Published online Dec 7, 2013. doi: 10.3748/wjg.v19.i45.8203
Revised: November 7, 2013
Accepted: November 18, 2013
Published online: December 7, 2013
Processing time: 77 Days and 16.1 Hours
Helicobacter pylori (H. pylori) produce an enzyme known as γ-glutamyl transpeptidase (HpGGT) that is highly conserved and common to all strains. HpGGT has been gaining increasing attention as an important virulence factor of the bacterium, having been demonstrated to be an important colonization factor in several animal models and has also recently been strongly associated with the development of peptic ulcer disease. From the results of various independent researcher groups, it is clear that HpGGT acts through several pathways to damage gastric epithelial cells including the induction of apoptosis and cell cycle arrest, production of reactive oxygen species leading to DNA damage, promotion of inflammation by increasing cyclooxygenase-2 and interleukin-8 expression, and upregulation of heparin-binding epidermal growth factor-like growth factor resulting in cell survival and proliferation. In addition, the potential role of HpGGT in promoting gastric carcinogenesis will also be discussed in this review. Apart from affecting the gastric epithelium, HpGGT also has immunomodulatory actions on host immune cells where it displays an antiproliferative effect on T cells by inducing cell cycle arrest and also works with other H. pylori virulence factors to skew dendritic cells towards a tolerogenic phenotype, possibly contributing to the persistence of the pathogen in the gastric mucosa.
Core tip:Helicobacter pylori produce γ-glutamyl transpeptidase (HpGGT), an important virulence factor associated with the development of peptic ulcer disease. HpGGT acts through several pathways to damage gastric epithelial cells including induction of apoptosis and cell cycle arrest, production of reactive oxygen species, promotion of inflammation and upregulation of heparin-binding epidermal growth factor-like growth factor which may then lead to carcinogenesis. HpGGT also has immunomodulatory actions on immune cells where it displays an antiproliferative effect on T cells and skews dendritic cells towards a tolerogenic phenotype, possibly contributing to the persistence of the pathogen in the gastric mucosa.
-
Citation: Ling SSM, Yeoh KG, Ho B.
Helicobacter pylori γ-glutamyl transpeptidase: A formidable virulence factor. World J Gastroenterol 2013; 19(45): 8203-8210 - URL: https://www.wjgnet.com/1007-9327/full/v19/i45/8203.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i45.8203
Helicobacter pylori (H. pylori) is a Gram-negative, spiral-shaped bacterium that selectively colonizes the human gastric mucosa. It has been reported to chronically infect at least half of the world’s population[1,2] and may persist for life in the absence of appropriate treatment. H. pylori is a major etiological factor of a range of gastroduodenal diseases including chronic gastritis[3] and peptic ulcer disease[4], and has been closely associated with the development of mucosa-associated lymphoid tissue lymphoma[5] and even gastric cancer[6].
Since the first isolation of H. pylori in 1983[7], numerous virulence factors of the pathogen have been identified including the extensively studied cytotoxin-associated gene A (CagA)[8] and vacuolating cytotoxin (VacA)[9]. In western countries, strains harbouring CagA and VacA (with s1/mL alleles) have been strongly associated with peptic ulcer disease and gastric cancer[10,11]. However, their relevance in East Asia remains unclear as such correlations were not apparent[12,13]. From these observations, it can be inferred that CagA and VacA are probably not the only factors contributing to H. pylori pathogenesis. There is thus a constant search for other pathogenic factors that could aid in the virulence of the bacterium. One such factor is H. pyloriγ-glutamyl transpeptidase (HpGGT) which has been gaining increasing attention in recent years and will be the main focus of this review.
Similar to mammalian GGTs, HpGGT catalyzes reactions in which a γ-glutamyl moiety is transferred from γ-glutamyl compounds, such as glutathione, to amino acids (transpeptidation) or water (hydrolysis)[14]. HpGGT is first translated in a single-chain precursor form which is inactive. The proenzyme then undergoes intramolecular autocatalytic cleavage, resulting in a catalytically active heterodimer comprising a large (40 kDa) and small (20 kDa) subunit. Interestingly, the amino acid sequence of HpGGT is considerably different from the GGTs of other bacterial species, sharing only 52.5%, 47.7% and 38% amino acid sequence identities with Escherichia coli, Pseudomonas aeruginosa and Bacillus subtilis GGTs, respectively[15]. Among different H. pylori strains however, HpGGT is highly conserved with > 97% sequence homology between isolates[16]. Notably, HpGGT is also constitutively expressed and is commonly found in all H. pylori strains[15], suggesting its importance in the physiology of the bacterium. In further support of this, a subsequent study by Gong and Ho[17] demonstrated the importance of HpGGT in the growth of H. pylori where strains with higher GGT activity exhibited more profuse growth compared to those having lower GGT activity. Indeed, it was later found that one of the main physiological functions of HpGGT is to metabolize extracellular glutathione and glutamine (substrates that it is unable to uptake directly) as a source of glutamate which is then taken up by the bacterium and subsequently incorporated into the tricarboxylic acid cycle[18].
Although not essential for in vitro survival, two pioneer studies on HpGGT had earlier demonstrated the enzyme to be an important virulence factor of the gastric pathogen[15,19]. Using the Swiss specific pathogen-free murine model, Chevalier et al[15] first described HpGGT to be essential for colonization as H. pylori SS1 GGT-deficient mutants could not be recovered from the mice stomachs from 3-60 d post-infection. Interestingly, McGovern et al[19] later showed using two different animal models, namely gnotobiotic piglets and C57BL/6 mice, that although the H. pylori HpM5 ggt-isogenic mutants were still able to colonize the animals, the bacterial load was significantly reduced compared to the parental strain. The differences in animal models and H. pylori strains used by both groups could have contributed to the variations observed but nevertheless, both studies had consistently shown that the presence of HpGGT provides an advantage to the bacterium in colonization.
The clinical importance of HpGGT was reported by our group in 2010 where H. pylori isolates from patients with peptic ulcer disease (n = 54) were found to have significantly higher GGT activity (P < 0.001) compared to those cultured from patients with non-ulcer dyspepsia (n = 44)[16]. Furthermore, no correlation was observed between HpGGT and other known virulence genes such as cagA, vacA, iceA and babA, suggesting a causal link between HpGGT and gastroduodenal diseases. The exact mechanisms detailing how the presence of HpGGT leads to disease development have not been fully elucidated. However, several pathways involving both gastric epithelial cells as well as immune cells have been put forward by various groups and these will be discussed in this review.
H. pylori-induced apoptosis of gastric epithelial cells both in vitro and in vivo had earlier been described by many researchers[20-22], however the bacterial factor(s) responsible were not clearly defined. By analyzing various H. pylori membrane fractions capable of inducing apoptotic cell death in AGS cells, HpGGT was later found to be one of the leading factors involved in the induction of apoptosis by H. pylori[23]. The pathway by which this occurs is mitochondria-mediated as evident from the accompanying activation of caspases 9 and 3, upregulation of pro-apoptotic Bax and downregulation of antiapoptotic Bcl-2 and Bcl-xL as well as the release of cytochrome c from the mitrochondria into the cytosolic space[24]. In addition, it has also been shown by Kim et al[25] that HpGGT inhibits cell cycle progression at the G1-S phase transition and the authors have suggested that this dysregulation results in the enhancement of apoptosis.
The underlying mechanism as to how HpGGT triggers apoptosis was not addressed in these earlier studies. Interestingly, we had recently reported that exposure of gastric cells to purified native HpGGT resulted in the formation of reactive oxygen species (ROS), in particularly H2O2[16] which is a known inducer of apoptosis[26-28]. Accordingly, we and others have shown that pro-oxidant products generated by HpGGT through glutathione degradation triggered apoptosis in gastric epithelial cells[16,29], hence providing the link between HpGGT and its ability to induce apoptotic cell death. This model also corroborates with earlier observations whereby H. pylori infection was found to be associated with excessive ROS levels[30,31] and diminished glutathione levels in the infected gastric mucosa[32].
Intriguingly, apart from gastric cells, HpGGT has also recently been shown to be capable of inducing mitochondria-mediated apoptosis in a human cholangiocarcinoma cell line[33]. This suggests that HpGGT-induced apoptosis is not only restricted to gastric epithelial cells and may possibly occur via a common pathway across different cell types. Hence, future studies investigating the effects of HpGGT on other cell lines may be of particular interest.
H. pylori-infected subjects develop an inflammatory and immune response towards the pathogen characterized by infiltration of the mucosa by polymorphonuclear and mononuclear leukocytes as well as neutrophils[34]. However, this response is ineffective in clearing the bacteria, thereby resulting in chronic gastric inflammation[35]. With regard to the role of HpGGT in inflammation, Busiello et al[36] showed by using the MKN28 gastric cell line that HpGGT upregulates cyclooxygenase-2 (COX-2) expression and its enzymatic product prostaglandin E2, whose role in inflammation has been well established[37]. Notably, COX-2 has been found to be overexpressed in various types of cancer including gastric carcinoma[38-40] and has roles in promoting cell proliferation, angiogenesis and metastasis[41-43].
In addition, our group had also previously reported that purified native HpGGT stimulated the activation of the transcription factor NF-κB, leading to increased expression and secretion of the pro-inflammatory chemokine interleukin-8 (IL-8) from both AGS and primary gastric epithelial cells[16]. HpGGT-induced IL-8 production in gastric cells may thus contribute to the recruitment of immune cells to the sites of infection and the maintenance of chronic inflammation in the gastric mucosa. Importantly, H. pylori infection has been associated with elevated levels of gastric IL-8[44,45], a potent neutrophil recruitment factor thought to play a pivotal role in the immunopathogenesis of H. pylori infections[46]. Collectively, these results strongly support the contributory role of HpGGT in pro-inflammatory processes.
HpGGT upregulates the expression of heparin-binding epidermal growth factor-like growth factor (HB-EGF), a member of the EGF-like growth factor family of proteins and a ligand of epidermal growth factor receptor (EGFR)[36]. HB-EGF is first synthesized as a membrane-anchored precursor which is subsequently cleaved at the cell surface, yielding the mature, soluble form[47]. Binding of soluble HB-EGF to EGFR activates the Raf/Ras/MEK/Erk and phosphoinositide-3-kinase (PI3K)/Akt pathways which promote cell survival and proliferation[48,49]. Importantly, expression of HB-EGF has been reported to be increased in various cancer types including hepatic[50], breast[51], ovarian[52] and gastric cancer[53]. Furthermore, both expression and protein shedding of HB-EGF have been found to be increased in H. pylori infections[54] and this has been suggested to contribute to gastric cancer progression by promoting epithelial-mesenchymal transition[55]. Till date, the definitive role of HpGGT-induced HB-EGF expression in gastric cells has not been clearly elucidated but its potential role in carcinogenesis would certainly be an area worth investigating in future studies.
It seems contradictory for HpGGT to have both apoptosis- and survival-promoting properties. However, both effects may play different roles during the various events of carcinogenesis. HpGGT-induced apoptosis has been suggested to be important particularly in the early events of carcinogenesis[23]. This is because an increase in the rate of apoptosis in a subpopulation of cells could induce a secondary hyperproliferative response where the gastric mucosa attempts to maintain its cell mass[56]. Hyperproliferation, coupled with DNA damage induced by HpGGT[16], could then potentially lead to an increase in the mutation rates of important tumor suppressor genes in these cells, resulting in their transformation to a malignant phenotype. In tumor cells that have become apoptosis-resistant, it is then possible that HpGGT-induced COX-2 upregulation in these cells contribute to their continuous survival and proliferation. This postulation is partially supported by the finding that HpGGT-dependent induction of COX-2 mRNA is higher in MKN28 cells compared to AGS cells as observed by Busiello et al[36]. Although AGS and MKN28 cells are both carcinoma cells lines, MKN28 cells have a mutation in p53, an important tumor suppressor involved in the control of cell cycle progression and apoptosis[57]. Thus, it is plausible that COX-2-induced cell proliferation affects apoptosis-resistant tumor cells to a greater extent, leading to the survival and proliferation of these cancerous cells.
Apart from directly influencing gastric epithelial cells, an increasing body of evidence pointing to the role of HpGGT in modulating the immune response is emerging. Being a secreted bacterial protein[58], the possibility of HpGGT interacting with other non-gastric cells is highly possible especially since H. pylori is capable of disrupting gastric epithelial barrier function[59]. Interestingly, the effects of HpGGT on immune cells have been investigated in various studies and have yielded important results and implications.
In one of the earlier studies investigating the effects of HpGGT on immune effector cells, Schmees et al[60] found that HpGGT was capable of abrogating the proliferation of both primary and immortalized human T cells. A corresponding cell cycle arrest at the G1 phase was observed in these cells which possibly occurred due to disruption of a Ras-dependent signalling pathway. Intriguingly, inhibition of T cell proliferation by HpGGT was found in the same study to be mediated by an apoptosis-independent mechanism which is different from that observed in gastric epithelial cells, suggesting that separate mechanisms exist in both cell types. HpGGT-induced inhibition of T cell proliferation has been proposed to have immunosuppressive effects which contribute to the persistence of H. pylori infections[60]. Interestingly, in a separate study by Beigier-Bompadre et al[61], HpGGT-dependent antiproliferative effect on T cells was found to be modulated by bacterial cholesterol/cholesterol α-glucoside content, suggesting that HpGGT works with other H. pylori factors to shape the immune response during an infection.
Working together with H. pylori lipopolysaccharide and vacuolating cytotoxin (VacA), HpGGT was recently reported to upregulate microRNA-155 (miR-155) expression in CCRF-CEM cells, the first study to investigate the regulation of miRNAs by H. pylori in T cells[62]. Clinically, miR-155 has been shown to be induced upon H. pylori infection[63] and has also been associated with the development of diffuse large B-cell lymphoma[64,65]. In addition, HpGGT-induced miR-155 expression in both CCRF-CEM cells and primary human peripheral blood mononuclear cells was found to be dependent on forkhead box P3 (Foxp3) and requires activation of the cyclic adenosine monophosphate cascade[62]. Foxp3 is a transcription factor thought to be the master regulator in the development of regulatory T cells (Treg)[66], a subset of T cells with a suppressive activity on immune responses[67]. In support of this, mice infected with ggt-isogenic mutants were found to have lower Treg counts compared to wild type-infected mice[68]. Hence, it was suggested that HpGGT may play an important role in the modulation of the immune system[62].
The ability of H. pylori to reprogram dendritic cells (DCs) towards a tolerogenic phenotype has been implicated in the development of immune tolerance and favors persistence of the bacteria in the gastric mucosa[69]. Recently, it has been reported that both VacA and HpGGT play critical roles in DC reprogramming by interfering with their maturation and that this occurred in a manner independent of their suppressive effects on T cells[68]. The underlying mechanisms dictating how both factors prevent DC maturation and promote tolerization were not clearly elucidated in the study but it is known that they act via non-redundant pathways since neither of the respective isogenic mutants was capable of rescuing the effect of the other.
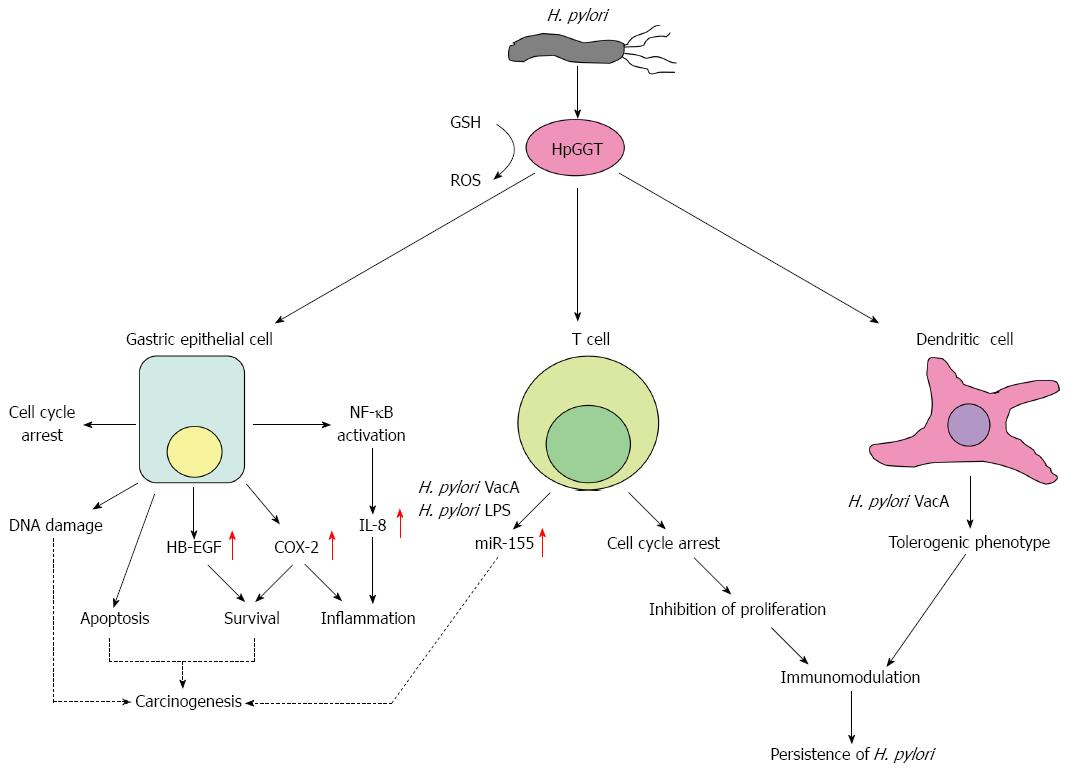
H. pylori produces a potent virulence factor, HpGGT, which causes injury to host cells through multiple ways (illustrated in Figure 1 and summarized in Table 1), many of which have been implicated in carcinogenesis. To gastric epithelial cells, it induces mitochondrial-dependent apoptosis, cell cycle arrest and production of the pro-inflammatory IL-8. To T cells, it inhibits their proliferation and upregulates miR-155 expression while to DCs, it skews them towards a tolerogenic phenotype. Taken together, it is clear that HpGGT plays an important role in the pathogenesis of H. pylori by directly damaging gastric epithelial cells and also in modulating the immune response towards the bacterium, resulting in persistent colonization by the organism. Despite the relatively numerous reports on its effects on the host, much of the underlying mechanisms of how such effects are brought about by HpGGT remain ill-defined. Future studies on the molecular mechanisms responsible for the actions of HpGGT will be required to better understand the role of HpGGT in the pathogenesis of H. pylori. This will be particularly important in the consideration of HpGGT as a viable anti-H. pylori target. In addition, it could also be worthwhile to evaluate the efficacy of HpGGT as a potential vaccine candidate against H. pylori infections especially since the protein is present in all H. pylori strains.
| Ref. | Study description | Main findings |
| Peptic ulcer disease | ||
| Gong et al[16] (2010) | Comparison of GGT activity between H. pylori isolates from PUD (n = 54) vs NUD (n = 44) patients. | HpGGT is associated with PUD as strains isolated from PUD patients had significantly higher HpGGT activity compared to those from NUD patients (P < 0.001). |
| Gastric epithelium damage by apoptosis | ||
| Shibayama et al[23] (2003) | Identification of apoptosis-inducing factors from H. pylori by testing different purified membrane fractions of the bacteria on AGS cells. | HpGGT is a leading factor in H. pylori-mediated apoptosis induction. |
| Kim et al[24] (2007) | Determination of the pathway involved in HpGGT-induced apoptosis by analyzing levels of caspase-9, -3, Bax, Bcl-2, Bcl-xL and cytochrome c release in AGS cells upon treatment with recombinant HpGGT. | HpGGT induces apoptosis via a mitochondria-mediated pathway. |
| Kim et al[25] (2010) | Examination of the effects of recombinant HpGGT on cell cycle progression in AGS cells. | HpGGT induces cell cycle arrest at the G1-S phase transition. (The authors propose this dysregulation enhances apoptosis induction) |
| Gong et al[16] (2010) | Investigation of the effects of HpGGT-induced H2O2 production on apoptosis. AGS cells were incubated with purified native HpGGT and NAC (H2O2 inhibitor) and the activities of caspase-3, -8 and -9 were measured. | HpGGT-mediated oxidative stress is required for HpGGT-associated apoptosis. |
| Promotion of inflammation | ||
| Busiello et al[36] (2004) | Purification and identification of secreted H. pylori factors involved in the upregulation of COX-2 expression in MKN28 cells. | HpGGT is able to upregulate COX-2 expression and its enzymatic product, prostaglandin E2. |
| Gong et al[16] (2010) | Determination of the ability of HpGGT to induce IL-8 production in AGS and primary gastric epithelial cells. | Purified native HpGGT activates NF-κB and upregulates IL-8 production in gastric epithelial cells. |
| Upregulation of heparin-binding epidermal growth factor-like growth factor | ||
| Busiello et al[36] (2004) | Investigation of the ability of HpGGT to upregulate HB-EGF expression in MKN28 cells and elucidating the underlying host cellular pathways involved using specific pathway inhibitors. | HpGGT upregulates HB-EGF expression via activation of a phosphatidylinositol-3 kinase and p38 kinase-dependent signalling transduction pathway. Increase in HB-EGF promotes cell survival and proliferation. |
| Modulation of host immune response | ||
| Schmees et al[60] (2007) | Purification and identification of H. pylori factors responsible for inhibition of T cell proliferation. | HpGGT inhibits T cell proliferation by inducing cell cycle arrest in the G1 phase, possibly through the disruption of a Ras-dependent signalling pathway. |
| Beigier-Bompadre et al[61] (2011) | Characterization of the interdependent effects of VacA, HpGGT and bacterial cholesterol on T cell proliferation using H. pylori and relevant mutants. | HpGGT antiproliferative activity on T cells is modulated by the bacterial cholesterol/cholesterol α-glucoside content. |
| Fassi Fehri et al[62] (2010) | Identification of H. pylori factors involved in the regulation of miRNAs in T cells using miRNA profiling. | HpGGT works with H. pylori VacA and lipopolysaccharide to upregulate miRNA-155 expression in CCRF-CEM cells. This was dependent on Foxp3 transcription factor and requires activation of the cAMP cascade. |
| Oertli et al[68] (2013) | Determination of the role of HpGGT and VacA in dendritic cell reprogramming and development of immune tolerance using in vitro and in vivo models. | Both HpGGT and VacA independently interfere with dendritic cell maturation, possibly contributing to dendritic cell tolerization and hence promoting the persistence of H. pylori infection. |
P- Reviewers: Huerta-Franco MR, Lee YC, Ulasoglu C, Zhu YL S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 762] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 2. | Linz B, Balloux F, Moodley Y, Manica A, Liu H, Roumagnac P, Falush D, Stamer C, Prugnolle F, van der Merwe SW. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445:915-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 720] [Cited by in RCA: 637] [Article Influence: 35.4] [Reference Citation Analysis (3)] |
| 3. | Cover TL, Blaser MJ. Helicobacter pylori and gastroduodenal disease. Annu Rev Med. 1992;43:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 154] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Peterson WL. Helicobacter pylori and peptic ulcer disease. N Engl J Med. 1991;324:1043-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 296] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1229] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 6. | De Koster E, Buset M, Fernandes E, Deltenre M. Helicobacter pylori: the link with gastric cancer. Eur J Cancer Prev. 1994;3:247-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 454] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791-5795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 933] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 9. | Cover TL, Blaser MJ. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10570-10575. [PubMed] |
| 10. | Atherton JC, Peek RM, Tham KT, Cover TL, Blaser MJ. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997;112:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 406] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 11. | van Doorn LJ, Figueiredo C, Sanna R, Plaisier A, Schneeberger P, de Boer W, Quint W. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology. 1998;115:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 412] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999;37:2274-2279. [PubMed] |
| 13. | Zheng PY, Hua J, Yeoh KG, Ho B. Association of peptic ulcer with increased expression of Lewis antigens but not cagA, iceA, and vacA in Helicobacter pylori isolates in an Asian population. Gut. 2000;47:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Boanca G, Sand A, Barycki JJ. Uncoupling the enzymatic and autoprocessing activities of Helicobacter pylori gamma-glutamyltranspeptidase. J Biol Chem. 2006;281:19029-19037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Chevalier C, Thiberge JM, Ferrero RL, Labigne A. Essential role of Helicobacter pylori gamma-glutamyltranspeptidase for the colonization of the gastric mucosa of mice. Mol Microbiol. 1999;31:1359-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 155] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Gong M, Ling SS, Lui SY, Yeoh KG, Ho B. Helicobacter pylori gamma-glutamyl transpeptidase is a pathogenic factor in the development of peptic ulcer disease. Gastroenterology. 2010;139:564-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Gong M, Ho B. Prominent role of gamma-glutamyl-transpeptidase on the growth of Helicobacter pylori. World J Gastroenterol. 2004;10:2994-2996. [PubMed] |
| 18. | Shibayama K, Wachino J, Arakawa Y, Saidijam M, Rutherford NG, Henderson PJ. Metabolism of glutamine and glutathione via gamma-glutamyltranspeptidase and glutamate transport in Helicobacter pylori: possible significance in the pathophysiology of the organism. Mol Microbiol. 2007;64:396-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (4)] |
| 19. | McGovern KJ, Blanchard TG, Gutierrez JA, Czinn SJ, Krakowka S, Youngman P. gamma-Glutamyltransferase is a Helicobacter pylori virulence factor but is not essential for colonization. Infect Immun. 2001;69:4168-4173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Moss SF, Calam J, Agarwal B, Wang S, Holt PR. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 312] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Wagner S, Beil W, Westermann J, Logan RP, Bock CT, Trautwein C, Bleck JS, Manns MP. Regulation of gastric epithelial cell growth by Helicobacter pylori: offdence for a major role of apoptosis. Gastroenterology. 1997;113:1836-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 212] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Konturek PC, Pierzchalski P, Konturek SJ, Meixner H, Faller G, Kirchner T, Hahn EG. Helicobacter pylori induces apoptosis in gastric mucosa through an upregulation of Bax expression in humans. Scand J Gastroenterol. 1999;34:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Shibayama K, Kamachi K, Nagata N, Yagi T, Nada T, Doi Y, Shibata N, Yokoyama K, Yamane K, Kato H. A novel apoptosis-inducing protein from Helicobacter pylori. Mol Microbiol. 2003;47:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Kim KM, Lee SG, Park MG, Song JY, Kang HL, Lee WK, Cho MJ, Rhee KH, Youn HS, Baik SC. Gamma-glutamyltranspeptidase of Helicobacter pylori induces mitochondria-mediated apoptosis in AGS cells. Biochem Biophys Res Commun. 2007;355:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Kim KM, Lee SG, Kim JM, Kim DS, Song JY, Kang HL, Lee WK, Cho MJ, Rhee KH, Youn HS. Helicobacter pylori gamma-glutamyltranspeptidase induces cell cycle arrest at the G1-S phase transition. J Microbiol. 2010;48:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Li J, Huang CY, Zheng RL, Cui KR, Li JF. Hydrogen peroxide induces apoptosis in human hepatoma cells and alters cell redox status. Cell Biol Int. 2000;24:9-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Mao Y, Song G, Cai Q, Liu M, Luo H, Shi M, Ouyang G, Bao S. Hydrogen peroxide-induced apoptosis in human gastric carcinoma MGC803 cells. Cell Biol Int. 2006;30:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Singh M, Sharma H, Singh N. Hydrogen peroxide induces apoptosis in HeLa cells through mitochondrial pathway. Mitochondrion. 2007;7:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Flahou B, Haesebrouck F, Chiers K, Van Deun K, De Smet L, Devreese B, Vandenberghe I, Favoreel H, Smet A, Pasmans F. Gastric epithelial cell death caused by Helicobacter suis and Helicobacter pylori γ-glutamyl transpeptidase is mainly glutathione degradation-dependent. Cell Microbiol. 2011;13:1933-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Davies GR, Simmonds NJ, Stevens TR, Sheaff MT, Banatvala N, Laurenson IF, Blake DR, Rampton DS. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut. 1994;35:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 302] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 31. | Ding SZ, Minohara Y, Fan XJ, Wang J, Reyes VE, Patel J, Dirden-Kramer B, Boldogh I, Ernst PB, Crowe SE. Helicobacter pylori infection induces oxidative stress and programmed cell death in human gastric epithelial cells. Infect Immun. 2007;75:4030-4039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Shirin H, Pinto JT, Liu LU, Merzianu M, Sordillo EM, Moss SF. Helicobacter pylori decreases gastric mucosal glutathione. Cancer Lett. 2001;164:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Boonyanugomol W, Chomvarin C, Song JY, Kim KM, Kim JM, Cho MJ, Lee WK, Kang HL, Rhee KH, Sripa B. Effects of Helicobacter pylori γ-glutamyltranspeptidase on apoptosis and inflammation in human biliary cells. Dig Dis Sci. 2012;57:2615-2624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Dundon WG, Nishioka H, Polenghi A, Papinutto E, Zanotti G, Montemurro P, Del GG, Rappuoli R, Montecucco C. The neutrophil-activating protein of Helicobacter pylori. Int J Med Microbiol. 2002;291:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Robinson K, Argent RH, Atherton JC. The inflammatory and immune response to Helicobacter pylori infection. Best Pract Res Clin Gastroenterol. 2007;21:237-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | Busiello I, Acquaviva R, Di Popolo A, Blanchard TG, Ricci V, Romano M, Zarrilli R. Helicobacter pylori gamma-glutamyltranspeptidase upregulates COX-2 and EGF-related peptide expression in human gastric cells. Cell Microbiol. 2004;6:255-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986-1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2847] [Cited by in RCA: 2717] [Article Influence: 194.1] [Reference Citation Analysis (0)] |
| 38. | Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183-1188. [PubMed] |
| 39. | Okami J, Yamamoto H, Fujiwara Y, Tsujie M, Kondo M, Noura S, Oshima S, Nagano H, Dono K, Umeshita K. Overexpression of cyclooxygenase-2 in carcinoma of the pancreas. Clin Cancer Res. 1999;5:2018-2024. [PubMed] |
| 40. | Lim HY, Joo HJ, Choi JH, Yi JW, Yang MS, Cho DY, Kim HS, Nam DK, Lee KB, Kim HC. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res. 2000;6:519-525. [PubMed] |
| 41. | Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336-3340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 1039] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 42. | Tatsuguchi A, Matsui K, Shinji Y, Gudis K, Tsukui T, Kishida T, Fukuda Y, Sugisaki Y, Tokunaga A, Tajiri T. Cyclooxygenase-2 expression correlates with angiogenesis and apoptosis in gastric cancer tissue. Hum Pathol. 2004;35:488-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Ma D, Liu M, Wang AP, Yang H. Cycloxygenase-2 is essential for the survival and proliferation of gastric cancer cells. Cell Biochem Biophys. 2011;61:637-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Gionchetti P, Vaira D, Campieri M, Holton J, Menegatti M, Belluzzi A, Bertinelli E, Ferretti M, Brignola C, Miglioli M. Enhanced mucosal interleukin-6 and -8 in Helicobacter pylori-positive dyspeptic patients. Am J Gastroenterol. 1994;89:883-887. [PubMed] |
| 45. | Fan XG, Chua A, Fan XJ, Keeling PW. Increased gastric production of interleukin-8 and tumour necrosis factor in patients with Helicobacter pylori infection. J Clin Pathol. 1995;48:133-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996;110:1744-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 236] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 47. | Goishi K, Higashiyama S, Klagsbrun M, Nakano N, Umata T, Ishikawa M, Mekada E, Taniguchi N. Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factor: conversion from juxtacrine to paracrine growth factor activity. Mol Biol Cell. 1995;6:967-980. [PubMed] |
| 48. | Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1330] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 49. | Polk DB, Peek RM. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 880] [Cited by in RCA: 856] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 50. | Inui Y, Higashiyama S, Kawata S, Tamura S, Miyagawa J, Taniguchi N, Matsuzawa Y. Expression of heparin-binding epidermal growth factor in human hepatocellular carcinoma. Gastroenterology. 1994;107:1799-1804. [PubMed] |
| 51. | Yotsumoto F, Oki E, Tokunaga E, Maehara Y, Kuroki M, Miyamoto S. HB-EGF orchestrates the complex signals involved in triple-negative and trastuzumab-resistant breast cancer. Int J Cancer. 2010;127:2707-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Tanaka Y, Miyamoto S, Suzuki SO, Oki E, Yagi H, Sonoda K, Yamazaki A, Mizushima H, Maehara Y, Mekada E. Clinical significance of heparin-binding epidermal growth factor-like growth factor and a disintegrin and metalloprotease 17 expression in human ovarian cancer. Clin Cancer Res. 2005;11:4783-4792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | Murayama Y, Miyagawa J, Shinomura Y, Kanayama S, Isozaki K, Yamamori K, Mizuno H, Ishiguro S, Kiyohara T, Miyazaki Y. Significance of the association between heparin-binding epidermal growth factor-like growth factor and CD9 in human gastric cancer. Int J Cancer. 2002;98:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Dickson JH, Grabowska A, El-Zaatari M, Atherton J, Watson SA. Helicobacter pylori can induce heparin-binding epidermal growth factor expression via gastrin and its receptor. Cancer Res. 2006;66:7524-7531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Yin Y, Grabowska AM, Clarke PA, Whelband E, Robinson K, Argent RH, Tobias A, Kumari R, Atherton JC, Watson SA. Helicobacter pylori potentiates epithelial: mesenchymal transition in gastric cancer: links to soluble HB-EGF, gastrin and matrix metalloproteinase-7. Gut. 2010;59:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 56. | Shirin H, Moss SF. Helicobacter pylori induced apoptosis. Gut. 1998;43:592-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Matozaki T, Sakamoto C, Matsuda K, Suzuki T, Konda Y, Nakano O, Wada K, Uchida T, Nishisaki H, Nagao M. Missense mutations and a deletion of the p53 gene in human gastric cancer. Biochem Biophys Res Commun. 1992;182:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Bumann D, Aksu S, Wendland M, Janek K, Zimny-Arndt U, Sabarth N, Meyer TF, Jungblut PR. Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect Immun. 2002;70:3396-3403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 162] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 59. | Wroblewski LE, Shen L, Ogden S, Romero-Gallo J, Lapierre LA, Israel DA, Turner JR, Peek RM. Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology. 2009;136:236-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 60. | Schmees C, Prinz C, Treptau T, Rad R, Hengst L, Voland P, Bauer S, Brenner L, Schmid RM, Gerhard M. Inhibition of T-cell proliferation by Helicobacter pylori gamma-glutamyl transpeptidase. Gastroenterology. 2007;132:1820-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 61. | Beigier-Bompadre M, Moos V, Belogolova E, Allers K, Schneider T, Churin Y, Ignatius R, Meyer TF, Aebischer T. Modulation of the CD4+ T-cell response by Helicobacter pylori depends on known virulence factors and bacterial cholesterol and cholesterol α-glucoside content. J Infect Dis. 2011;204:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Fassi Fehri L, Koch M, Belogolova E, Khalil H, Bolz C, Kalali B, Mollenkopf HJ, Beigier-Bompadre M, Karlas A, Schneider T. Helicobacter pylori induces miR-155 in T cells in a cAMP-Foxp3-dependent manner. PLoS One. 2010;5:e9500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 63. | Xiao B, Liu Z, Li BS, Tang B, Li W, Guo G, Shi Y, Wang F, Wu Y, Tong WD. Induction of microRNA-155 during Helicobacter pylori infection and its negative regulatory role in the inflammatory response. J Infect Dis. 2009;200:916-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 64. | Kluiver J, Poppema S, de Jong D, Blokzijl T, Harms G, Jacobs S, Kroesen BJ, van den Berg A. BIC and miR-155 are highly expressed in Hodgkin, primary mediastinal and diffuse large B cell lymphomas. J Pathol. 2005;207:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 518] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 65. | Rai D, Karanti S, Jung I, Dahia PL, Aguiar RC. Coordinated expression of microRNA-155 and predicted target genes in diffuse large B-cell lymphoma. Cancer Genet Cytogenet. 2008;181:8-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 66. | Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5576] [Cited by in RCA: 5857] [Article Influence: 266.2] [Reference Citation Analysis (0)] |
| 67. | Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2532] [Cited by in RCA: 2559] [Article Influence: 121.9] [Reference Citation Analysis (0)] |
| 68. | Oertli M, Noben M, Engler DB, Semper RP, Reuter S, Maxeiner J, Gerhard M, Taube C, Müller A. Helicobacter pylori γ-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc Natl Acad Sci USA. 2013;110:3047-3052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 69. | Zhang M, Liu M, Luther J, Kao JY. Helicobacter pylori directs tolerogenic programming of dendritic cells. Gut Microbes. 2010;1:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |