s Hospital, Shanghai Jiaotong University. Twenty-four male BALB/c mice weighing 25 g were purchased from Shanghai Laboratory Animal Center and housed at room temperature (25 °C) with a 12-h light/dark cycle. All animals were given free access to rodent chow and tap water ad libitum and acclimated for ≥ 3 d prior to the experiment.
Following 12 h fasting, all experimental mice were randomly divided into two groups, the SAP group (n = 12) and the control group (n = 12). An experimental SAP mouse model was established as previously reported[23]. In the SAP group, intraperitoneal injection of 50 μg/kg caerulein dissolved in normal saline (Bachem, Bubendorf, Switzerland) was repeated at hourly intervals for 6 h followed by intraperitoneal injection of 10 mg/kg Escherichia coli lipopolysaccharide (LPS) dissolved in normal saline (Sigma-Aldrich, St Louis, MO, United States). In the control group, the same volume of normal saline in place of caerulein and LPS was injected at hourly intervals for 6 h.
All animals were euthanized by injecting 3% intraperitoneal pentobarbital (0.1 mL/100 g; Dongchang Chemical, Shanghai, China) at 3 h after the last injection. Blood samples and pancreas and distal ileal segment specimens were collected. Blood samples were centrifuged at 3000 rpm for 8 min at 4 °C within 2 h after collection, and the supernatants were collected and stored at -80 °C for further experiments. Pancreas and intestine specimens were immediately fixed in 10% buffered formaldehyde (Dongchang Chemical) for further histological examination. Mucosal tissues of the distal ileal segment, 3-5 cm long, were stripped, snap frozen in liquid nitrogen, and stored at -80 °C for further experiments.
Histological examination
Paraffin-embedded pancreatic and intestinal tissues were cut into 5-μm sections. Sections were stained with hematoxylin and eosin (Sigma-Aldrich) and examined using a light microscope (Olympus, Tokyo, Japan). Acute pancreatic damage, including pancreatic edema, acinar cell necrosis, adipose necrosis and hemorrhage, parenchymal inflammation, and extravascular infiltration, was evaluated according to Schmidt’s criteria[24], with a maximum score of 16. Intestinal epithelial barrier damage, including changes in mucosal cells, mucosal structure, parenchymal hemorrhage, and inflammatory cell infiltration, was semiquantitatively evaluated using the following scale: (0) no injury; (1) local atrophy and necrosis of mucosal cells; (2) patchy shedding of mucosal cells, hemorrhage, inflammatory cell infiltration, and intact mucosal structure; (3) large areas of shedding and necrosis of mucosal cells, inflammatory cell infiltration, and destroyed mucosal structure; and (4) extensive necrosis of mucosal cells, inflammatory cell infiltration, loss of mucosal structure, and patchy hemorrhage. All experiments were performed in duplicate.
Serum amylase, diamine oxidase, and tumor necrosis factor-α assays
Serum amylase (a biomarker of acute pancreatic damage) concentration was measured using a commercially available biochemical kit (Kehua, Shanghai, China) as instructed by the manufacturer; serum diamine oxidase (DAO) (a biomarker of intestinal barrier dysfunction) concentration was determined using ultraviolet UV spectrophotometer; and serum tumor necrosis factor (TNF)-α (a biomarker of systemic inflammation and acute phase reaction) level was determined using an ELSIA kit (R and D Systems, Minneapolis, MN, United States). All experiments were performed in duplicate.
Western blotting assay of ZO-1 and E-cadherin
Intestinal epithelial specimens were homogenized in ice-cold lysis buffer (Sigma-Aldrich). The soluble lysate was mixed with the loading buffer containing 40% glycerol (Sigma-Aldrich), 200 mmol/L dithiothreitol (Sigma-Aldrich) and 0.04% bromophenol blue (Kehua, Shanghai, China), and boiled for 5 min. SDS-PAGE (Sigma-Aldrich) was used to separate the proteins, and the electroblotting technique was used to transfer the proteins to a polyvinylidene fluoride membrane. The blots were blocked with 5% bovine serum albumin (Beyotime, Jiangsu, China) at room temperature for 1 h and incubated with primary antibodies against ZO-1 (1:200, polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA, United States) and E-cadherin (1:1000, polyclonal; Cell Signaling Technology, Danvers, MA, United States) at 4 °C overnight. The blots were further incubated with horseradish-peroxidase-conjugated secondary antibodies (Abcam, Cambridge, United Kingdom) for 1 h. The Fujifilm chemiluminescence detection system (Fuji, Tokyo, Japan) was used to detect and visualize the immunoblot bands. The band density of the target protein was quantified using Image J software (http://rsbweb.nih.gov/ij) and normalized to that of β-actin (internal control). All experiments were performed in duplicate.
miR-155 real-time reverse transcriptase polymerase chain reaction
Total RNA samples were isolated from mouse intestinal epithelial specimens using the mirVana miRNA isolation kit (Ambion, Austin, TX, United States). RNA was reversely transcribed into cDNA using the TaqMan miRNA reverse transcription kit (Applied Biosystems, Foster City, CA, United States). miR-155 was detected using stem-loop reverse transcription primers, and U6 served as an internal control. Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was performed using the TaqMan miRNA assay kit (Applied Biosystems) and quantitated using the 7900HT sequence detection system (Applied Biosystems). The thermal cycling was set as follows: denaturation at 95 °C for 15 s, annealing at 60 °C for 1 min, and extension at 60 °C for 1 min, for a total of 40 cycles. Threshold cycle (Ct) was defined as the fractional cycle number of fluorescence that passed through a given threshold. The 2-ΔΔCt method was used to determine the relative miR-155 expression level[25]. All experiments were performed in duplicate.
miRNA target gene prediction
Possible target genes for miR-155 were predicted using the miRTarBase database (http://mirtarbase.mbc.nctu.edu.tw), a database curating experimentally validated miRNA-target interactions, and two computational methods, namely, RNA22 (http://cbcsrv.watson.ibm.com/rna22.html) and PicTar (http://pictar.mdc-berlin.de). The structural analyses were conducted to confirm the AJC-associated target genes for miR-155. The expression of the possible target gene was validated at the transcriptional and post-transcriptional levels.
Real-time RT-PCR and Western blotting of Ras homolog gene family, member A
Ras homolog gene family, member A (RhoA) mRNA samples were extracted from intestinal epithelial specimens using the TRIzol reagent (Invitrogen, San Diego, CA, United States) and reverse transcribed into cDNA with the high-capacity cDNA reverse transcription kit (Applied Biosystems). cDNA was determined by qRT-PCR using the SYBR Green PCR Master Mix kit (Applied Biosystems). PCR amplification was performed using the 7900HT sequence detection system (Applied Biosystems), and the thermal cycling was set as follows: denaturation at 95 °C for 1 min, annealing at 60 °C for 1 min, and extension at 75 °C for 1 min, for a total of 40 cycles. All primers were designed using the Primer Premier software (PREMIER Biosoft, Palo Alto, CA, United States) and synthesized by Sangon (Shanghai, China). The sequences of primers were as follows: for RhoA, sense, AGCTTGTGGTAAGACATGCTTG, antisense, GTGTCCCATAAAGCCAACTCTAC; for β-actin (internal control), sense, CCAGGCACCAGGGCGTGATG, antisense, CGGCCAGCCAGGTCCAGACG. The 2-ΔΔCt method was also used to determine the relative RhoA expression level[25]. All experiments were performed in duplicate.
RhoA western blotting assay was performed as mentioned above and using a primary antibody against RhoA (1:2000, polyclonal; Abcam) with β-actin as the internal control.
Statistical analysis
Statistical analysis was performed using the PASW Statistics version 18.0 software (SPSS Inc., Hong Kong, China). All data were expressed as mean ± SD. The means were compared using the two independent samples Student’s t test, one-way analysis of variance. P < 0.05 was considered statistically significant.
RESULTS
Sequential intraperitoneal injection of caerulein and LPS induces experimental SAP
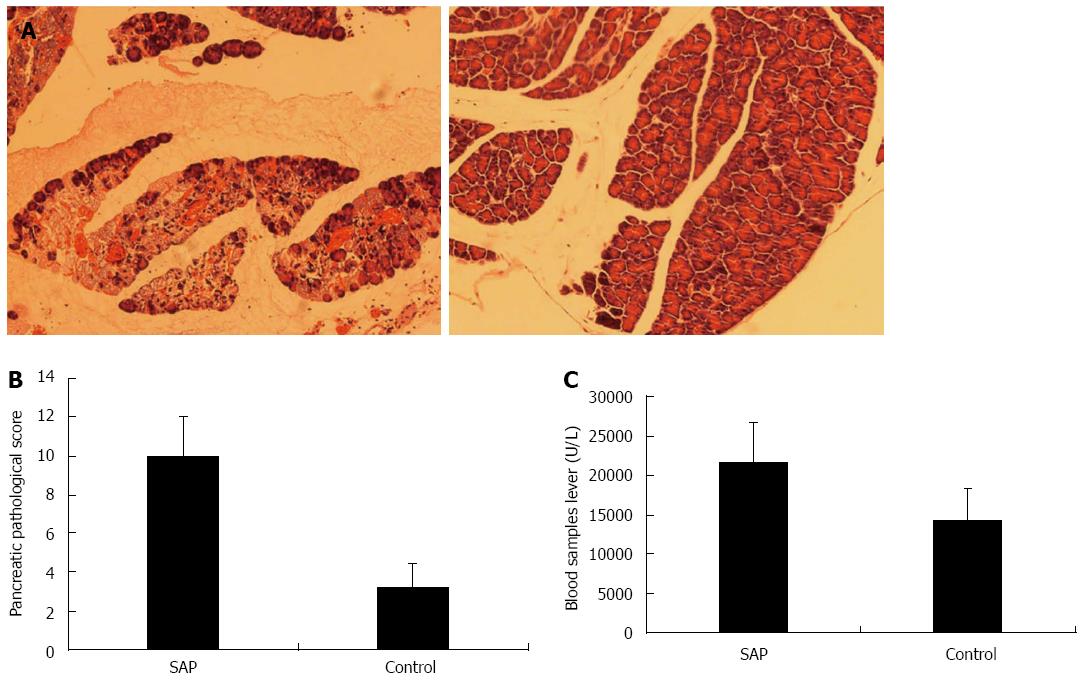
The control group showed no obvious abnormality in the pancreatic tissue on gross and microscopic examination (Figure 1A, right panel). In contrast, the SAP group exhibited patchy hemorrhage and necrosis of the pancreas (Figure 1A, left panel). Moreover, scattered saponification spots on macroscopic examination corresponded to inflammatory cell infiltration with swollen acinar cells and intercellular space widening on microscopy in the SAP group. The Schmidt’s acute pancreatic damage score was significantly higher in the SAP than the control group (10.0 ± 2.0 vs 3.2 ± 1.2, P < 0.01; Figure 1B). Serum amylase level was also significantly higher in the SAP than control group (21.6 ± 5.1 U/mL vs 14.3 ± 4.2 U/mL, P < 0.01; Figure 1C). These results showed that sequential intraperitoneal injection of caerulein and LPS successfully induced experimental SAP in mice, manifesting as pancreatic hemorrhage, necrosis, and inflammatory cell infiltration, as well as hyperamylasemia.
Figure 1 Pancreas histology and serum amylase level in severe acute pancreatitis and control mice.
A: Representative photomicrographs of the pancreas in severe acute pancreatitis (SAP) (left panel) and control (right panel) mice (hematoxylin-eosin, × 100); B: Schmidt’s acute pancreatic damage scores in SAP and control mice, P < 0.01 vs control; C: Serum amylase levels in SAP and control mice, P < 0.01 vs control.
Caerulein and LPS induces intestinal barrier dysfunction in experimental SAP
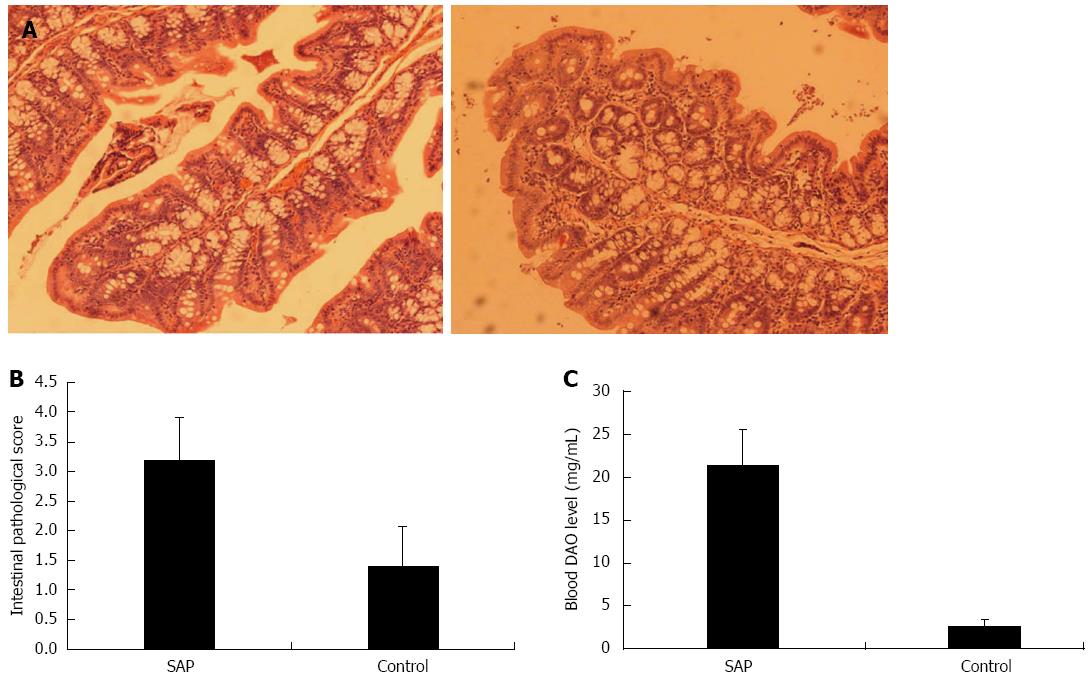
Histological examination in the control group showed mild intestinal mucosal congestion and shedding (Figure 2A, right panel), while the SAP group exhibited marked intestinal mucosal congestion and hemorrhage, inflammatory cell infiltration, glandular atrophy, and necrosis and shedding of epithelial cells (Figure 2A). The intestinal epithelial barrier damage score was significantly higher in the SAP than control group (3.2 ± 0.7 vs 1.4 ± 0.7, P < 0.01; Figure 2B), and serum DAO level was also significantly higher in the SAP group (21.4 ± 4.1 mg/mL vs 2.6 ± 0.8 mg/mL, P < 0.01; Figure 2C). These results showed that sequential intraperitoneal injection of caerulein and LPS also induced intestinal barrier damage in experimental SAP mice.
Figure 2 Intestine histology and serum diamine oxidase levels in severe acute pancreatitis and control mice.
A: Representative photomicrographs of the intestine in severe acute pancreatitis (SAP) (left panel) and control (right panel) mice (hematoxylin-eosin, × 100); B: Intestinal barrier damage scores in SAP and control mice, P < 0.01 vs control; C: serum diamine oxidase (DAO) levels in SAP and control mice, P < 0.01 vs control.
Massive TNF-α release in experimental SAP
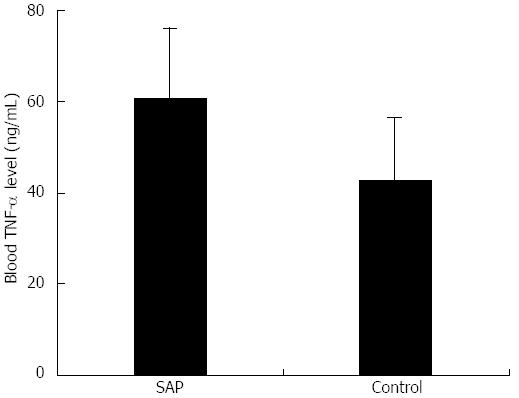
Serum TNF-α concentration in the SAP group was significantly higher than in the control group (61.0 ± 15.1 ng/mL vs 42.9 ± 13.9 ng/mL, P < 0.01; Figure 3). This showed that a systemic inflammatory response occurred in experimental SAP and resulted in massive release of TNF-α, a major proinflammatory cytokine.
Figure 3 Serum tumor necrosis factor-α levels in severe acute pancreatitis and control mice.
Serum tumor necrosis factor-α level was measured using ELISA; P < 0.01 vs control. SAP: Severe acute pancreatitis.
ZO-1 and E-cadherin are underexpressed in experimental SAP intestinal epithelia
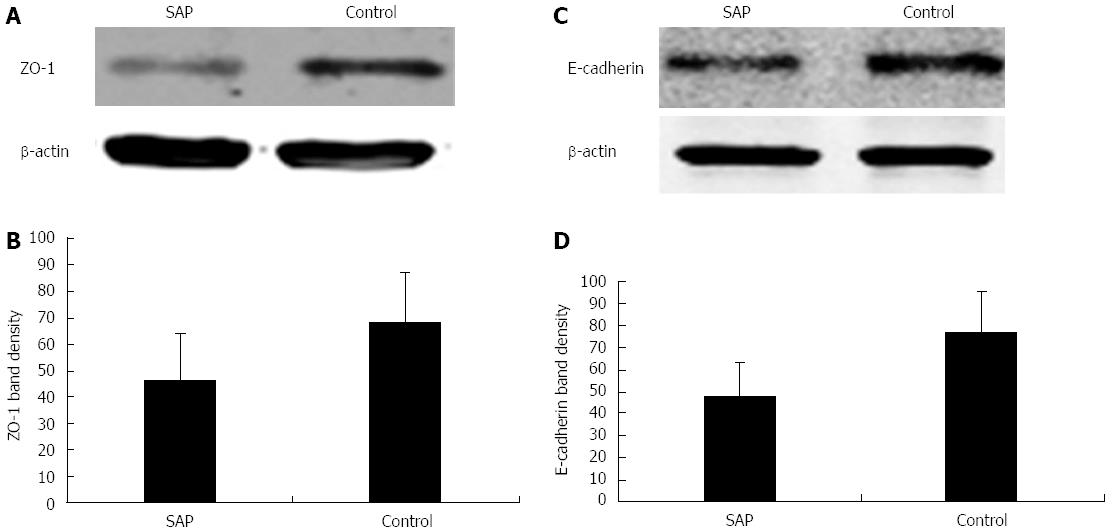
Western blotting analysis showed that the expressions of ZO-1 (46 ± 18 fold vs 68 ± 19 fold, P < 0.01; Figure 4A and B) and E-cadherin (48 ± 15 fold vs 77 ± 18 fold, P < 0.01; Figure 4C and D) were significantly downregulated in experimental SAP intestinal epithelia as compared to the control group. These results showed that ZO-1 and E-cadherin, two major component proteins of the AJC, were damaged in experimental SAP with intestinal barrier dysfunction.
Figure 4 Expressions of zonula occludens-1 and E-cadherin in the intestinal epithelia of severe acute pancreatitis and control mice.
A: Representative western blots showing zonula occludens (ZO)-1 downregulation in SAP mice, with β-actin as an internal control; B: Quantification of ZO-1 expression in SAP and control mice, bP < 0.01 vs control; C: Representative western blots showing E-cadherin downregulation in SAP mice, with β-actin as an internal control; D: Quantification of E-cadherin expression in SAP and control mice, P < 0.01 vs control. SAP: Severe acute pancreatitis.
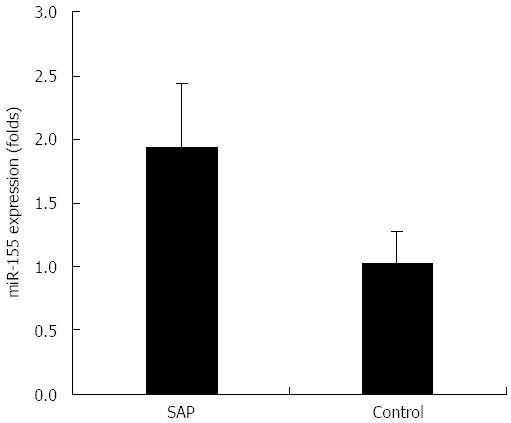
miR-155 is overexpressed in experimental SAP intestinal epithelia
As shown in Figure 5, miR-155 was significantly overexpressed in intestinal epithelia in the SAP group as compared to the control group (1.94 ± 0.50 fold vs 1.03 ± 0.23 fold, P < 0.01). This result showed that miR-155 overexpression might dysregulate AJC protein expression and contribute to intestinal barrier dysfunction in experimental SAP mice.
Figure 5 TaqMan miRNA real-time reverse transcriptase polymerase chain reaction for miRNA-155 expression in severe acute pancreatitis and control mice.
Quantification of miRNA (miR)-155 expression level was normalized to that of U6 expression; P < 0.01 vs control. SAP: Severe acute pancreatitis.
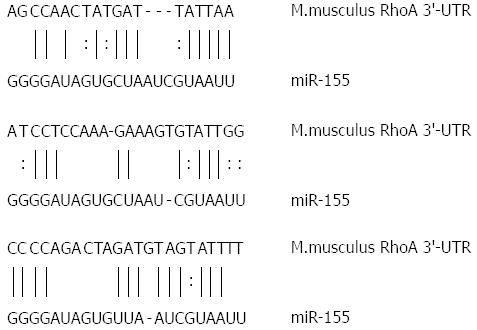
RhoA is a target gene for miR-155
miRNA regulates its target mRNA by binding to its specific binding site in the 3’-untranslated region (UTR). Prediction with miRTarBase, RNA22, and PicTar analytical and computational tools showed that RhoA gene encoding a small GTPase protein known to regulate the actin cytoskeleton in the formation of stress fibers and cell division was one of the possible target genes for miR-155. Moreover, structural analysis of miR-155 and RhoA mRNA confirmed that the latter contained three miR-155-specific binding sites in the 3’-UTR (Figure 6). This result showed that miR-155 overexpression might dysregulate AJC protein expression through the RhoA signaling pathway in experimental SAP with intestinal barrier dysfunction.
Figure 6 Sequence of miRNA-155 and 3′-untranslated region of Ras homolog gene family, member A gene.
Ras homolog gene family, member A (RhoA) mRNA contains three miR-155 specific binding sites in the 3′-untranslated region (UTR). Vertical lines and columns indicate the Watson-Crick and wobble base pairing, respectively.
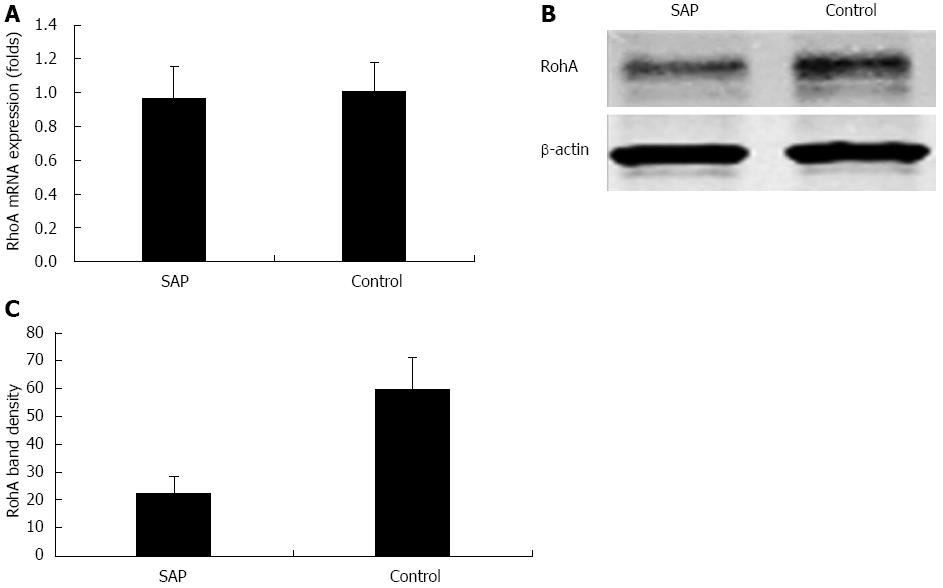
RhoA is underexpressed in experimental SAP intestinal epithelia
RhoA mRNA expression was not significantly upregulated or downregulated in SAP intestinal epithelia as compared to the control group (0.97 ± 0.18 fold vs 1.01 ± 0.17 fold, P > 0.05; Figure 7A). However, western blotting analysis showed that RhoA protein expression was significantly underexpressed in experimental SAP intestinal epithelia (22.7 ± 5.8 fold vs 59.6 ± 11.6 fold, P < 0.01; Figure 7B and C). This result suggested that miR-155 overexpression negatively regulated the target gene RhoA expression at the post-transcriptional level, and post-transcriptional RhoA underexpression was implicated in AJC protein dysregulation in experimental SAP with intestinal barrier dysfunction.
Figure 7 Expressions of Ras homolog gene family, member A mRNA and protein in the intestinal epithelia of severe acute pancreatitis and control mice.
A: Real-time reverse transcriptase polymerase chain reaction showing the relative level of Ras homolog gene family, member A (RhoA) mRNA expression normalized to that of β-actin, P > 0.05 vs control; B: Representative western blots showing RhoA protein expression downregulation in severe acute pancreatitis (SAP) mice, with β-actin as an internal control; C: Quantification of RhoA protein expression in SAP and control mice, P < 0.01 vs control.
DISCUSSION
Intestinal barrier dysfunction is closely associated with poor prognosis in SAP patients, and is believed to be the primary cause of SAP progression[26-28]. It has remained unknown until now how intestinal barrier dysfunction occurs in SAP. To the best of our knowledge, the present work was the first study to report the implication of miR-155 dysregulation in SAP with intestinal barrier dysfunction. Our results showed that a systemic inflammatory response occurred in experimental SAP, as indicated by massive TNF-α release, and miR-155 overexpression was seen in SAP intestinal epithelia with barrier dysfunction. These pathogenetic processes were accompanied with significant underexpression of RhoA, a target gene for miR-155, as well as ZO-1 and E-cadherin, two major component proteins of AJC; all of which contributed to intestinal barrier dysfunction in experimental SAP.
Serum DAO level is regulated by the intestinal mucosal permeability, and is believed to a reliable biomarker of intestinal barrier function[29,30]. Our results showed that serum DAO level increased significantly in experimental SAP. Intestinal barrier dysfunction manifested histologically as necrosis and shedding of epithelial cells in the intestinal mucosal layer as observed in experimental SAP mice. All this biochemical and pathological evidence confirmed that intestinal barrier damage occurred in experimental SAP.
TJs and adherens junctions (AJs) are among the key protein complexes of the AJC[7,31]. ZO-1 and E-cadherin are the major component proteins in TJs and AJs, respectively[15]. In TJs, as a cytoskeletal protein, ZO members, including ZO-1, ZO-2, and ZO-3, are linked to the transmembrane protein through its intracellular domains and connected with adjacent epithelial cells via its extracellular domains[32]. E-cadherin, a transmembrane protein, is connected to adjacent cells via its extracellular domains and linked with the cytoskeleton by binding to β-catenin via its intracellular domains. These two AJC proteins tightly crosslink epithelial cells in the intestinal mucosa, and maintain the intestinal barrier[8]. The AJC is the key structure for maintenance of intestinal barrier function[4-8]. The underexpression of ZO-1 and E-cadherin in experimental SAP could result in AJC disruption and consequently compromise intestinal barrier dysfunction.
Our study results showed that miR-155 was significantly overexpressed in experimental SAP intestinal epithelia. miRNA is an endogenous non-coding RNA, at a length of 21-25 nucleotides, which plays a crucial role in the post-transcriptional regulation of gene expression. Moreover, miRNA, especially miR-155, is known to be implicated in regulating the systemic inflammatory response[33], which normally occurs at the early stage of SAP[17-21]. Multiple biological functions of miR-155 have been identified, including induction of Toll-like receptor (TLR) activation in monocytes/macrophages, regulation of LPS-induced non-specific immunity, and feedback modulation of the TLR signaling pathway[22,34]. More importantly, miR-155 expression can be induced by inflammatory cytokines, such as TNF-α and interferons[17,22,34-37]. TNF-α, one of the most important early proinflammatory mediators, is the first factor that is massively released into the circulation and plays a key role in systemic inflammatory response syndrome[38,39]. TNF-α is also destructive for the AJC in other inflammatory diseases. Sasaki et al[40] reported that the increase in serum TNF-α in inflammatory bowel disease induced by enteric bacteria downregulated expression of ZO-1 protein and E-cadherin and disrupted the AJC. Fries[41] found that excessive TNF-α release decreased the protein synthesis of TJs in inflammatory bowel disease, which could be treated with anti-TNF-α therapy by improving TJ protein synthesis. Ma et al[42] demonstrated that increased serum TNF-α inhibited ZO-1 protein synthesis in Crohn’s disease intestinal epithelial cells and consequently resulted in AJC damage. TNF-α release increased in response to systemic inflammation in SAP, which was accompanied by significant overexpression of miR-155 in SAP intestinal epithelia. These results suggested that excessive TNF-α release caused AJC damage by dysregulating miR-155 expression in SAP epithelial cells.
miRNA exerts a regulatory effect by acting on target genes. miRNA binds to argonaute proteins to form the RNA-induced silencing complex, inhibits the transcription of the target genes by acting on its specific binding site in the 3’-UTR[43], and leads to reduced protein synthesis of the target genes[44]. The presence of a specific structural pairing between miRNA and its target gene allows for structural analysis to predict the target genes for miRNA. In this study, RhoA was determined to be one of the possible target genes for miR-155. RhoA mRNA contains three miR-155-specific binding sites in the 3’-UTR, which are highly conserved among multiple species, including rats, mice and humans. RhoA, a small GTPase belonging to the Rho family[45], is the major regulator of AJC expression[46-48]. RhoA promotes the formation and maintenance of the AJC, and RhoA underexpression leads to disrupted AJC[49-51] by blocking protein synthesis of TJs[52,53] and AJs[54]. Our results showed that RhoA protein rather than mRNA was significantly underexpressed in SAP intestinal epithelia. This indicates that miR-155 overexpression in SAP intestinal epithelia dysregulates RhoA gene expression at the post-transcriptional instead of transcriptional level.
In conclusion, miR-155 plays a crucial role in the structural and functional regulation of the AJC in the intestinal epithelial cells. In SAP, excessive inflammatory response leads to excessive release of inflammatory cytokines, such as TNF-α, and induces miR-155 overexpression in the intestinal epithelia. miR-155 overexpression inhibits RhoA protein synthesis by dysregulating RhoA gene expression at the post-transcriptional level, and consequently downregulates expressions of ZO-1 and E-cadherin; two major component proteins of the AJC. This pathogenetic process eventually results in intestinal barrier dysfunction in SAP.
COMMENTS
Background
Intestinal barrier dysfunction is believed to result in endotoxemia and multiple organ failure, complicating severe acute pancreatitis (SAP). The apical junction complex (AJC) is the mainstay structure that maintains the intestinal barrier and prevents enteric endotoxemia, while AJC damage can lead to intestinal barrier dysfunction. However, the mechanisms underlying AJC damage complicating SAP remain unknown.
Research frontiers
miRNA (miR) is known to maintain epithelial junctions and polarity, as well as cytoskeletal remodeling, by regulating protein synthesis. miR-dysregulation-induced proinflammatory factors are reported to contribute to AJC disruption in inflammatory bowel disease via an unknown signaling pathway. miR-155 is a major inflammatory mediator emerging at the early stage of the inflammatory process. There is a knowledge gap in the current literature as to whether miR-155 dysregulation is implicated in intestinal barrier dysfunction complicating SAP, and through which signaling pathway dysregulated miR-155 expression interrupts AJC component protein synthesis.
Innovations and breakthroughs
This successfully created an experimental SAP mouse model with complicating intestinal barrier dysfunction by inducing intraperitoneal inflammatory response, which was validated histologically and biochemically. This study reported for the first time that miR-155 was overexpressed in experimental SAP intestinal epithelia as induced by excess circulating tumor necrosis factor (TNF)-α. RhoA, a small GTPase belonging to the Rho family, is the major regulator of AJC component protein expression. Their target gene prediction test showed that RhoA gene was one of the target genes for miR-155 and was underexpressed in experimental SAP intestinal epithelia at the post-transcriptional level. Downregulated RhoA expression was also accompanied by underexpression of zonula occludens (ZO)-1 and E-cadherin, which are component proteins of tight junctions and adherens junctions - two major constituents of the AJC. Release of proinflammatory factors, such as TNF-α, dysregulates miR-155 expression in intestinal epithelia and consequently disrupts protein synthesis of AJC components, possibly via the TNF-α/miR-155/RhoA/ZO-1/E-cadherin signaling pathway.
Applications
The results suggest that miR-155 is a key mediator implicated in intestinal barrier dysfunction complicating SAP and other inflammatory gastrointestinal diseases. Inhibitor targeting of the miR-155 signaling pathway may be an effective alternative for treating inflammatory gastrointestinal diseases by repairing intestinal epithelial barrier.
Terminology
The AJC is a functional unit located at the cell apex and connects the epithelial cells, which is composed of tight junctions and zonula adherens, and desmosomes, and functions to regulate cell polarity, tissue integrity, and intercellular adhesion and permeability. miR-155 is encoded by miR-155 host gene and plays a critical role in regulating inflammatory responses implicated in inflammatory, infectious, and neoplastic disorders. miRTarBase is a computational bioinformatics database accumulating > 3000 miR-target interactions, which are collected by manual survey of pertinent literature through data mining of the text and systematically filtering miR functional studies, and miR-target interactions are normally validated by experimental reporter genes, Western blotting, and microarray and gene sequencing analyses.
Peer review
The authors demonstrated evidence of SAP. miR-155 expression increased compared to the controls and was associated with a decrease in ZO-1, E-cadherin, and RhoA expression. The authors conclude that these results suggest that SAP decreases intestinal barrier function by decreasing synthesis of key junctional complex proteins via miR-155 signaling.