INTRODUCTION
Portal hypertension is the most severe complication that develops in cirrhotic patients and is a leading cause of mortality worldwide[1]. Ascites, hepatorenal syndrome, life-threatening gastroesophageal bleeding, portosystemic encephalopathy and sepsis, derived from shunting of portal blood into the systemic circulation through neoformed collateral vessels, are the most serious and frequent clinical complications[1].
Portal hypertension is defined as an increase in portal blood pressure and is determined from the hepatic venous pressure gradient or pressure difference between the portal vein and the inferior vena cava[2]. The impairments arising from this pathological increase in portal pressure constitute the portal hypertensive syndrome[3].
EXPERIMENTAL PORTAL HYPERTENSIVE MODEL
The partial portal vein ligation experimental model in the rat is generally used to study portal hypertension since it has the lowest degree of hepatic impairment because portal hypertension is extrahepatic[4]. The surgical technique is simple and is based on making a calibrated stenosis of the portal vein[5]. If it is assumed that the intensity of the portal hypertension is determined by the resistance to the inflow produced by the constriction of the portal vein, this model of prehepatic portal hypertension could be improved by increasing the initial resistance to blood flow. With this objective in mind, we have modified this surgical technique by increasing the length of the stenosed portal tract with three equidistant calibrated stenosis[6].
This experimental model causes minimal alternations in the liver, thus making a more selective study possible for the pathological changes characteristic of prehepatic portal hypertension[4,6]. The experimental model of partial portal vein ligation is generally studied in the short-term, i.e., 2-4 wk[4]. However, studying the late phases could be of great interest since the mechanisms involved and the related complications could be more similar to those found in chronic liver diseases in humans[1,2] which are related to the chronicity of portal hypertension, among other factors.
INFLAMMATORY RESPONSE RELATED TO PORTAL HYPERTENSION
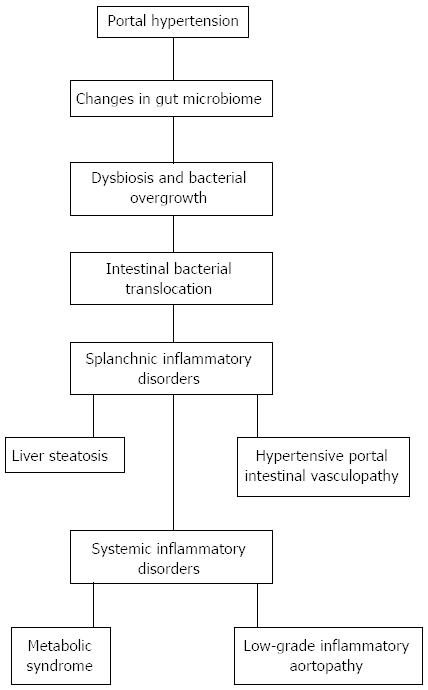
Much evidence shows how inflammation contributes to the initiation and maintenance of portal hypertension[7,8]. Furthermore, early and chronic partial portal vein ligated-rats show hemodynamic and metabolic impairments, where the etiopathogeny is of an inflammatory nature[7,8]. Consequently, it could be hypothesized that chronic hemodynamic, vascular and metabolic changes in rats with prehepatic portal hypertension could have an inflammatory origin, most probably subsequent to splanchnic inflammation. In this way, the endothelial inflammatory mechanotransduction induced by portal hypertension could be the first step in the production of an inflammatory response in the intestinal wall. Thus, the early splanchnic endothelial disorder could induce, in turn, an inflammatory intestinal phenotype that would be linked to both phenomena: the gut microbiota alteration as well as the vasomotor impairments that are responsible for the splanchnic hyperdynamic circulation (Figure 1).
Figure 1 Splanchnic and systemic alterations in rats with prehepatic portal hypertension produced by triple partial portal vein stenosis.
Furthermore, prehepatic portal hypertension induces the development of portal hypertensive enteropathy with inflammatory cell infiltration, particularly mast cells[9,10], which is reduced by the prophylactic administration of anti-inflammatory drugs, like budesonide and ketotifen[8,11]. Since the basic structural alteration found in portal hypertensive enteropathy is angiogenesis, the very appropriate name of “hypertensive portal intestinal vasculopathy” has been proposed[12].
The formation of new blood vessels could be a key mechanism in the pathogenesis of prehepatic portal hypertension[8]. Mast cells are involved in the regulation of physiological and pathological vasculogenesis by producing mediators, such as heparin, histamine, tryptase, transforming growth factor-β1, tumor necrosis factor-α (TNF-α), interleukins and cytokines, such as vascular endothelial growth factor[13]. The ability of mast cells to promote the synthesis and selective release of different angiogenic mediator molecules[14] would explain their participation in the splanchnic remodeling related to experimental prehepatic portal hypertension[8-10]. Lastly, intestinal mast cells are also a potent source of multiple chemokines and play an important role in immune regulation[15,16].
We have previously shown that prehepatic portal hypertension in the rat induces liver steatosis and causes changes in lipid and carbohydrate metabolism similar to those produced in chronic inflammatory conditions described in metabolic syndrome in humans[17-19]. Long-term portal hypertensive rats show a decrease in plasma adrenocorticotrophic hormone and corticosterone[20]. Glucocorticoids, such as cortisol and corticosterone, are pluripotent hormones that are vital in the host adaptation to stress. They are also essential for maintaining normal vascular tone, endothelial integrity and vascular permeability. Thus, the decrease in corticosterone in portal hypertensive rats could have deleterious effects on vascular systems. In addition, cortisol clearance is increased in individuals with fatty liver, and cortisol clearance is in turn inversely correlated with insulin sensitivity[21]. Therefore, in rats with portal hypertension, in which liver steatosis is present, a decreased stress responsiveness could be related to an impaired metabolic feedback system. The decreased neuroendocrine response to stress and systemic chronic inflammation would be another link between disordered lipid metabolism and inflammation in the evolution of this experimental model[22]. So, rats with long-term portal hypertension presenting systemic low-grade inflammation and decreased responsiveness to stress could inappropriately switch carbohydrate metabolism to predominant lipid metabolism, thus inducing body energy imbalance and ultimately, hepatic steatosis and metabolic syndrome[18,19]. Consequently, data from animal models proved that mast cells are directly involved in diet-induced obesity, diabetes and metabolic syndrome[23].
The association of liver steatosis and metabolic syndrome with inflammation is well documented[24]. Hence, we could hypothesize that inflammation, probably of splanchnic origin, could be the pathophysiologic link between the metabolic syndrome with liver steatosis and aortic vascular disease in portal hypertensive rats. The association between metabolic and atherogenic alterations with a proinflammatory aortic phenotype could suggests the possibility that portal hypertension might constitute a novel risk factor for cardiovascular disease[20,25] (Figure 1).
PORTAL HYPERTENSIVE INTESTINAL MICROBIOME
Bacterial intestinal translocation occurs in acute portal hypertension[26] and in chronic portal hypertension in the adult partial portal vein ligated rat[27] when there are associated precipitating factors, such as hemorrhagic shock[28]. However, in chronic (1 mo) prehepatic portal hypertension by triple partial portal vein ligation, there are gut microflora alterations with less positive cultures of Enterococci and lactic acid bacteria, associated with bacterial translocation to the mesenteric lymph nodes[29]. Bacterial overgrowth in the intestinal tract may be the most important factor in bacterial translocation, in particular when associated with splanchnic inflammation and increased intestinal permeability, secondary to portal hypertension[29]. It has also been proposed that bacterial translocation may render the gut a “cytokine-releasing” organ that, at the same time, would induce nitric oxide overproduction and the development of the hyperdynamic circulatory state. At the same time, this is one of the progressive characteristics of portal hypertension[30]. Bacterial overgrowth could also be caused by delayed transit, mucosal hypoperfusion and oxidative damage, which increases intestinal permeability and induces the transmural passage of bacteria in portal hypertensive rats[31,32].
Although microbial communities reside on all mammal body surfaces, including the skin and respiratory, gastrointestinal and genitourinary tracts, the largest collection of microbes reside in the gut[33]. The intestinal microbiome is considered more than just a simple organ of the mammal body. Cooperative interactions between intestinal microbes and their hosts typically involve microbial participation in host functions such as defense, metabolism, and reproduction[34]. However, communications between the host and its gut microbiota are altered in pathophysiological processes, especially if associated with inflammation, including portal hypertension[35]. Diseases mediated by the inflammatory response could induce a change in the relationship of the rat body with gut microbiota, the significance of which is unknown[35].
Inflammatory conditions, such as splanchnic inflammation related to portal hypertension, could induce a change in the mammalian organism and gut microbiota relationship. In particular, splanchnic inflammation not only could alter gut microbiota composition, but also cause epithelial and endothelial permeability of the intestinal bacteria to increase bacterial products, such as toxins[34]. Bacterial translocation secondary to portal hypertension could lead to bacterial overgrowth and disruption of gut homeostasis[29]. This results in a “leaky gut” syndrome, with translocation of gut bacteria and bacterial products, also called pathogen-associated molecular patterns or PAMPs, to systemic sites, that finally results in systemic complications[34-37].
Gut microbiota in rats with portal hypertension could contribute to the development of liver steatosis and metabolic syndrome. The gut microbiota may be involved in hepatologic conditions including non-alcoholic fatty liver disease[33]. Thus, bacterial products, including endotoxins, can affect Kupffer cells, hepatocytes and hepatic stellate cells, which participate in the initiation and progression of non-alcoholic fatty liver disease[38]. Gut microbiota could also contribute to the development of hypertensive portal intestinal vasculopathy. Recent results suggest that the increased intestinal vascularization is mediated through microbiota-induced angiopoietin-1 expression in the intestinal epithelium[39].
Gut microbiome responses to portal hypertension could be a central event in the pathogenesis of splanchnic, i.e., liver steatosis and enteropathy, and systemic inflammatory conditions. However, it has been also suggested that microbiome changes could alter host-microbiome interactions to mitigate disease[33] (Figure 1).
WOUND-LIKE INFLAMMATORY AORTIC RESPONSE
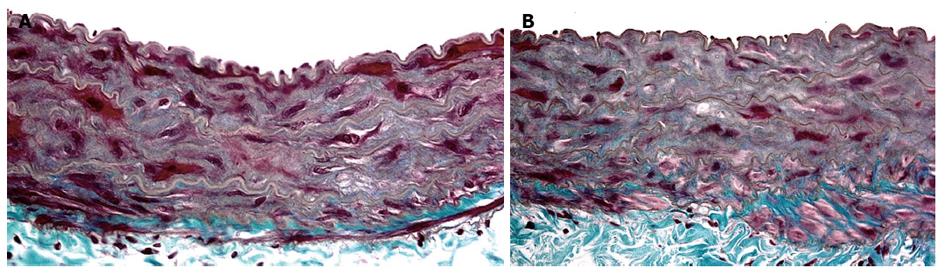
Long-term experimental prehepatic portal hypertension represents a risk factor of an inflammatory nature for aortic disease development[20,25]. Triple partial portal vein ligation in the rat induces an abdominal aortic inflammatory response 22 mo after the operation. These portal hypertensive rats show significant histological changes, in particular in the middle layer of the aortic wall. The elastic fibers lose their orderly circumferential arrangement. The interstitial connective tissue is enlarged with fibrosis and associated with a dramatic decrease in the number of smooth muscle cells. Finally, immature collagen also increases with the degeneration of connective tissue[20] (Figure 2).
Figure 2 Aortic abdominal wall microscopic images in long-term (22 mo) sham-operated rat (A) and in a triple partial portal vein ligated-rat (B).
The aortic wall in the rat with portal hypertension is enlarged, has more fibrosis, with collagen deposition in the middle layer, a greater loss of the smooth muscular cell nucleus and much thinner elastic fibers than in the aortic wall of the sham-operated rat. They are also are distributed in an irregular manner (Masson, × 40).
In this experimental model of long-term triple portal vein ligated rats, the abdominal aortic proinflammatory response could be attributed to oxidative stress. In this way, the increased aortic reduced-nicotinamide-adenine dinucleotide phosphate [NAD(P)H] oxidase activity[20] could be associated with reactive oxygen species production and promote aortic inflammation[40]. Also oxidative stress mediated by NAD(P)H oxidase has been associated with risks factors for inflammation and atherosclerosis[41].
In chronic portal hypertensive rats, over-activation of endothelial nitric oxide synthase (eNOS) might cause aortic nitric oxide overproduction[20,25]. Upregulation of eNOS has also been seen in the aorta of short-term portal vein stenosed and biliary cirrhotic rats, respectively[42]. It has been suggested that an increased basal release of nitric oxide has a major role in the pathogenesis of vasodilation and vascular hypocontractility associated with portal hypertension[7]. Lipopolysaccharide (LPS) administration to cirrhotic rats increases aortic eNOS activation but, on the contrary, decreases eNOS protein expression and activity in superior mesenteric arteries. These results may explain the worsening of the hyperdynamic state in cirrhosis during septic shock by direct LPS-induced eNOS activation in large systemic vessels, and its inhibition in concomitant small splanchnic vasculature[43].
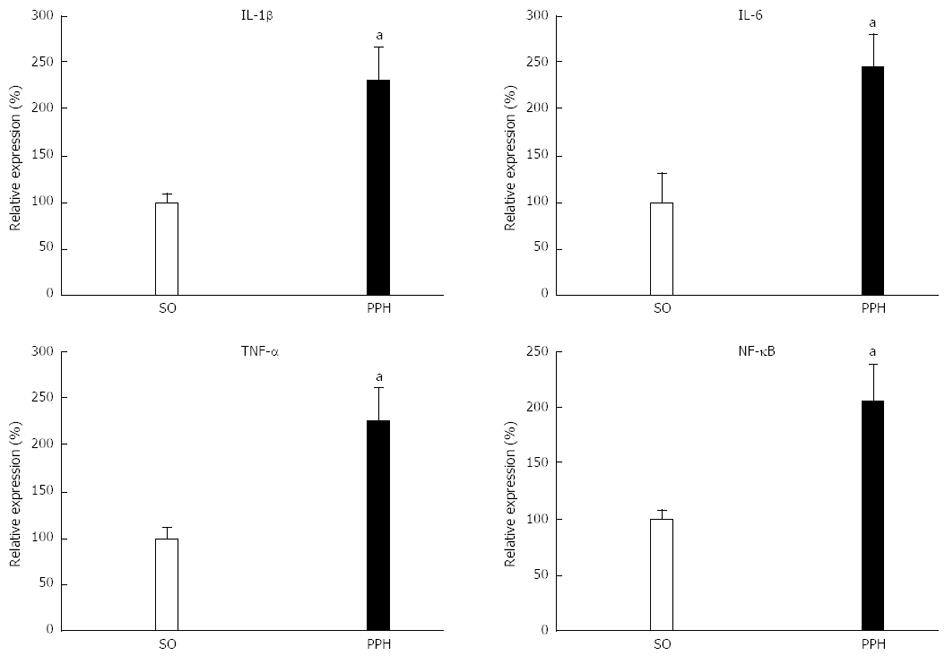
The essential role of reactive oxygen species in the chronic inflammatory response has led to the view that reactive oxygen species promote inflammation[44]. Reactive oxygen species can increase the expression of inducible genes leading to the synthesis of cytokines, chemokines, chemokine receptors and adhesion molecules. These actions rely on transcription factors, such as nuclear factor κB (NF-κB)[44]. Aortic overproduction of proinflammatory cytokines, including TNF-α, interleukin-1β (IL-1β), and IL-6 in chronic portal hypertensive rats, associated with an increased NF-κB/NF-κB inhibitor (IκB) ratio supports the existence of a proinflammatory abdominal aortic response. If so, reactive oxygen species or TNF-α could induce activation of the Iκκ (IκB kinase) complex resulting in phosphorylation of IκB, subsequent translocation of NF-κB to the nucleus and expression of NF-κB responsive genes (Figure 3).
Figure 3 Aortic inflammatory mediators in triple partial vein ligated-rats at 22 mo after postoperative evolution.
Increased aortic mRNA expression of tumoral necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6, are associated with the increased expression of mRNA levels of the nuclear factor κB (NF-κB)/NF-κB inhibitor (IκB) ratio. SO: Sham-operated rats; PPH: Prehepatic portal hypertensive rats. aP < 0.05, statistically significant value in regards to SO-rats.
Proinflammatory cytokines, cytokine-dependent pathways and immune cells have been implicated in the development of cardiovascular diseases, i.e., atherosclerosis, coronary artery disease, chronic heart failure and hypertension[44]. Thus, a large body of evidence supports the involvement of common proinflammatory cytokines in the development and progression of a systemic low-grade inflammation affecting the cardiovascular system. In addition, these low-grade inflammatory cardiovascular diseases can be aggravated when a new inflammatory process, either of infectious origin or autoimmune nature, is added[45,46]. This is the reason why it could also be considered that prehepatic portal hypertension produces a low degree inflammatory cardiovascular response, which could be aggravated when a new inflammatory process (acute-over-chronic) is added, particularly infections or hepatic insufficiency[8].
Mast cells stand out among the potential mediators of the low-grade inflammatory response supposedly involved in metabolic and vascular diseases in the experimental model of prehepatic portal hypertension[3,9]. Nonetheless, these results obtained in the short-term evolution of portal hypertensive rats cannot be extrapolated to long-term portal hypertensive rats. Thus, a study of the role of the splanchnic subpopulations of mast cells in chronic portal hypertensive rats would be interesting, given that the gut, particularly with impaired intestinal barrier function, plays an important pathophysiological role in chronic inflammation in cardiovascular diseases[47]. In particular, mast cells play a key role in experimental atherosclerosis and can modulate the inflammatory aortic response through numerous proinflammatory mediators, including TNF-α, IL-6 and metalloproteinases[48,49]. In addition, mast cell chymase also functions as an angiotensin-converting enzyme, particularly in rodents and therefore contributes to aortic fibrosis[50] (Figure 4).
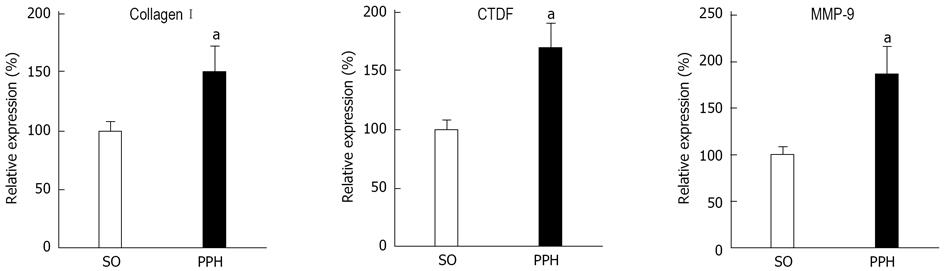
Figure 4 Aortic profibrogenic mediators in triple partial vein ligated-rats at 22 mo of postoperative evolution.
Increased abdominal aortic expression of matrix metalloproteinase (MMP)-9, collagen I and connective tissue growth factor (CTGF). SO: Sham-operated rats. PHH: Prehepatic portal hypertensive-rats. aP < 0.05, statistically significant value in regards to SO-rats.
Lastly, enhanced aortic mRNA expression of oxidative and inflammatory mediators are associated with increased aortic expression of collagen I and connective tissue growth factor (CTGF) in long-term portal hypertensive rats[20,25]. Increased aortic CTGF expression could regulate aorta collagen remodeling, and therefore enhance the synthesis of extracellular matrix proteins, particularly type I collagen. In turn, CTGF could activate the NF-κB pathway and increase proinflammatory gene expression[51]. These results suggest the existence of a proinflammatory and a profibrotic aortic phenotype in long-term prehepatic portal hypertensive rats (Figures 4 and 5).
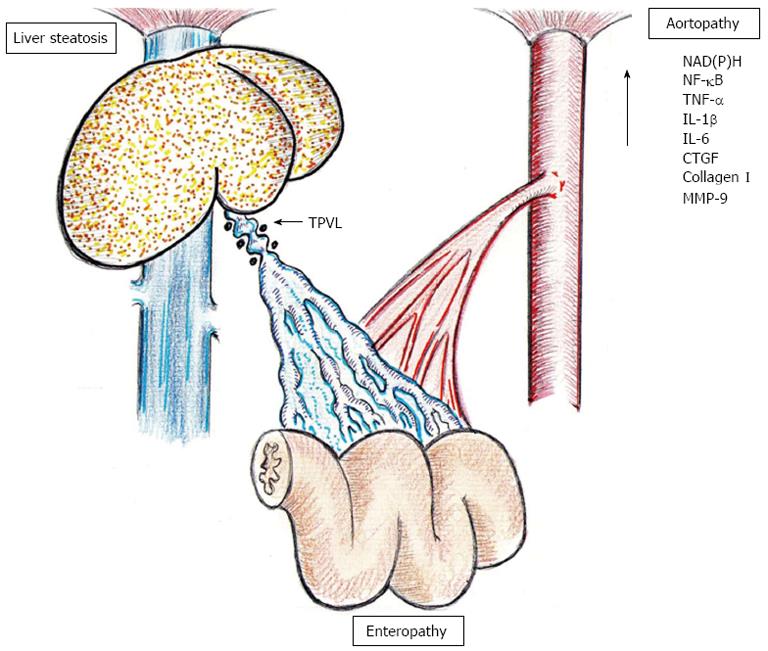
Figure 5 Chronic splanchnic alterations, liver steatosis and enteropathy, secondary to long-term triple partial portal vein stenosed rats are associated with oxidative stress, inflammatory cytokines and profibrogenic mediators in abdominal aorta.
NAD(P)H: Reduced-nicotinamide-adenine dinucleotide phosphate; MMP: Matrix metalloproteinases; TPVL: Triple partial portal vein stenosed; NF-κB: Nuclear factor κB; TNF-α: Tumor necrosis factor-α; IL: Interleukin; CTGF: connective tissue growth factor; MMP-9: Matrix metallopeptidase 9.
CONCLUSION
In summary, rats with long-term portal hypertension with liver steatosis and hypertensive portal intestinal vasculopathy also suffer a low-grade abdominal aortic inflammation associated with fibrosis.
ACKNOWLEDGMENTS
We would like to thank Maria Elena Vicente for her excellent assistance in preparing the manuscript and Elizabeth Mascola, a professional linguistic reviewer, as well as a native English speaker, for translating it into English.
P- Reviewers: Genesca J, Gentilucci UV S- Editor: Wen LL L- Editor: Cant MR E- Editor: Zhang DN