Published online Nov 28, 2013. doi: 10.3748/wjg.v19.i44.8099
Revised: September 23, 2013
Accepted: October 19, 2013
Published online: November 28, 2013
Processing time: 128 Days and 19.2 Hours
AIM: To investigate H2B monoubiquitination (uH2B) and H3K4 di- and tri-methylation (H3K4-2me, H3K4-3me) levels and their clinical significance in gastric cancer (GC).
METHODS: Immunohistochemistry (IGC) was used to detect the differential levels of uH2B, H3K4-2me and H3K4-3me modifications in GC specimens from chemo/radiotherapy-naïve patients who underwent potentially curative surgical resection (n = 159) and in a random sampling of non-tumor gastric epithelium specimens (normal controls, n = 20). The immunohistochemistry (IHC)-detected modifications were classified as negative, low-level, or high-level using a dual-rated (staining intensity and percentage of positively-stained cells) semi-quantitative method. The relationships between uH2B modification levels and clinicopathological parameters of GC were assessed by a Wilcoxon rank sum test (pairwise comparisons) and the Kruskal-Wallis H test (multiple comparisons). The correlation between uH2B modification and survival was estimated by Kaplan-Meier analysis, and the role of uH2B as an independent prognostic factor for survival was assessed by multivariate Cox regression analysis.
RESULTS: The presence and level of H3K4-2me and H3K4-3me IHC staining was similar between the normal controls and GC specimens. In contrast, the level of uH2B was significantly lower in the malignant gastric tissues (vs normal control tissues) and decreased along with increases in dedifferentiation (well differentiated > moderately differentiated > poorly differentiated). The level of uH2B correlated with tumor differentiation (P < 0.001), Lauren’s diffuse- and intestinal-type classification (P < 0.001), lymph node metastasis (P = 0.049) and tumor-node-metastasis stage (P = 0.005). Patients with uH2B+ staining had higher 5-year survival rates than patients with uH2B-staining (52.692 ± 2.452 vs 23.739 ± 5.207, P < 0.001). The uH2B level was an independent prognostic factor for cancer-specific survival (95%CI: 0.237-0.677, P = 0.001).
CONCLUSION: uH2B displays differential IHC staining patterns corresponding to progressive stages of GC. uH2B may contribute to tumorigenesis and could be a potential therapeutic target.
Core tip: The abundant H2B monoubiquination (uH2B) modification detected by immunohistochemistry (IHC) in normal human gastric epithelium is decreased in malignant gastric cancer specimens, and the decreasing trend is correlated with decreased tumor differentiation, Lauren’s classification intestinal-type, presence of lymph node metastasis, and TNM stage. Positive uH2B staining is associated with higher 5-year survival. Multivariate analysis identified uH2B modification level as an independent prognostic factor for gastric cancer-specific survival. Collectively, these findings indicate the clinical significance of IHC-detected uH2B differential staining patterns as a potential prognostic biomarker in early stage gastric cancer.
- Citation: Wang ZJ, Yang JL, Wang YP, Lou JY, Chen J, Liu C, Guo LD. Decreased histone H2B monoubiquitination in malignant gastric carcinoma. World J Gastroenterol 2013; 19(44): 8099-8107
- URL: https://www.wjgnet.com/1007-9327/full/v19/i44/8099.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i44.8099
Focused public health efforts to increase awareness of gastric cancer (GC) and implementation of screening programs to detect malignancy in asymptomatic patients have led to a decline in the overall mortality of this disease worldwide. Asian countries continue to report the highest incidence rates of GC and these cases have worse prognosis. The low overall 5-year survival rate of GC cases in China (about 40%)[1,2] highlights the particular burden facing these nations’ healthcare systems and the impact on the overall social and economic well-being of their citizens.
The aggressive nature of GC remains a particular challenge to clinical management of this malignancy, and surgical resection of the affected tissues is the only effective treatment, with chemo/radiotherapy providing some benefit as adjuvant treatment. However, the efficacy of GC surgery is reliant upon the disease stage at which it is applied. Delays associated with incorrect or mis-diagnosis of the generally non-specific clinical symptoms in early stage GC (when the tumor is localized and has low risk of metastasis) can completely preclude surgery. Indeed, it has been reported that > 30% of GC patients in China are diagnosed at malignancy stages that are too far advanced for resection to be a feasible (benefit:risk) option[3].
One way to improve timely diagnosis in GC patients is to develop more accurate and sensitive methods of screening. Biomarkers, such as epigenetic modifications, are good candidates for such tests as they are detectable in serum samples and may reflect not only the presence of disease, but also its prognosis (when differential levels correspond to progressive stages of tumor pathology). In addition, diagnostic and prognostic biomarkers represent putative molecular targets of therapeutic strategies and may be exploited to develop more effective, less invasive and more individualized therapies against these aggressive tumors.
Several forms of epigenetic modifications exist, and their various alterations to the chromatin structure affect gene expression and have been implicated in pathological processes underlying a multitude of disease conditions, including tumorigenesis[4,5]. In particular, the post-translational modifications (PTMs) of histones, including acetylation, methylation, phosphorylation and ubiquitination, function as regulators of DNA-associated signaling networks required for normal physiological processes[6], such as cell growth, cycling, and movement - all important features of human cancer[6-9].
Compared to the other histone modifications, ubiquitination is less well studied and its specific roles in many types of tumors remain to be precisely defined. Focused research efforts involving monoubiquitination of lysine 120 on histone H2B (uH2B), however, have begun to elucidate its regulatory mechanism and its downstream effects under normal physiological conditions. Upon catalyzation by ubiquitin-conjugating enzyme (Rad6) and ubiquitin-protein ligase (RNF20)[10,11], uH2B acts to promote or suppress gene transcription[12,13]. Intriguingly, recruitment of RNF20 to gene promoter regions, mediated by transactivators such as Gal4 or p53, has been shown to be required for full induction of transcription of genes related to cancer, such as p21 and MDM2. Furthermore, de-regulation of uH2B has been suggested as an etiology of cancer development[14,15].
The current study was designed to investigate the potential roles of three forms of histone modification, uHB and di- and tri-methylation at H3 lysine 4 (H3K4-2me and H3K4-3me, respectively), in gastric carcinoma and in relation to its clinicopathological features. Detecting cancer type- and stage-specific differential immunohistochemistry (IHC)-staining patterns of histone modifications may represent a useful biomarker-based prognostic method and provide novel insights into potentially manipulable targets of anti-GC molecular therapies.
One-hundred-and-fifty-nine formalin-fixed, paraffin-embedded GC tissue specimens obtained from gastrectomy or upper-gastrointestinal endoscopy performed at the Department of Gastrointestinal Surgery and Digestive Endoscopy Center of West China Hospital between January 2006 to January 2007 were selected for analysis. The GC specimens included 23 well-differentiated, 55 moderately-differentiated and 81 poorly-differentiated tumors. According to the Lauren classification system, 60 were intestinal-type and 59 were diffuse-type GC. According to staging by the tumor-node-metastasis (TNM) system, 15 were at stage I, 20 were at stage II, 99 were at stage III and 25 were at stage IV.
According to the medical records, all GC specimens were obtained during potentially curative surgical resection, and none of the patients had received preoperative chemotherapy or radiotherapy. Follow-up data was available for all patients until December 2012 or until death.
In addition, 20 non-tumor gastric mucosa specimens, including sections from normal and inflammatory epithelium, were randomly selected for use as normal controls.
The GC and normal control specimens (5 μm) were deparaffinized and incubated with 0.3% hydrogen peroxide in 28% methanol for 30 min to quench the endogenous peroxidase activity. Following EDTA/high-pressure antigen retrieval, the sections were exposed to 1% bovine serum albumin for 20 min to block non-specific binding sites and then to primary antibodies against uH2B, H3K4-2me and H3K4-3me (Cat. No. 05-1312, 05-1338, and 05-1339 respectively; Millipore, Billerica, MA, United States) for 30 min. An additional 15 min post-antibody blocking step was carried out before exposure to the PowerVision+ poly-horseradish peroxidase (HRP)-anti-mouse/rabbit IgG secondary antibodies (Leica Biosystems, Newcastle, United Kingdom) for 30 min and HRP antibody (VECTASTAIN®; Vector Laboratories Inc., Berlingame, CA, United States) for 30 min. Immunoreactivity was visualized upon exposure to the DAB chromogen. The processed tissue sections were then counterstained with hematoxylin, dehydrated and mounted.
The degree of uH2B, H3K4-2me and H3K4-3me immunostaining in each specimen was assessed by two investigators (Yang JL and Wang YP) working independently, as described below. The two sets of results were compared and in the case of disagreement, the section was re-examined by both investigators simultaneously with discussion to achieve a consensus score.
For each processed specimen, three high-power (× 200) magnification fields encompassing an average of 1000 cells (range: 800-1200) were selected (BX51 microscope; Olympus, Tokyo, Japan) and image obtained (FAST 1394 camera with accompanying QCapture suite software; QImaging, Surrey, BC, Canada) to capture an overall representation of different staining densities. An immunoreactivity score (IRS) for each of the three modifications detected was calculated as the product of staining intensity (SI) multiplied by percentage of positively-stained cells (PP)[16]. SI was defined according to a four-point gradient scale, where no staining = 0, weak-coloring (light yellow) = 1, moderate-coloring (bright yellow) = 2, and strong-coloring (brown) = 3. PP was defined according to a four-point positive/negative scale, where 0-9% positive cells = 0, 10%-25% positive cells = 1, 26%-50% positive cells = 2, 51%-75% positive cells = 3, and > 75% positive cells = 4.
The triplicate IRS scores for each of the three detected modifications were averaged for each specimen and used to classify the degree of uH2B, H3K4-2me and H3K4-3me immunostaining as follows: no modification: 0; low-level modification; 1-5; high-level modification: ≥ 6.
All statistical analyses were performed by the SPSS software suite, version 13.0 (SPSS Inc., Chicago, IL, United States). The relationships between uH2B modification levels and clinicopathological parameters of GC were examined by a Wilcoxon rank sum test (for pairwise comparisons) and the Kruskal-Wallis H test (for multiple comparisons). The correlation between uH2B modification and survival was estimated by Kaplan-Meier analysis. The role of uH2B as an independent prognostic factor for survival was assessed by multivariate Cox regression analysis. The threshold for statistical significance was set as P < 0.05.
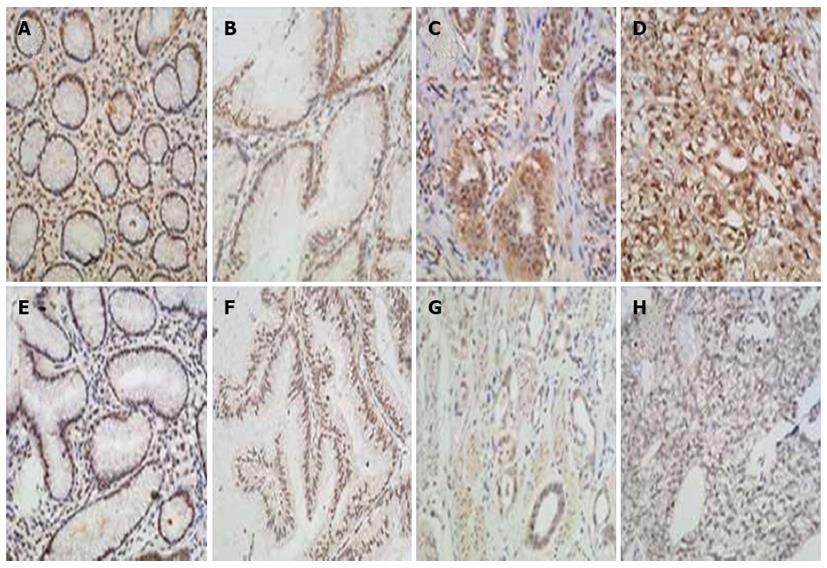
The IHC staining patterns of H3K4-2me and H3K4-3me were similar between the GC and normal control tissues, with the nuclear staining distributed evenly, regardless cancer status or tumor differentiation (Figure 1). In contrast, the uH2B staining patterns and IRS scores were remarkably different between the GC and the normal control tissues, as well as between the different classes of tumor differentiation (Figure 2). All 20 non-tumor mucosa specimens showed high-level uH2B modification (≥ 6 IRS). The amount of GC specimens with high-level uH2B modification decreased in conjunction with increasing level of tumor dedifferentiation, with IRS scores ≥ 6 seen in 65.2% (15/23) of well-differentiated GC tumors, 47.2% (26/55) of moderately-differentiated GC tumors, and 2.4% (2/81) of poorly-differentiated GC tumors. Moreover, this trend of decreased uH2B with increased degree of differentiation was statistically significant (P < 0.001, Table 1), suggesting that uH2B may play a role in maintenance of tumor differentiation.
| Parameter | n | IHC Staining Level | u/H1 | P value | ||
| Negative | Low | High | ||||
| Age (yr) | 3059.000 | 0.969 | ||||
| < 60 | 93 | 10 | 62 | 21 | ||
| ≥ 60 | 66 | 15 | 29 | 22 | ||
| Sex | 2728.000 | 0.373 | ||||
| Male | 100 | 13 | 59 | 28 | ||
| Female | 59 | 12 | 32 | 15 | ||
| Tumor differentiation | 40.376 | < 0.001 | ||||
| Well | 23 | 1 | 7 | 15 | ||
| Moderate | 55 | 7 | 22 | 26 | ||
| Poor | 81 | 17 | 62 | 2 | ||
| Lauren classification | 933.000 | < 0.001 | ||||
| Intestinal | 60 | 6 | 21 | 33 | ||
| Diffuse | 59 | 17 | 35 | 7 | ||
| Lymph node metastasis | 2330.000 | 0.049 | ||||
| Absent | 53 | 2 | 35 | 16 | ||
| Present | 106 | 23 | 56 | 27 | ||
| TNM stage | 12.896 | 0.005 | ||||
| I | 15 | 1 | 6 | 8 | ||
| II | 20 | 2 | 15 | 3 | ||
| III | 99 | 14 | 55 | 30 | ||
| IV | 25 | 8 | 15 | 2 | ||
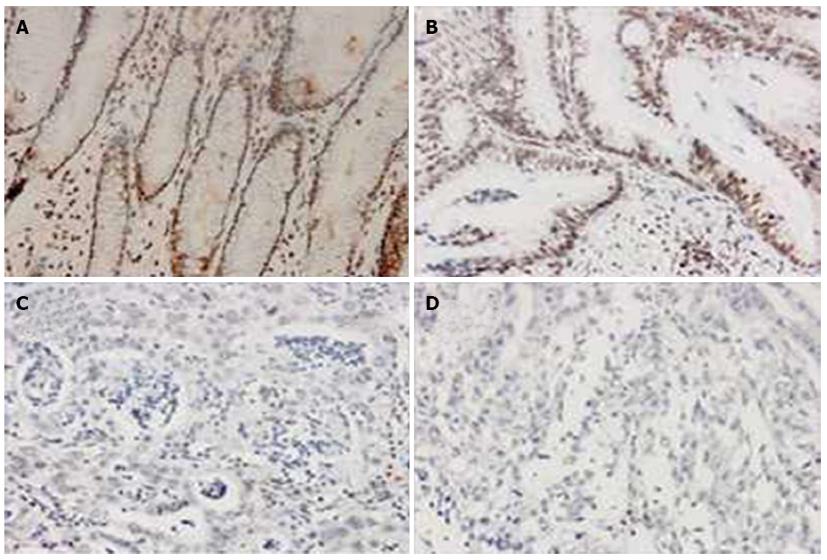
When the GC specimens were divided by the Lauren classification, significantly more of the intestinal-type samples showed positive uH2B staining than the diffuse-type samples [90.0% (54/60) vs 71.2% (42/59), P < 0.05; Figure 3]. In addition, significantly more of the intestinal-type tumors showed high-level modification [55.0% (33/60) vs low-level modification: 35.0% (21/60), P < 0.05; Figure 3], and this pattern was significantly different from that seen in the diffuse-type tumors [vs high-level modification in diffuse-type: 11.9% (7/59), P < 0.001; Table 1].
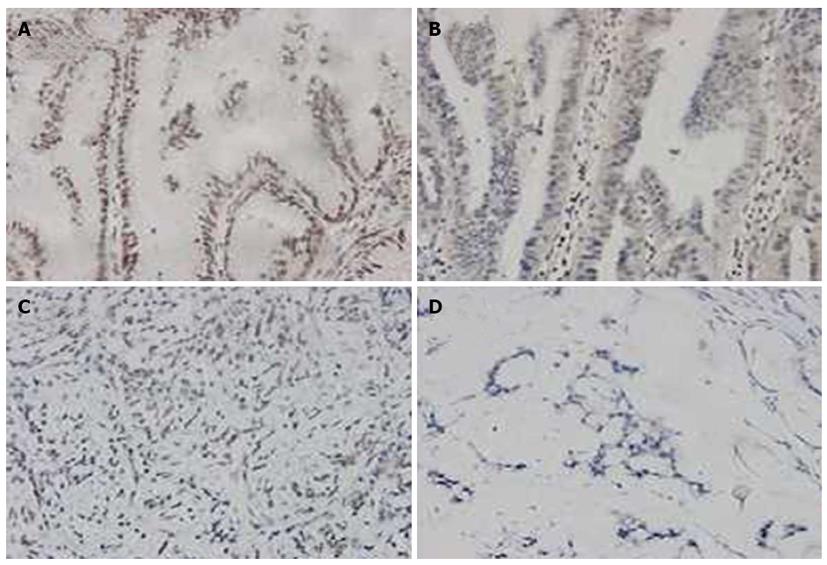
When the GC specimens were divided by TNM stages, a statistically significant trend in differential uH2B modification level was observed. As shown in Figure 4, the frequency of high-level uH2B modification was 53.3% (8/15) in stage I tumors, 15.0% (3/20) in stage II tumors, 30.3% (30/99) in stage III tumors, and 8.0% (2/25) in stage IV tumors. The difference in frequency of high-level uH2B modification detected in stage I and stage IV tumors reached statistical significance (P = 0.005; Table 1).
In addition, GC cases with lymph node metastasis showed a significantly lower frequency of high-level uH2B modification [25.4% (27/106) vs no lymph node metastasis: 30.2% (16/53), P = 0.049; Table 1].
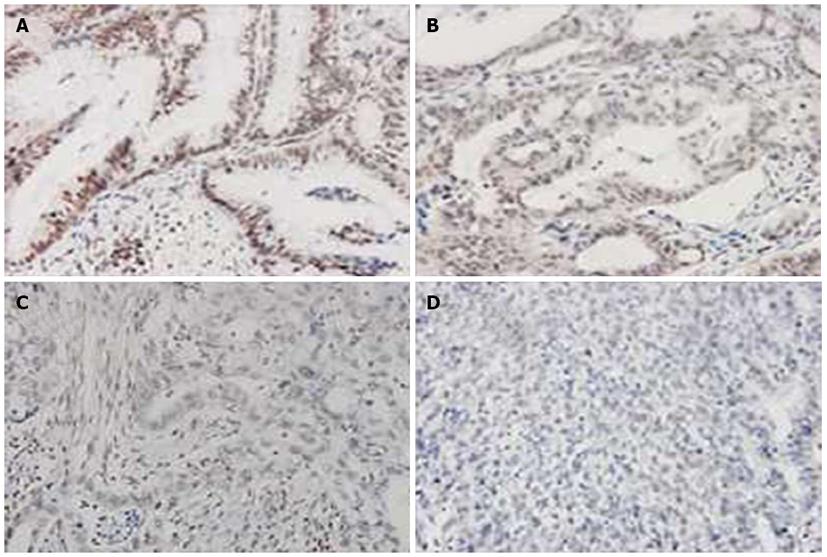
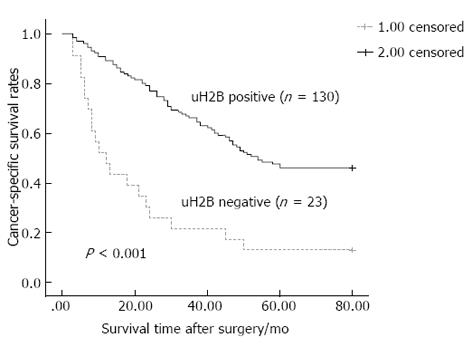
Of the 159 GC patients treated with surgical resection, 96 (60.4%) died from GC-related causes during the follow-up period and six died from non-GC causes. When the overall patient population was divided by presence of uH2B staining, GC-related deaths were found to have occurred in a significantly higher proportion of patients with negative uH2B staining than those with positive uH2B staining [88.0% (22/25) vs 55.2% (74/134), P < 0.05]. The cumulative 5-year cancer-specific survival rate was 43.4%. Moreover, the 5-year survival rate of patients with positive uH2B staining was significantly higher than that of patients with negative uH2B staining (52.69 ± 2.45 vs 23.74 ± 5.21, P < 0.001; Figure 5).
According to Cox multivariate regression, uH2B modification level is an independent prognostic factor for cancer-specific survival of GC patients. The risk of death in patients with negative uH2B staining was 2.5-times (1:0.4) that of patients with positive uH2B modification (RR = 0.40, 95%CI: 0.237-0.677, P = 0.001; Table 2).
| Variable | Comparison | RR | 95% CI | P value |
| Age (yr) | < 60 vs≥ 60 | 0.761 | 0.493-1.174 | 0.217 |
| Sex | male vs female | 0.961 | 0.618-1.494 | 0.858 |
| Tumor differentiation | Well vs moderate, poor | 0.497 | 0.301-0.819 | 0.006 |
| Lymph node metastasis | Present vs absent | 3.274 | 1.728-6.201 | < 0.001 |
| TNM stage | I vs II, III, IV | 1.695 | 1.112-2.583 | 0.014 |
| uH2B modification | IHC stain positive vs negative | 0.400 | 0.237-0.677 | 0.001 |
In this study, immunohistochemical detection of human GC samples was performed as a semi-quantitative approach to measure the H2B monoubiquitination at lysine 121 and investigate its potential clinical significance with regards to diagnosis (GC vs control tissues) and prognosis (progressive stages of GC tumorigenesis). To the best of our knowledge, this study provides the first evidence of correlation between uH2B modification level and clinicopathological and prognostic features of human GC, including tumor differentiation, Lauren’s classification, lymph node metastasis, and TNM stage but not with sex or age (data not shown).
The modification of H2B at lysine 121, shown by the percentage of positivity and intensity of immunohistochemical detection, was significantly less robust in tumors of lower differentiation level. The degree of differentiation is considered to be strongly associated with the malignancy of cancer; therefore, this result indicates that reduced uH2B may be correlated with a worse prognosis. The more frequent and intense staining of uH2B observed in intestinal-type tumors in the current study, compared to the diffuse-type tumors, suggests that loss of uH2B may contribute to GC tumor progression. From a histological perspective, the composition of intestinal-type GC tumors includes a remarkable amount of ductal structures, displaying a better differentiation than the diffuse-type GC tumors that may be related to the better prognosis of the former tumor type[17,18]. Finally, the current observation of lower uH2B modification level in higher grade TNM stages, which tend to be more aggressive and invasive towards the inner tissues and more metastatic, suggest that this modification may be a useful predictive biomarker of the invasive potential of a GC specimen.
The collected results of the current study indicate that the progressive stages of GC are accompanied by differential uH2B modification levels that are detectable by IHC. Prenzel et al[19] reported a similar finding for human specimens of breast cancer. Specifically, the abundant uH2B signals detected by IHC in normal mammary epithelium and benign breast tumors were absent in most malignant and metastatic breast tumors. Urasaki et al[20] also demonstrated drastically reduced uH2B modification levels in breast, colon and lung cancer cells, as compared to the abundant expression in matched normal control tissues. Thus, loss of uH2B may lead to progression and metastasis of tumors, in general.
The mechanisms underlying tumor-related decreases in uH2B remain unknown. Besides the known Rad6 and RNF20 regulatory enzymes[10,11], other de/ubiquitinating enzymes are likely to be involved in the dynamic process of uH2B promotion of tumorigenesis. For example, the ubiquitin-specific protease 22 (USP22), a member of the recently identified polycomb/cancer stem cell signature[21], has been shown to deubiquitinate H2Aub1 or H2Bub1 in vitro, suggesting functions in epigenetic regulation, cancer progression and transcription activation[22,23]. If USP22 plays a role in the cancer-related differential uH2B modifications, then corresponding changes in USP22 expression may be detected.
In fact, studies of USP22 expression level in cancer have demonstrated significant upregulation in malignant tumors (compared to the normal low or moderate levels in non-cancerous skeletal, heart, muscle, liver and lung tissues)[24] and significant correlations to tumor relapse, invasion depth, pathological stage and lymph node metastasis[25]. Moreover, a study of primary GC showed that upregulated USP22 protein expression was related to lymph node metastasis[26]. Future investigations may elucidate a role for USP22 in the de-regulation of uH2B, particularly in GC.
Another potential regulator of tumor-related decreases in uH2B is the ubiquitin-specific peptidase 49 (USP49) complex, which specifically deubiquitinates histone H2B in vivo to enhance the stability of a nucleosome spanning the affected exons[27]. Again, further research is required to determine whether USP49 acts as a histone H2B-specific deubiquitinase to promote tumorigenesis.
While the current study provided clear evidence of decreased uH2B in malignant and poorly-differentiated human GC specimens, the physiological significance of the loss of this histone code remains unclear. The critical roles of H3K4-2me and H3K4-3me modifications in gene expression and cellular viability are well established; however, the requirement for uH2B in these processes remains controversial. Genome-wide studies have shown that uH2B is associated mostly with actively transcribed genes in mammals, suggesting that H2B is universally required for gene transcription. However, loss of the H2B-specific RNF20/40 enzyme produced only moderate effects on a fraction of the transcriptome and no overall effects on cell viability[28]. This apparent differential function of H3K4 modifications and the uH2B modification is in line with the observations of the current study of GC tissues, whereby the uH2B level showed a unique gradual decrease from the benign to malignant stages.
In conclusion, the compelling evidence of uH2B not being required for viability of malignant cells in gastric carcinoma provided by the current study indicates that the decrease of uH2B might be an early event in GC and could be a counteracting factor against carcinogenesis of GC.
Gastric cancer (GC) remains a significant healthcare burden worldwide, with high mortality rates in countries with the highest incidences, such as China. Surgery is currently the only effective treatment, but must be applied in the early stages when the tumor is less aggressive. Unfortunately, the asymptomatic nature of early stage GC leads to missed diagnosis and precludes early surgical management. There is an urgent need to identify sensitive biomarkers that accurately diagnose GC at the early stage, as well as indicate prognosis for a particular patient. Such factors may also represent novel targets of molecular therapies, helping to overcome the limitations and risks associated with surgical resection.
The physiological and pathological roles of monoubiquitination on lysine 120 of histone H2B (uH2B) remain to be fully elucidated. As a general transcriptional regulator, de-regulation of uH2B may contribute to tumorigenesis and cancer progression by promoting expression of cancer-associated genes or suppressing expression of anti-tumor genes. Investigating the differential level of uH2B in specific cancer types, such as GC, will provide insights into its clinicopathological and prognostic significance.
In this study, immunohistochemical (IHC) analysis of human GC specimens was used to detect the tumor-related levels of uH2B modification. Application of a dual-rated semi-quantitative method to score the IHC results allowed for statistical correlation analysis of uH2B modification level and tumor-related features. The GC-related decrease in uH2B modification level was positively correlated with extent of tumor dedifferentiation, intestinal-type tumors, occurrence of lymph node metastasis, worse TNM stage and lower 5-year survival rate. IHC-detected differential uH2B modification may be developed as clinically useful prognostic biomarker in early GC.
The uH2B differential staining pattern detected by IHC that accompanies progressive stages of GC not only indicated a potentially important role for this histone modification in carcinogenesis, but also suggested its potential as a target of molecular therapy.
uH2B, the monoubiquitination on lysine 120 of histone H2B, is a general transcriptional regulator, and its deregulation has been implicated in various cancers. IHC is a well-established laboratory detection method that exploits the antibody-antigen binding reaction to identify and visualize the location and level of a target protein in tissues or individual cells.
This paper is an interesting article regarding the role of histone modification events in the development of GC. The results of the paper show that decrease of uH2B might be an early event in GC, which could be a counteracting factor against carcinogenesis of gastric cancer. Furthermore, histone modification plays important roles in understanding the pathogenesis of gastric carcinoma, and could be a potential therapeutic target in the future
P- Reviewers: Alshehabi Z, Kim H S- Editor: Zhai HH L- Editor: Stewart GJ E- Editor: Liu XM
| 1. | Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12:17-20. [PubMed] |
| 2. | Wang YY, Ye ZY, Zhao ZS, Li L, Wang YX, Tao HQ, Wang HJ, He XJ. Clinicopathologic significance of miR-10b expression in gastric carcinoma. Hum Pathol. 2013;44:1278-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Wang CS, Chao TC, Jan YY, Jeng LB, Hwang TL, Chen MF. Benefits of palliative surgery for far-advanced gastric cancer. Chang Gung Med J. 2002;25:792-802. [PubMed] |
| 4. | Calcagno DQ, Gigek CO, Chen ES, Burbano RR, Smith Mde A. DNA and histone methylation in gastric carcinogenesis. World J Gastroenterol. 2013;19:1182-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 5. | Fukuda H, Sano N, Muto S, Horikoshi M. Simple histone acetylation plays a complex role in the regulation of gene expression. Brief Funct Genomic Proteomic. 2006;5:190-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Misri S, Pandita S, Kumar R, Pandita TK. Telomeres, histone code, and DNA damage response. Cytogenet Genome Res. 2008;122:297-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Chi P, Allis CD, Wang GG. Covalent histone modifications--miswritten, misinterpreted and mis-erased in human cancers. Nat Rev Cancer. 2010;10:457-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 925] [Cited by in RCA: 845] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 8. | Sawan C, Vaissière T, Murr R, Herceg Z. Epigenetic drivers and genetic passengers on the road to cancer. Mutat Res. 2008;642:1-13. [PubMed] |
| 9. | Suganuma T, Workman JL. Signals and combinatorial functions of histone modifications. Annu Rev Biochem. 2011;80:473-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 368] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 10. | Koken MH, Reynolds P, Jaspers-Dekker I, Prakash L, Prakash S, Bootsma D, Hoeijmakers JH. Structural and functional conservation of two human homologs of the yeast DNA repair gene RAD6. Proc Natl Acad Sci USA. 1991;88:8865-8869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 180] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Kim J, Hake SB, Roeder RG. The human homolog of yeast BRE1 functions as a transcriptional coactivator through direct activator interactions. Mol Cell. 2005;20:759-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 238] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 12. | Henry KW, Wyce A, Lo WS, Duggan LJ, Emre NC, Kao CF, Pillus L, Shilatifard A, Osley MA, Berger SL. Transcriptional activation via sequential histone H2B ubiquitylation and deubiquitylation, mediated by SAGA-associated Ubp8. Genes Dev. 2003;17:2648-2663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 558] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 13. | Hwang WW, Venkatasubrahmanyam S, Ianculescu AG, Tong A, Boone C, Madhani HD. A conserved RING finger protein required for histone H2B monoubiquitination and cell size control. Mol Cell. 2003;11:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 353] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 14. | Espinosa JM. Histone H2B ubiquitination: the cancer connection. Genes Dev. 2008;22:2743-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Johnsen SA. The enigmatic role of H2Bub1 in cancer. FEBS Lett. 2012;586:1592-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Li JC, Yang XR, Sun HX, Xu Y, Zhou J, Qiu SJ, Ke AW, Cui YH, Wang ZJ, Wang WM. Up-regulation of Krüppel-like factor 8 promotes tumor invasion and indicates poor prognosis for hepatocellular carcinoma. Gastroenterology. 2010;139:2146-2157.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 18. | Qiu MZ, Cai MY, Zhang DS, Wang ZQ, Wang DS, Li YH, Xu RH. Clinicopathological characteristics and prognostic analysis of Lauren classification in gastric adenocarcinoma in China. J Transl Med. 2013;11:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | Prenzel T, Begus-Nahrmann Y, Kramer F, Hennion M, Hsu C, Gorsler T, Hintermair C, Eick D, Kremmer E, Simons M. Estrogen-dependent gene transcription in human breast cancer cells relies upon proteasome-dependent monoubiquitination of histone H2B. Cancer Res. 2011;71:5739-5753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 760] [Reference Citation Analysis (0)] |
| 20. | Urasaki Y, Heath L, Xu CW. Coupling of glucose deprivation with impaired histone H2B monoubiquitination in tumors. PLoS One. 2012;7:e36775. [PubMed] |
| 21. | Zhang XY, Varthi M, Sykes SM, Phillips C, Warzecha C, Zhu W, Wyce A, Thorne AW, Berger SL, McMahon SB. The putative cancer stem cell marker USP22 is a subunit of the human SAGA complex required for activated transcription and cell-cycle progression. Mol Cell. 2008;29:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Zhao Y, Lang G, Ito S, Bonnet J, Metzger E, Sawatsubashi S, Suzuki E, Le Guezennec X, Stunnenberg HG, Krasnov A. A TFTC/STAGA module mediates histone H2A and H2B deubiquitination, coactivates nuclear receptors, and counteracts heterochromatin silencing. Mol Cell. 2008;29:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 297] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 23. | Pijnappel WW, Timmers HT. Dubbing SAGA unveils new epigenetic crosstalk. Mol Cell. 2008;29:152-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Lee HJ, Kim MS, Shin JM, Park TJ, Chung HM, Baek KH. The expression patterns of deubiquitinating enzymes, USP22 and Usp22. Gene Expr Patterns. 2006;6:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Li J, Wang Z, Li Y. USP22 nuclear expression is significantly associated with progression and unfavorable clinical outcome in human esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2012;138:1291-1297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Yang DD, Cui BB, Sun LY, Zheng HQ, Huang Q, Tong JX, Zhang QF. The co-expression of USP22 and BMI-1 may promote cancer progression and predict therapy failure in gastric carcinoma. Cell Biochem Biophys. 2011;61:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Zhang Z, Jones A, Joo HY, Zhou D, Cao Y, Chen S, Erdjument-Bromage H, Renfrow M, He H, Tempst P. USP49 deubiquitinates histone H2B and regulates cotranscriptional pre-mRNA splicing. Genes Dev. 2013;27:1581-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Shema E, Tirosh I, Aylon Y, Huang J, Ye C, Moskovits N, Raver-Shapira N, Minsky N, Pirngruber J, Tarcic G. The histone H2B-specific ubiquitin ligase RNF20/hBRE1 acts as a putative tumor suppressor through selective regulation of gene expression. Genes Dev. 2008;22:2664-2676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |