Published online Nov 28, 2013. doi: 10.3748/wjg.v19.i44.7852
Revised: October 25, 2013
Accepted: November 12, 2013
Published online: November 28, 2013
Processing time: 74 Days and 14.7 Hours
Hepatitis C virus (HCV) frequently elicits only mild immune responses so that it can often establish chronic infection. In this case HCV antigens persist and continue to stimulate the immune system. Antigen persistence then leads to profound changes in the infected host’s immune responsiveness, and eventually contributes to the pathology of chronic hepatitis. This topic highlight summarizes changes associated with chronic hepatitis C concerning innate immunity (interferons, natural killer cells), adaptive immune responses (immunoglobulins, T cells, and mechanisms of immune regulation (regulatory T cells). Our overview clarifies that a strong anti-HCV immune response is frequently associated with acute severe tissue damage. In chronic hepatitis C, however, the effector arms of the immune system either become refractory to activation or take over regulatory functions. Taken together these changes in immunity may lead to persistent liver damage and cirrhosis. Consequently, effector arms of the immune system will not only be considered with respect to antiviral defence but also as pivotal mechanisms of inflammation, necrosis and progression to cirrhosis. Thus, avoiding Scylla - a strong, sustained antiviral immune response with inital tissue damage - takes the infected host to virus-triggered immunopathology, which ultimately leads to cirrhosis and liver cancer - the realm of Charybdis.
Core tip: This topic highlight on the immunopathogenesis of chronic hepatitis C addresses changes in innate immunity (interferons and natural killer cells), adaptive immunity and immunoregulation (regulatory T cells). Our review provides a succinct but comprehensive overview and presents the concept, that effective antiviral immunity is associated with pronounced acute liver damage, while during chronic infection the arms of immunity will acquire new functions, which will cause and maintain tissue damage. Thus, the immune response becomes part of the mechanisms that eventually lead to progressive inflammation, liver cirrhosis and death in chronic hepatitis C.
- Citation: Spengler U, Nischalke HD, Nattermann J, Strassburg CP. Between Scylla and Charybdis: The role of the human immune system in the pathogenesis of hepatitis C. World J Gastroenterol 2013; 19(44): 7852-7866
- URL: https://www.wjgnet.com/1007-9327/full/v19/i44/7852.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i44.7852
Scylla and Charybdis were two immortal and irresistible sea monsters in Greek mythology believed to live on either side of the Strait of Messina between Sicily and Italy. Scylla was a six-headed supernatural creature - probably reflecting a shoal that devoured whatever came within her reach, and Charybdis was a whirlpool off the coast of Sicily. Avoiding Charybdis meant passing too close to Scylla and vice versa. According to Homer, the Greek hero Odysseus opted for Scylla when passing the strait, and had to sacrifice six of his companions rather than to risk the loss of his vessel in the whirlpool. Thus, being “between Scylla and Charybdis” means to be forced to make a choice between two equally unpleasant evils.
This allegory matches the challenge of the human immune system when defending against a viral infection, such as hepatitis C which has a high potential to establish chronic persistence. On one hand a strong and efficient immune response rapidly clears the virus; accepting the risk of severe tissue damage from immune-mediated destruction. On the other hand a less vigorous response allows for viral persistence and facilitates a low-level smoldering inflammation, which eventually results in progressive liver disease and ultimately death of the individual. In line with this analogy, acute self-limited hepatitis C is frequently associated with symptomatic disease and jaundice, while chronic hepatitis C often establishes in the absence of any characteristic symptoms[1,2]. Studies in various expression systems (cell culture or transgenic mice) indicate that hepatitis C virus (HCV) is not directly cytopathic, and viral replication may occur in the absence of any detectable inflammatory reaction[3,4]. On the other hand, chronic hepatitis C is associated with liver cell damage and intrahepatic inflammatory infiltrates. Of note, hepatocellular damage coincides with the onset of an immune response during acute infection but not with that of viral replication[5]. Thus, activation of the immune response is a pivotal factor for the pathogenic processes in hepatitis C leading to progressive tissue injury. Ultimately, hepatic inflammation and progressive fibrosis in chronic hepatitis C may result in cirrhosis and carry a high risk for hepatocellular carcinoma.
HCV is a hepacivirus of the Flaviviridae family. Its genome consists of a single strand positive sense RNA. After cell entry the viral genome is translated into a single polyprotein which is co- and post-translationally cleaved into structural and non-structural proteins by host peptidases and two virus-encoded proteases. Replication involves generation of an antigenomic replication intermediate, and probably intermediate double-stranded RNA (ds-RNA) products, which can trigger intracellular pattern recognition receptors. The new viral genomes are packaged into viral particles by the viral non-structural proteins, which then are released from hepatocytes in association with host lipoproteins. Thus, HCV circulates in blood as a lipoprotein-coated virus[6]. During replication HCV is sensed by pattern recognition receptors (PRRs) in the host cell which detect pathogen-associated molecular patterns within viral products. This process then leads to coordinated activation of innate and adaptive immune responses. Both arms of the immune response work together in an integrated fashion to recognize and defend against HCV infection.
Innate responses to HCV comprise both cellular responses, such as recognition of non-self by various types of natural killer (NK) cells and humoral components, such as induction of a variety of cytokines, especially interferons. These various elements of innate immunity act in a highly integrated fashion as do innate and adaptive immune responses. Thus, development of adaptive B and T cell immunity is shaped by the initial innate responses, in particular interferons and other inflammatory and immunoregulatory cytokines that are induced by viral invasion[7]. However, despite these immune defences, hepatitis C becomes chronic in about 70%-80% of acute infections[8]. Failing immunity and continued viral persistence lead to sustained inflammatory host responses which then become the key mechanism for tissue injury in chronic hepatitis C.
Three types of PRRs are known to detect HCV: (1) the retinoic acid inducible gene-I (RIG-I)-like receptors, RIG-I and melanoma differentiation antigen 5, which sense viral RNA in the cytosol; (2) toll-like receptors (TLRs), such as TLR3, which detects ds-RNA fragments in the endosomal compartment; and (3) the non-traditional pattern recognition receptor protein kinase R (PKR), which binds ds-RNA binding and upon activation promotes interaction with mitochondrial antiviral signaling protein (MAVS) to trigger innate immunity[9].
RIG-I signaling is initiated by binding of the HCV PAMP RNA which consists of an exposed 5’triphosphate and the 3’poly-U/UC-rich untranslated region of the HCV RNA[10,11]. These regions are located at opposite ends of the viral genome but are brought together by intra-genomic interactions. In this configuration the viral RNA comes into close contact with RIG-I and induces conformational changes of RIG-I. RIG-I activation leads to the formation of a multi-component complex with MAVS (also termed interferon beta promoter stimulator protein 1 or card adaptor inducing interferon beta, cardiff). Finally, the interferon signaling cascade results in the activation of multiple transcription factors, such as interferon-regulatory factor-3 (IRF-3) and nuclear factor kappa B and production of multiple pro-inflammatory cytokines[12].
HCV dsRNA intermediates, which occur late in HCV replication, have been identified as ligands for TLR3[13]. TLR3 signals are transmitted by the adaptor molecule TIR-domain-containing-adaptor-inducing-interferon-β (TRIF) and also lead to production of interferons and pro-inflammatory cytokines[14]. TLR3 mediated interferon and cytokine responses are considered a secondary innate immune defense after initial RIG-I activation to establish an antiviral state and trigger T cell recruitment in HCV infection.
The ligand for PKR is the structured RNA at the internal ribosomal entry site (IRES) of HCV RNA[15,16]. Binding of HCV RNA induces phosphorylation of the α-subunit of the eukaryotic initiation factor 2 (eIF2α). In addition, RNA binding also triggers a kinase-independent signal transduction cascade involving MAVS which finally activates interferon-β and interferon-stimulated genes (ISGs)[9,16].
Although HCV can be detected effectively by RIG-I, TLR3 and PKR, it frequently establishes chronic persistence in up to 80% of patients, because it has evolved several mechanisms to counter-act innate immunity. The multi-functional HCV NS3/NS4A protease is a key component of the HCV evasion strategy from innate immunity. Studies in Huh-7 cells indicate that HCV initially activates the RIG-I pathway which is shut down as infection progresses and NS3/NS4 abundance increases[17]. In addition to proteolytically processing the HCV polyprotein, NS3/NS4A can block RIG-I signaling, because it cleaves MAVS from intracellular membranes[18-21]. This cleavage prevents signal transduction, abrogates interferon induction and facilitates progression to chronic infection. However, other hepatotropic viruses, such as hepatitis A virus also encode proteases that can cleave MAVS but in general do not become chronic[22]. Thus, MAVS cleavage alone is not sufficient for viral chronicity. Nevertheless, cleavage of MAVS has been demonstrated in the livers of patients with chronic hepatitis C, and patients with cleaved MAVS revealed reduced interferon pathway activation, although this inverse correlation was rather weak[23].
The NS3/NS4A protease can also cleave TRIF[24], the adaptor protein of the TLR3 pathway, and the relative abundance of this protein is reduced after HCV infection, probably as a result of degradation following its cleavage by NS3/NS4A[25]. Although details are insufficiently understood at present, blocking of the TLR3 pathway by HCV also seems to contribute to establishing chronic infection. TLR3-independent sensing of RNA which signals via TRIF has also been described, and is likewise blocked by NS3/NS4A targeting of TRIF[26]. Finally HCV proteins E2, NS3, NS4A and NS5 provide several strategies to interfere both with PKR signaling and PKR-regulated inhibition of translation[27-29]. However, these interactions are complex and the exact mechanisms how they support HCV persistence are still unclear.
Continued triggering of PRR pathways in chronic hepatitis C is likely to contribute to immunopathology, such as hepatic inflammation, fibrosis progression and HCV-associated malignancy. In this context it is interesting to note that HCV proteins core and NS3 also trigger TLR 1-2 and 2-6 dimers[30,31], and there is evidence from genetic epidemiology and functional in vitro studies that HCV-TLR interactions might contribute to hepatic fibrogenesis and cirrhosis[32,33], development of liver cancer[34], HCV-associated autoimmunity and B cell lymphoma[35].
HCV recognition by PRRs ultimately leads to induction of antiviral cytokines termed interferons (IFNs). Type I IFNs (several interferons-α and interferon-β) bind to the ubiquitously expressed type I interferon receptor, while type III IFNs [IFN-λ1 alias interleukin (IL)-29, IFN-λ2 alias IL-28A, IFN-λ3 alias IL-28B] have their own receptor consisting of the IL10R2 chain (IL-10 receptor beta chain) and a unique IFN-λ receptor chain with a limited expression mainly on hepatocytes[36,37]. The type II interferon IFN-γ has its own IFN-γ receptor. All IFN receptors transmit signals from the cell surface to the nucleus via the Jak-STAT pathway to activate interferon stimulated genes (ISGs). Specifically type I and III IFNs induce IFN-stimulated gene factor 3 consisting of phosphorylated STAT1 and 2 proteins and IRF9 which activate the IFN-stimulated response elements (ISRE) of multiple genes contributing to antiviral activity[38-40].
IFN signaling is regulated by suppressors, such as suppressor of cytokine signaling and ubiquitin specific peptidase 18 (USP18) which provide important negative feed-back loops[41-43]. USP18 is a protease cleaving ISG15 from its target proteins, also including STAT1[44]. ISG15 is conjugated to STAT1 by the sequential action of several enzymes[45]. This so-called ISG-ylation and its de-conjugation by USP18 modify signal transduction pathways and immune responsiveness[46,47]. However, recently it has been recognized that USP18 suppresses IFN-signaling independently from its de-conjugating activity by interfering with the interaction between Jak1 and the type I IFN receptor[48]. USP18 is a major mediator of unresponsiveness to type 1 IFNs in liver cells[49]. However, it does not inhibit signal transduction of type II and III IFNs[50].
Activation of the endogenous IFN system in the liver exerts little anti-HCV activity, and it has been well established that patients with high activation of the endogenous IFN system respond poorly to IFNα based therapies[51-55]. It has been proposed that expression of HCV proteins inhibits binding of activated STATs to ISRE[56], and Jak-STAT signaling was found to be inhibited both in HCV transgenic mice and liver biopsies from patients with hepatitis C[57,58]. Beyond that, phosphorylation and activation of STAT3 is involved in the antiviral IFN activity[59], and STAT3 expression was found to be reduced in HCV-infected livers[60]. Indeed, HCV core protein can prevent STAT3 phosphorylation[57,60,61], and this has been associated with HCV resistance to IFN-α[62]. Next, HCV-induced PKR activation inhibits cap-dependent translation of antiviral host proteins at the ribosomes owing to phosphorylation of eIF2α while production of HCV proteins is not impaired, because translation occurs via an IRES-dependent mechanism[63]. Of note, most studies on endogenous ISG induction in hepatitis C were based on steady state mRNA level measurements rather than determination of protein concentrations[51-55]. Finally, HCV proteins might directly inhibit ISG antiviral effector functions apart from their inhibition of ISG translation. This concept is supported by experimental evidence from knock-out mice which demonstrated that expression of the USP18 leads to a long-term refractory state towards IFNα stimulation[49]. Likewise, strong USP18 expression was found in many hepatocytes of patients with chronic hepatitis C and high endogenous IFN activity, when histological specimens were studied[64]. At present the cellular sources and involved types of IFNs that maintain long-term ISG expression in chronic hepatitis C are still a matter of debate. IFN-λs are strong candidates as triggers of ISG induction in patients with chronic hepatitis C and endogenous activation of the IFN system, because, unlike all other IFN types, IFN-λ mRNA is readily detected in liver biopsies[53], and their action is not inhibited by USP18[50].
In patients with chronic hepatitis C endogenous ISG induction varies considerably between individuals, and this variability, as well as differential responsiveness to exogenous IFN-α is attributed to a combination of viral and host factors. For instance, difficult-to-treat HCV genotypes 1 and 4 induce high levels of endogenous IFN expression in hepatocytes resulting in an IFN-insensitive state that attenuates treatment responses[65]. Of note, endogenous ISG induction in Kupffer cells, the resident liver macrophages, is also a strong predictor of treatment responsiveness[66]. However, the relationship between baseline ISG induction and treatment outcome is opposite to that observed in hepatocytes: Virtually all non-responders lack baseline induction of ISGs whereas strongly induced ISG expression is found in responders[67]. This finding suggests that ISG induction in Kupffer cells may have a protective role for the host concerning both spontaneous HCV elimination and treatment outcomes.
Apart from viral factors genome-wide association studies have identified single nucleotide polymorphisms (SNPs) upstream of the IFNL3 gene on chromosome 19q13, which are associated with outcomes of HCV infection both under IFN-based therapy of chronic hepatitis C[68-70] and disease evolution during acute HCV infection[71,72]. Although some initial studies failed to find a relationship between the SNPs and IFNL3 mRNA expression[71,73], it has meanwhile become clear that SNPs in this region alter IFN-λ expression levels[53,68,70,74-76], and the unfavorable minor alleles result in less IFNL3 expression. Thus, it is quite unlikely that these SNPs simply reflect linkage disequilibrium with some other gene. However, the molecular and cellular mechanisms that underlie this association between outcomes of HCV infection and the IFNL3 gene locus are not yet understood. It has been proposed that the unfavorable IFNL3 variants may lead to compromised innate immune functions in particular with respect to natural killer cell activity[77,78]. However, given the fact that NK cells do not express type III IFN receptors this hypothesis needs refining[79]. In addition, a dinucleotide polymorphism upstream of the IFNL3 gene has been described, which can create or disrupt an alternative open reading frame giving rise to a new gene, termed IFNL4[80,81]. Although it has been proposed that loss of IFNL4 expression should be protective against HCV, it is as yet not clear if the putative IFN-λ4 gene product plays any role for differential immune responses to HCV infection.
NK cells constitute a first line of defence against viral infections. They rapidly recognize and lyse virus-infected cells, inhibit viral replication but also exert immune-regulatory functions. NK cells constitute approximately 30% of resident lymphocytes in a normal liver, and may account for as many as 60% of lymphocytes in HCV infection[82].
Activation of natural killer cells results from the integration of multiple activating and inhibitory signals via specific receptors. The most important NK cell receptors (and their cognate ligands) comprise the killer immunoglobulin-like receptor (KIR) family (ligands: HLA-A, -B and -C), the CD94-NKG2A/C complex (ligand: HLA-E), NKG2D (ligands: MIC-A and MIC-B and others) and the natural cytotoxicity receptors NKp30, NKp44 and NKp46[83]. In addition, part of these receptors also exerts immune-regulatory functions in subsets of T lymphocytes. NK cells are activated, when there is a relative reduction of inhibitory signals, e.g., down-regulated MHC class I expression on virus-infected cells, or a relative increase in signals from activating receptors, e.g., binding of antibody-coated antigens[84]. However, conventional MHC class I expression is not substantially reduced in hepatitis C, and it has been proposed that NK cell functions might be altered by binding of HCV-derived peptides to non-polymorphic restriction molecules, such as HLA-E[85,86]. NK cells are recruited to inflammatory sites by a variety of chemokines and can also be stimulated by cytokines, such as IFN-α and ILs 8, 12, 15 and 18[87].
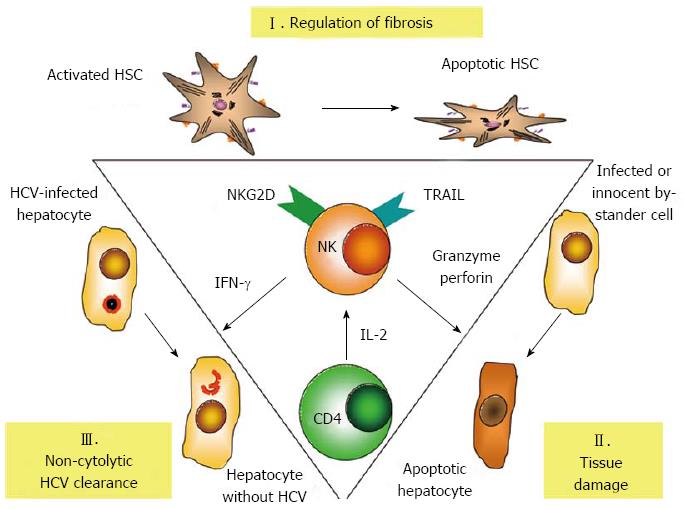
Activated NK cells with potent de-granulation and substantial cytokine production have been described in acute HCV infection[88,89], and there is accumulating evidence to suggest that NK cells play an important role in the antiviral immune response to hepatitis C and later on also in the immune-mediated pathogenesis of chronic hepatitis C. NK cells can inhibit HCV replication in vitro both by IFN-γ mediated non-cytolytic as well as granzyme/perforin and TRAIL-mediated cytotoxic mechanisms[90]. While HCV-infected hepatocytes up-regulate expression of TRAIL receptors[91], in vivo IFNγ-mediated clearance of HCV might be more important than direct cytolysis, because cytolytic elimination of all HCV-infected hepatocytes would lead to extensive liver damage[92]. Multi-functional NK cells are also detectable early after HCV exposure in health-care workers and iv drug users, who do not proceed to develop acute hepatitis; suggesting a potentially protective role of NK cells in early HCV infection[93,94]. Further support for a protective role of NK cells in HCV infection comes from genetic studies, where genes encoding the inhibitory receptor KIR2DL3 and its ligand human leucocyte antigen group 1 (HLA-C1) seem to favour both spontaneous and treatment-induced elimination of HCV[95,96]. Since the affinity between inhibitory KIR2DL3 and HLA-C1 is weaker than all other combinations, it is reasonable to assume that a lower threshold of activation is needed to trigger KIR2DL3 NK cell responses in HLA-C1 homozygous individuals[88,97]. Finally, NK cells can become more activated upon IFN-based therapy and may contribute to HCV elimination by TRAIL-mediated cytotoxic mechanisms[98]. Interestingly, responsiveness in this setting again depends on the endogenous IFN-α activation state, since a rapid first phase HCV decline is associated with strong induction of STAT1 phosphorylation, whereas non-responders exhibit reduced STAT1 induction[99]. Chronic exposure of NK cells to IFN-α results in preferential STAT1 over STAT4 phosphorylation, which is associated with increased STAT1-dependent cytotoxicity but reduced STAT4-dependent IFN-γ production[99-101]. These findings correspond to NK cell phenotypes and functional differentiation seen at later stages in IFN-α responders and non-responders[100,102], although patients who achieve a sustained virological response also exhibit substantial NK cell cytotoxicity[103].
NK cells in chronic hepatitis C have been reported to also express altered patterns of NK receptors (Figure 1). Although reported patterns are somewhat inconsistent and may vary between peripheral blood and the liver, altered expression on NK cells has been reported for receptors NKp30, NKp44, NKp46, NKG2A, NKG2C, NKG2D and CD122[100,104-107]. In addition, NK cells express the tetraspanin CD81, a co-receptor of HCV, and in vitro binding of the HCV envelope 2 (E2) protein to CD81 has been shown to block antiviral functions of NK cells and to alter their migratory behaviour[108-111]. However, the experimental setting of these studies involved cross-linking of HCV E2 on plastic plates, whereas NK cells exposed to intact virions did not exhibit altered functionality[112]. Thus, it remains to be elucidated if cross-linking of CD81 by HCV E2 affects functions of NK cells to facilitate chronic infection. A particularly interesting NK cell receptor is NKp46, since it is considered a major activating receptor in hepatitis C, which also has a role in the regulation of adaptive immunity: High expression of NKp46 defines a NK cell subset with high cytotoxic activity and IFN-γ production that accumulates in the liver in chronic hepatitis C[113,114]. Of note, recently Pembroke et al[115] confirmed intrahepatic enrichment of NKp46+ NK cells in chronic hepatitis C and reported a high (> 80%) frequency of NKp46+ cells in the liver to be associated with pronounced inflammation in histology. Another important finding of this study was the observation, that expression of NKp46 could predict responses to IFN therapy. Patients with chronic hepatitis C, who successfully cleared their HCV infection, had lower mean frequencies of activated NKp46+ NK cells than patients who did not respond to therapy. The possible identification of NKp46 as a marker of both IFN-un-responsiveness and hepatic inflammation bears some similarity to the paradoxical relationship between IFN-un-responsiveness and high baseline ISG expression and may be linked to chronic endogenous interferon exposure. On the other hand, unlike Pembroke et al[115] the group of Golden-Mason[113] reported increased NKp46 expression in white female Americans as opposed to male African-Americans and proposed that a high proportion of functionally active NKp46+ NK cells could explain their higher response to IFN therapy. Thus, the precise role of NKp46+ NK still remains elusive.
Finally, NK cell-mediated cytotoxicity against hepatic stellate cells (HSC) may contribute to the regulation of intrahepatic fibrosis in hepatitis C. HSC store vitamin A, reside in the space of Dissé, and produce extracellular matrix proteins upon activation, e.g., upon TLR stimulation, exposure to cytokines or reactive oxygen species[116]. HSC activation leads to trans-differentiation into myofibroblasts, which in the mouse also alters the balance in the expression between activating and inhibitory NK cell receptor ligands, so that they become target cells for NKG2D-, TRAIL- and granzyme-mediated killing by NK cells[117,118]. NKG2D- and TRAIL-mediated killing by NK cells has now also been reported for human HSC in chronic hepatitis C[119], and CXCR3 + CD56Bright as well as NKp46+ NK cells express particularly high cytotoxic capacity against HSC in chronic hepatitis C[114,120]. Importantly, when other processes, such as CD4 T cell depletion in HIV/HCV co-infection interfere with the regulation of hepatic fibrosis by NK cells, this may result in accelerated fibrosis progression[121].
A coordinated immune response involving both antibodies and T cell responses is normally required for efficient adaptive immunity. However, in hepatitis C the role of antibodies is complex: Circulating antibodies against structural and non-structural components are generated in virtually all patients irrespective from the outcome of HCV infection. A rapid induction of neutralizing antibodies early in the course of hepatitis C has been demonstrated to contribute to HCV clearance[122], but broad antibody responses usually occur at the stage of chronic infection and are not neutralizing[123,124]. Neutralizing antibodies frequently recognize the HCV envelope proteins[125-127]. However, these proteins have a high degree of mutational diversity, so that antibody responses are frequently directed against only a single strain or are easily evaded by viral mutations[124]. It is also quite likely that glycosylation of HCV proteins and the close association of the virus with lipoproteins further prevent antibody recognition. HCV antibodies are not required to clear HCV infection as has been demonstrated in patients with hypo-gammaglobulinemia[128]. HCV antibodies gradually disappear after successful HCV elimination[129]. Conversely, there is circumstantial evidence that HCV-specific cellular immune responses can protect individuals at high risk for hepatitis C without seroconversion[123,130,131]. Thus, adaptive cell-mediated immunity is considered a key mechanism for resolution of primary HCV infection[132]. Cell-mediated immunity involves CD8+ cytolytic T lymphocytes (CTL), which recognize linear HCV peptides of 8 to 11 amino acids in length bound to self HLA class I molecules, and CD4+ T helper lymphocytes, which respond to longer viral peptides bound to class II molecules. Single source outbreaks further support a clear relationship between distinct HLA types and the outcome of HCV infection: patients with HLA-A3, HLA-B27, and HLA-B57 exhibit greater chances to develop protective immunity, thus strengthening the importance of effective antigen presentation and the generation of efficient antigen-specific T cell responses for immune control of HCV infection[133-136].
The most conclusive experiments to suggest an important role for T cells in protective immunity against HCV stem from chimpanzee experiments: Depletion of CD8+ T cells in animals, which had recovered from previous hepatitis C, resulted in prolonged viraemia, and viral clearance was correlated to recovery of HCV-specific CD8+ T cells[137]. Likewise, depletion of CD4+ T cells resulted in abrogation of a previously protective immune response[138]. In acute hepatitis C strong HCV-specific CTL[139,140] and TH1 type CD4+ T helper cell responses[141] have consistently been reported to be closely associated with a self-limited course of HCV infection. Moreover, several groups have reported an inverse relationship between the strength of the CTL response and HCV viral loads[142-144] further suggesting that in principle it is possible for cellular immunity to control HCV infection[145]. A substantial proportion of individuals who ultimately develop chronic hepatitis C also generate HCV-specific CD4(+) and CD8(+) T cell responses during the early acute phase of infection and may transiently gain some control over HCV[140,146-149]. However, early T cell responses decline to almost undetectable levels later on, and initial control over HCV replication is lost. If present, HCV-specific CD4+ and CD8+ T cells are detected at only low frequency in peripheral blood although they are somewhat enriched in the liver[150,151]. Thus, chronic hepatitis C is characterized by a progressive functional exhaustion and ultimately loss of HCV-specific CD4+ and CD8+ T cells[152,153].
Exhausted T cells exhibit a couple of characteristic abnormalities: They show increased expression of inhibitory receptors, such as programmed death-1 (PD-1), cytotoxic T lymphocyte antigen 4 (CTLA-4), T cell immunoglobulin and mucin domain-containing molecule 3, corresponding to up-regulated expression of their cognate ligands in the liver[154-159]. Conversely, functional recovery of HCV-specific T cells can be achieved experimentally by the combined blockade of CTLA-4 and PD-1 signalling[157,160].
HCV replicates by an RNA-dependent RNA polymerase which has a high error rate and consequently generates considerable genomic diversity of HCV and T cell escape mutations. Mutations that affect CD8+ T cell epitopes and proteasomal processing have been observed in several HCV single source outbreaks[161-163]. Due to the exhausted state of T cells new epitope variants rarely elicit strong CD8+ T cell responses at this stage of infection, and consequently further escape mutation to secondary epitopes are selected infrequently in man and the chimpanzee[146,164,165]. Protective T cells seem to target epitopes that do not allow for escape mutations owing to the associated loss of viral replication fitness[133-135]. Conversely, T cells that are not stimulated any more after HCV viral escape, do not show features of exhaustion[166]. Thus, prolonged exposure appears to be the mechanism that leads to T cell dysfunction in chronic hepatitis C, and T cell exhaustion in hepatitis C seems to follow the same pattern as has been first described in mice for lymphocytic choriomeningitis virus (LCMV) infection[167,168]. In this model, persistent high level viremia can be established in susceptible mouse strains by pathogenic virus variants. Initially, mice develop a robust T cell response but fail to eliminate the virus and subsequently exhibit a gradual decline of CD8+ and CD4+ T cell responses. T cells undergo T cell exhaustion in this model, and first lose production of IL-2, a cytokine which supports T cell proliferation. Then, cytotoxicity and production of tumour necrosis factor alpha and IFN-γ are lost sequentially. Finally, intracellular expression of pro-apoptotic factors, such as Bcl2-interacting mediator (Bim), is up-regulated both in the LCMV model and hepatitis C[169]. In analogy to the LCMV model, virus-specific CD4+ and CD8+ T cell responses decline in chronic hepatitis C but full exhaustion with deletion of antigen-specific CD8 T cells does not occur, because at least in vitro T cell responses can be rescued.
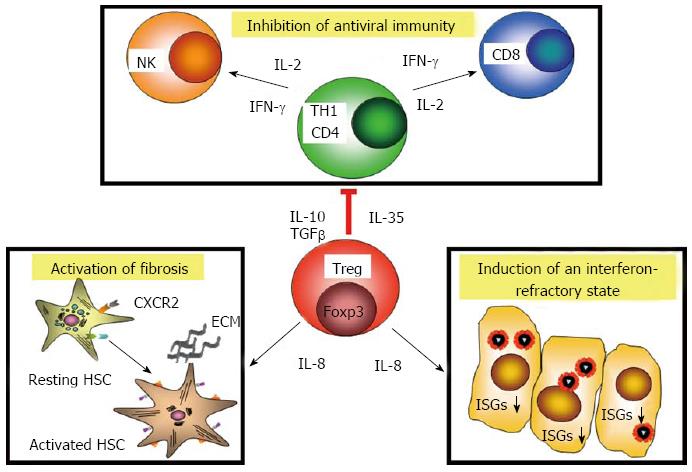
Recently CD8+ T cells have been reported in the livers of patients with chronic hepatitis C which were considered to represent CD8+ regulatory T cells, because they secrete IL-10 and suppress in vitro proliferation of liver-derived T cells[170]. In general, regulatory T cells (Tregs) actively control induction and activity of other immune cells by suppressing their functional activity via contact-dependent mechanisms and by release of immunosuppressive cytokines, such as IL-10 and transforming growth factor beta. The major cell type with these properties constitutes CD4+CD25highCD127- T cells, which express the transcription factor Foxp3 (forkhead box P3). They can be divided into thymus-derived natural regulatory T cells, that prevent autoreactivity to self-antigens and induced regulatory T cells, that are generated in the peripheral immune system as a regulatory response to antigenic stimulation. Foxp3+ Tregs were rarely detected in acute hepatitis C[171] and they are also not found in patients who had managed to resolve HCV infection[172], suggesting that effector T cells in acute and self-limited hepatitis C are not under active suppression by Tregs. In chronic hepatitis C, however, numbers of CD4+ Tregs were increased in the peripheral blood of patients, and depletion of CD4+ CD25+ T cells was associated with increased numbers and function of CD8+ T cells in in vitro assays[173-176]. Such regulatory T cells may reduce inflammatory activity and are considered to contribute importantly to preventing immune-mediated pathology in chronic hepatitis C. Functional analysis of regulatory T cell clones generated from patients with chronic hepatitis C revealed that Tregs were directed against HCV antigens and showed the same pattern of HLA class II restriction and epitope specificity as effector T cells[172]. Importantly, Treg clones from chronic hepatitis C inhibited in vitro proliferation and IFN-γ production of autologous reporter T cells via release of inhibitory cytokines, such as IL-10 and IL-35. Of note, intrahepatic regulatory T cells in chronic hepatitis C also produced substantial amounts of IL-8, and isolated Tregs as well as Treg clones activated fibrogenic genes of hepatic stellate cells in vitro[177]. High intrahepatic IL-8 mRNA levels in chronic hepatitis C have been linked with progression of fibrosis[178,179] and CD4+ Tregs are enriched in the liver[175,177,180-182]. Moreover, some but not all studies also reported a correlation between numbers of intrahepatic Tregs and the stage of fibrosis. Beyond that, IL-8 counter-acted the antiviral activity of IFN-α in the replicon model by down-regulation the expression of ISGs[183,184]. Moreover, in vitro studies suggest that part of the superior antiviral activity in IFN/ribavirin combination therapy may be due to preferential inhibition of Tregs by ribavirin[185] (Figure 2).
Thus, once the immune system has failed to clear HCV infection, regulatory T cells in chronic hepatitis C seem to exert multiple different effects: they dampen inflammatory responses associated with reduced antiviral activity of the immune system, facilitate HCV persistence, and also contribute to the regulation of fibrosis in the liver.
When an individual becomes infected with HCV, the immune system has to make a choice between Scylla and Charybdis. If it takes a course close to Scylla, it generates strong antiviral immune responses, which eliminates virus infected liver cells by the combined action of its several innate and adaptive defense mechanisms. This may cause extended liver damage and eventually liver failure. To avoid this risk, immune responses may be softer. Then, the virus has a chance to escape from control by immunity, and functions of innate and adaptive immune mechanisms become diverted owing to continued antigenic stimulation. An inflammatory state is induced, which, however, is refractory to stimulation by antiviral cytokines, and NK cells as well as cells in the adaptive immune system take over regulatory functions. Necro-inflammatory and pro-fibrotic activities maintained by diverted immune responses inevitably take a course towards Charybdis, and may ultimately result in liver cirrhosis, liver cancer and death of the individual. Thus, the immune system holds the steer to find the way between Scylla and Charybdis.
P- Reviewers: Auricchio S, Sakkas L S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
| 1. | Tillmann HL, Thompson AJ, Patel K, Wiese M, Tenckhoff H, Nischalke HD, Lokhnygina Y, Kullig U, Göbel U, Capka E. A polymorphism near IL28B is associated with spontaneous clearance of acute hepatitis C virus and jaundice. Gastroenterology. 2010;139:1586-1592, 1592.e1. [PubMed] |
| 2. | Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ulsenheimer A, Schraut WW, Schirren CA, Waechtler M, Backmund M. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 350] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 3. | Brillanti S, Foli M, Gaiani S, Masci C, Miglioli M, Barbara L. Persistent hepatitis C viraemia without liver disease. Lancet. 1993;341:464-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 123] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Alberti A, Morsica G, Chemello L, Cavalletto D, Noventa F, Pontisso P, Ruol A. Hepatitis C viraemia and liver disease in symptom-free individuals with anti-HCV. Lancet. 1992;340:697-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 256] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Farci P, Alter HJ, Shimoda A, Govindarajan S, Cheung LC, Melpolder JC, Sacher RA, Shih JW, Purcell RH. Hepatitis C virus-associated fulminant hepatic failure. N Engl J Med. 1996;335:631-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 95] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | André P, Perlemuter G, Budkowska A, Bréchot C, Lotteau V. Hepatitis C virus particles and lipoprotein metabolism. Semin Liver Dis. 2005;25:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1525] [Cited by in RCA: 1511] [Article Influence: 100.7] [Reference Citation Analysis (0)] |
| 8. | Seeff LB. The history of the “natural history” of hepatitis C (1968-2009). Liver Int. 2009;29 Suppl 1:89-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | McAllister CS, Samuel CE. The RNA-activated protein kinase enhances the induction of interferon-beta and apoptosis mediated by cytoplasmic RNA sensors. J Biol Chem. 2009;284:1644-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Uzri D, Gehrke L. Nucleotide sequences and modifications that determine RIG-I/RNA binding and signaling activities. J Virol. 2009;83:4174-4184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454:523-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 588] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 12. | Loo YM, Gale M. Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1581] [Cited by in RCA: 1435] [Article Influence: 102.5] [Reference Citation Analysis (0)] |
| 13. | Li K, Li NL, Wei D, Pfeffer SR, Fan M, Pfeffer LM. Activation of chemokine and inflammatory cytokine response in hepatitis C virus-infected hepatocytes depends on Toll-like receptor 3 sensing of hepatitis C virus double-stranded RNA intermediates. Hepatology. 2012;55:666-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 14. | Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 925] [Cited by in RCA: 952] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 15. | Shimoike T, McKenna SA, Lindhout DA, Puglisi JD. Translational insensitivity to potent activation of PKR by HCV IRES RNA. Antiviral Res. 2009;83:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Arnaud N, Dabo S, Akazawa D, Fukasawa M, Shinkai-Ouchi F, Hugon J, Wakita T, Meurs EF. Hepatitis C virus reveals a novel early control in acute immune response. PLoS Pathog. 2011;7:e1002289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Loo YM, Owen DM, Li K, Erickson AK, Johnson CL, Fish PM, Carney DS, Wang T, Ishida H, Yoneyama M. Viral and therapeutic control of IFN-beta promoter stimulator 1 during hepatitis C virus infection. Proc Natl Acad Sci USA. 2006;103:6001-6006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 324] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 18. | Baril M, Racine ME, Penin F, Lamarre D. MAVS dimer is a crucial signaling component of innate immunity and the target of hepatitis C virus NS3/4A protease. J Virol. 2009;83:1299-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Li XD, Sun L, Seth RB, Pineda G, Chen ZJ. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc Natl Acad Sci USA. 2005;102:17717-17722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 651] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 20. | Foy E, Li K, Sumpter R, Loo YM, Johnson CL, Wang C, Fish PM, Yoneyama M, Fujita T, Lemon SM. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc Natl Acad Sci USA. 2005;102:2986-2991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 430] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 21. | Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1976] [Cited by in RCA: 1921] [Article Influence: 96.1] [Reference Citation Analysis (0)] |
| 22. | Yang Y, Liang Y, Qu L, Chen Z, Yi M, Li K, Lemon SM. Disruption of innate immunity due to mitochondrial targeting of a picornaviral protease precursor. Proc Natl Acad Sci USA. 2007;104:7253-7258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 255] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 23. | Bellecave P, Sarasin-Filipowicz M, Donzé O, Kennel A, Gouttenoire J, Meylan E, Terracciano L, Tschopp J, Sarrazin C, Berg T. Cleavage of mitochondrial antiviral signaling protein in the liver of patients with chronic hepatitis C correlates with a reduced activation of the endogenous interferon system. Hepatology. 2010;51:1127-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Li K, Foy E, Ferreon JC, Nakamura M, Ferreon AC, Ikeda M, Ray SC, Gale M, Lemon SM. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci USA. 2005;102:2992-2997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 817] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 25. | Wang N, Liang Y, Devaraj S, Wang J, Lemon SM, Li K. Toll-like receptor 3 mediates establishment of an antiviral state against hepatitis C virus in hepatoma cells. J Virol. 2009;83:9824-9834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 26. | Zhang Z, Kim T, Bao M, Facchinetti V, Jung SY, Ghaffari AA, Qin J, Cheng G, Liu YJ. DDX1, DDX21, and DHX36 helicases form a complex with the adaptor molecule TRIF to sense dsRNA in dendritic cells. Immunity. 2011;34:866-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 298] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 27. | Taylor DR, Shi ST, Romano PR, Barber GN, Lai MM. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science. 1999;285:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 530] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 28. | Gale MJ, Korth MJ, Tang NM, Tan SL, Hopkins DA, Dever TE, Polyak SJ, Gretch DR, Katze MG. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology. 1997;230:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 608] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 29. | Noguchi T, Satoh S, Noshi T, Hatada E, Fukuda R, Kawai A, Ikeda S, Hijikata M, Shimotohno K. Effects of mutation in hepatitis C virus nonstructural protein 5A on interferon resistance mediated by inhibition of PKR kinase activity in mammalian cells. Microbiol Immunol. 2001;45:829-840. [PubMed] |
| 30. | Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J Leukoc Biol. 2007;82:479-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 31. | Dolganiuc A, Oak S, Kodys K, Golenbock DT, Finberg RW, Kurt-Jones E, Szabo G. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004;127:1513-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 235] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 32. | Coenen M, Nischalke HD, Krämer B, Langhans B, Glässner A, Schulte D, Körner C, Sauerbruch T, Nattermann J, Spengler U. Hepatitis C virus core protein induces fibrogenic actions of hepatic stellate cells via toll-like receptor 2. Lab Invest. 2011;91:1375-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Nischalke HD, Berger C, Luda C, Müller T, Berg T, Coenen M, Krämer B, Körner C, Trebicka J, Grünhage F. The CXCL1 rs4074 A allele is associated with enhanced CXCL1 responses to TLR2 ligands and predisposes to cirrhosis in HCV genotype 1-infected Caucasian patients. J Hepatol. 2012;56:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Nischalke HD, Coenen M, Berger C, Aldenhoff K, Müller T, Berg T, Krämer B, Körner C, Odenthal M, Schulze F. The toll-like receptor 2 (TLR2) -196 to -174 del/ins polymorphism affects viral loads and susceptibility to hepatocellular carcinoma in chronic hepatitis C. Int J Cancer. 2012;130:1470-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Feldmann G, Nischalke HD, Nattermann J, Banas B, Berg T, Teschendorf C, Schmiegel W, Dührsen U, Halangk J, Iwan A. Induction of interleukin-6 by hepatitis C virus core protein in hepatitis C-associated mixed cryoglobulinemia and B-cell non-Hodgkin’s lymphoma. Clin Cancer Res. 2006;12:4491-4498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1484] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 37. | Donnelly RP, Sheikh F, Kotenko SV, Dickensheets H. The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J Leukoc Biol. 2004;76:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 226] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 38. | Zhou Z, Hamming OJ, Ank N, Paludan SR, Nielsen AL, Hartmann R. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J Virol. 2007;81:7749-7758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 383] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 39. | Darnell JE. STATs and gene regulation. Science. 1997;277:1630-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2976] [Cited by in RCA: 3076] [Article Influence: 109.9] [Reference Citation Analysis (0)] |
| 40. | Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4322] [Cited by in RCA: 4587] [Article Influence: 148.0] [Reference Citation Analysis (0)] |
| 41. | Malakhov MP, Malakhova OA, Kim KI, Ritchie KJ, Zhang DE. UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J Biol Chem. 2002;277:9976-9981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 416] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 42. | Krebs DL, Hilton DJ. SOCS proteins: negative regulators of cytokine signaling. Stem Cells. 2001;19:378-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 645] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 43. | Malakhov MP, Kim KI, Malakhova OA, Jacobs BS, Borden EC, Zhang DE. High-throughput immunoblotting. Ubiquitiin-like protein ISG15 modifies key regulators of signal transduction. J Biol Chem. 2003;278:16608-16613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 44. | Liu LQ, Ilaria R, Kingsley PD, Iwama A, van Etten RA, Palis J, Zhang DE. A novel ubiquitin-specific protease, UBP43, cloned from leukemia fusion protein AML1-ETO-expressing mice, functions in hematopoietic cell differentiation. Mol Cell Biol. 1999;19:3029-3038. [PubMed] |
| 45. | Skaug B, Chen ZJ. Emerging role of ISG15 in antiviral immunity. Cell. 2010;143:187-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 46. | Chen L, Li S, McGilvray I. The ISG15/USP18 ubiquitin-like pathway (ISGylation system) in hepatitis C virus infection and resistance to interferon therapy. Int J Biochem Cell Biol. 2011;43:1427-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Zhang D, Zhang DE. Interferon-stimulated gene 15 and the protein ISGylation system. J Interferon Cytokine Res. 2011;31:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 268] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 48. | Malakhova OA, Kim KI, Luo JK, Zou W, Kumar KG, Fuchs SY, Shuai K, Zhang DE. UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. EMBO J. 2006;25:2358-2367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 361] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 49. | Sarasin-Filipowicz M, Wang X, Yan M, Duong FH, Poli V, Hilton DJ, Zhang DE, Heim MH. Alpha interferon induces long-lasting refractoriness of JAK-STAT signaling in the mouse liver through induction of USP18/UBP43. Mol Cell Biol. 2009;29:4841-4851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 50. | Makowska Z, Duong FH, Trincucci G, Tough DF, Heim MH. Interferon-β and interferon-λ signaling is not affected by interferon-induced refractoriness to interferon-α in vivo. Hepatology. 2011;53:1154-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 51. | Asselah T, Bieche I, Narguet S, Sabbagh A, Laurendeau I, Ripault MP, Boyer N, Martinot-Peignoux M, Valla D, Vidaud M. Liver gene expression signature to predict response to pegylated interferon plus ribavirin combination therapy in patients with chronic hepatitis C. Gut. 2008;57:516-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 52. | Chen L, Borozan I, Feld J, Sun J, Tannis LL, Coltescu C, Heathcote J, Edwards AM, McGilvray ID. Hepatic gene expression discriminates responders and nonresponders in treatment of chronic hepatitis C viral infection. Gastroenterology. 2005;128:1437-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 355] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 53. | Dill MT, Duong FH, Vogt JE, Bibert S, Bochud PY, Terracciano L, Papassotiropoulos A, Roth V, Heim MH. Interferon-induced gene expression is a stronger predictor of treatment response than IL28B genotype in patients with hepatitis C. Gastroenterology. 2011;140:1021-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 54. | Feld JJ, Nanda S, Huang Y, Chen W, Cam M, Pusek SN, Schweigler LM, Theodore D, Zacks SL, Liang TJ. Hepatic gene expression during treatment with peginterferon and ribavirin: Identifying molecular pathways for treatment response. Hepatology. 2007;46:1548-1563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 55. | Sarasin-Filipowicz M, Oakeley EJ, Duong FH, Christen V, Terracciano L, Filipowicz W, Heim MH. Interferon signaling and treatment outcome in chronic hepatitis C. Proc Natl Acad Sci USA. 2008;105:7034-7039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 545] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 56. | Heim MH, Moradpour D, Blum HE. Expression of hepatitis C virus proteins inhibits signal transduction through the Jak-STAT pathway. J Virol. 1999;73:8469-8475. [PubMed] |
| 57. | Blindenbacher A, Duong FH, Hunziker L, Stutvoet ST, Wang X, Terracciano L, Moradpour D, Blum HE, Alonzi T, Tripodi M. Expression of hepatitis c virus proteins inhibits interferon alpha signaling in the liver of transgenic mice. Gastroenterology. 2003;124:1465-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 58. | Duong FH, Filipowicz M, Tripodi M, La Monica N, Heim MH. Hepatitis C virus inhibits interferon signaling through up-regulation of protein phosphatase 2A. Gastroenterology. 2004;126:263-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 59. | Zhu H, Zhao H, Collins CD, Eckenrode SE, Run Q, McIndoe RA, Crawford JM, Nelson DR, She JX, Liu C. Gene expression associated with interferon alfa antiviral activity in an HCV replicon cell line. Hepatology. 2003;37:1180-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 60. | Larrea E, Aldabe R, Molano E, Fernandez-Rodriguez CM, Ametzazurra A, Civeira MP, Prieto J. Altered expression and activation of signal transducers and activators of transcription (STATs) in hepatitis C virus infection: in vivo and in vitro studies. Gut. 2006;55:1188-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Hosui A, Takehara T, Ohkawa K, Kanazawa Y, Tatsumi T, Yamaguchi S, Sakamori R, Hiramatsu N, Kanto T, Hayashi N. Suppressive effect on hepatocyte differentiation of hepatitis C virus core protein. Biochem Biophys Res Commun. 2006;346:1125-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 62. | Zhu H, Nelson DR, Crawford JM, Liu C. Defective Jak-Stat activation in hepatoma cells is associated with hepatitis C viral IFN-alpha resistance. J Interferon Cytokine Res. 2005;25:528-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Garaigorta U, Chisari FV. Hepatitis C virus blocks interferon effector function by inducing protein kinase R phosphorylation. Cell Host Microbe. 2009;6:513-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 64. | Dill MT, Makowska Z, Duong FH, Merkofer F, Filipowicz M, Baumert TF, Tornillo L, Terracciano L, Heim MH. Interferon-γ-stimulated genes, but not USP18, are expressed in livers of patients with acute hepatitis C. Gastroenterology. 2012;143:777-786.e1-6. [PubMed] |
| 65. | Chen L, Borozan I, Sun J, Guindi M, Fischer S, Feld J, Anand N, Heathcote J, Edwards AM, McGilvray ID. Cell-type specific gene expression signature in liver underlies response to interferon therapy in chronic hepatitis C infection. Gastroenterology. 2010;138:1123-1133.e1-3. [PubMed] |
| 66. | McGilvray I, Feld JJ, Chen L, Pattullo V, Guindi M, Fischer S, Borozan I, Xie G, Selzner N, Heathcote EJ. Hepatic cell-type specific gene expression better predicts HCV treatment outcome than IL28B genotype. Gastroenterology. 2012;142:1122-1131.e1. [PubMed] |
| 67. | Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1505] [Cited by in RCA: 1504] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 68. | Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2776] [Cited by in RCA: 2723] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 69. | Rauch A, Kutalik Z, Descombes P, Cai T, Di Iulio J, Mueller T, Bochud M, Battegay M, Bernasconi E, Borovicka J. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010;138:1338-1345, 1345.e1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 867] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 70. | Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1775] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 71. | Honda M, Sakai A, Yamashita T, Nakamoto Y, Mizukoshi E, Sakai Y, Yamashita T, Nakamura M, Shirasaki T, Horimoto K. Hepatic ISG expression is associated with genetic variation in interleukin 28B and the outcome of IFN therapy for chronic hepatitis C. Gastroenterology. 2010;139:499-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 344] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 72. | Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1706] [Cited by in RCA: 1687] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 73. | Urban TJ, Thompson AJ, Bradrick SS, Fellay J, Schuppan D, Cronin KD, Hong L, McKenzie A, Patel K, Shianna KV. IL28B genotype is associated with differential expression of intrahepatic interferon-stimulated genes in patients with chronic hepatitis C. Hepatology. 2010;52:1888-1896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 314] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 74. | Fukuhara T, Taketomi A, Motomura T, Okano S, Ninomiya A, Abe T, Uchiyama H, Soejima Y, Shirabe K, Matsuura Y. Variants in IL28B in liver recipients and donors correlate with response to peg-interferon and ribavirin therapy for recurrent hepatitis C. Gastroenterology. 2010;139:1577-1585, 1585.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 75. | Langhans B, Kupfer B, Braunschweiger I, Arndt S, Schulte W, Nischalke HD, Nattermann J, Oldenburg J, Sauerbruch T, Spengler U. Interferon-lambda serum levels in hepatitis C. J Hepatol. 2011;54:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 76. | Raglow Z, Thoma-Perry C, Gilroy R, Wan YJ. IL28B genotype and the expression of ISGs in normal liver. Liver Int. 2013;33:991-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Dring MM, Morrison MH, McSharry BP, Guinan KJ, Hagan R, O’Farrelly C, Gardiner CM. Innate immune genes synergize to predict increased risk of chronic disease in hepatitis C virus infection. Proc Natl Acad Sci USA. 2011;108:5736-5741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 78. | Naggie S, Osinusi A, Katsounas A, Lempicki R, Herrmann E, Thompson AJ, Clark PJ, Patel K, Muir AJ, McHutchison JG. Dysregulation of innate immunity in hepatitis C virus genotype 1 IL28B-unfavorable genotype patients: impaired viral kinetics and therapeutic response. Hepatology. 2012;56:444-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 79. | Krämer B, Eisenhardt M, Glässner A, Körner C, Sauerbruch T, Spengler U, Nattermann J. Do lambda-IFNs IL28A and IL28B act on human natural killer cells? Proc Natl Acad Sci USA. 2011;108:E519-E520; author reply E521-E322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Bibert S, Roger T, Calandra T, Bochud M, Cerny A, Semmo N, Duong FH, Gerlach T, Malinverni R, Moradpour D. IL28B expression depends on a novel TT/-G polymorphism which improves HCV clearance prediction. J Exp Med. 2013;210:1109-1116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 81. | Prokunina-Olsson L, Muchmore B, Tang W, Pfeiffer RM, Park H, Dickensheets H, Hergott D, Porter-Gill P, Mumy A, Kohaar I. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet. 2013;45:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 760] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 82. | Doherty DG, O’Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol Rev. 2000;174:5-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 284] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 83. | Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23:255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 459] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 84. | Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annu Rev Immunol. 2013;31:163-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 344] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 85. | Jinushi M, Takehara T, Tatsumi T, Kanto T, Miyagi T, Suzuki T, Kanazawa Y, Hiramatsu N, Hayashi N. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J Immunol. 2004;173:6072-6081. [PubMed] |
| 86. | Nattermann J, Nischalke HD, Hofmeister V, Ahlenstiel G, Zimmermann H, Leifeld L, Weiss EH, Sauerbruch T, Spengler U. The HLA-A2 restricted T cell epitope HCV core 35-44 stabilizes HLA-E expression and inhibits cytolysis mediated by natural killer cells. Am J Pathol. 2005;166:443-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 87. | Grégoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 411] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 88. | Amadei B, Urbani S, Cazaly A, Fisicaro P, Zerbini A, Ahmed P, Missale G, Ferrari C, Khakoo SI. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology. 2010;138:1536-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 89. | Pelletier S, Drouin C, Bédard N, Khakoo SI, Bruneau J, Shoukry NH. Increased degranulation of natural killer cells during acute HCV correlates with the magnitude of virus-specific T cell responses. J Hepatol. 2010;53:805-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 90. | Mondelli MU, Varchetta S, Oliviero B. Natural killer cells in viral hepatitis: facts and controversies. Eur J Clin Invest. 2010;40:851-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 91. | Zhu H, Dong H, Eksioglu E, Hemming A, Cao M, Crawford JM, Nelson DR, Liu C. Hepatitis C virus triggers apoptosis of a newly developed hepatoma cell line through antiviral defense system. Gastroenterology. 2007;133:1649-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 92. | Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 768] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 93. | Golden-Mason L, Cox AL, Randall JA, Cheng L, Rosen HR. Increased natural killer cell cytotoxicity and NKp30 expression protects against hepatitis C virus infection in high-risk individuals and inhibits replication in vitro. Hepatology. 2010;52:1581-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 94. | Werner JM, Heller T, Gordon AM, Sheets A, Sherker AH, Kessler E, Bean KS, Stevens M, Schmitt J, Rehermann B. Innate immune responses in hepatitis C virus-exposed healthcare workers who do not develop acute infection. Hepatology. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 95. | Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 926] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 96. | Suppiah V, Gaudieri S, Armstrong NJ, O’Connor KS, Berg T, Weltman M, Abate ML, Spengler U, Bassendine M, Dore GJ. IL28B, HLA-C, and KIR variants additively predict response to therapy in chronic hepatitis C virus infection in a European Cohort: a cross-sectional study. PLoS Med. 2011;8:e1001092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 97. | Moesta AK, Norman PJ, Yawata M, Yawata N, Gleimer M, Parham P. Synergistic polymorphism at two positions distal to the ligand-binding site makes KIR2DL2 a stronger receptor for HLA-C than KIR2DL3. J Immunol. 2008;180:3969-3979. [PubMed] |
| 98. | Ahlenstiel G, Edlich B, Hogdal LJ, Rotman Y, Noureddin M, Feld JJ, Holz LE, Titerence RH, Liang TJ, Rehermann B. Early changes in natural killer cell function indicate virologic response to interferon therapy for hepatitis C. Gastroenterology. 2011;141:1231-1239, 1239.e1-2. [PubMed] |
| 99. | Edlich B, Ahlenstiel G, Zabaleta Azpiroz A, Stoltzfus J, Noureddin M, Serti E, Feld JJ, Liang TJ, Rotman Y, Rehermann B. Early changes in interferon signaling define natural killer cell response and refractoriness to interferon-based therapy of hepatitis C patients. Hepatology. 2012;55:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 100. | Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, Ghany MG, Hoofnagle JH, Liang TJ, Heller T. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138:325-335.e1-2. [PubMed] |
| 101. | Miyagi T, Takehara T, Nishio K, Shimizu S, Kohga K, Li W, Tatsumi T, Hiramatsu N, Kanto T, Hayashi N. Altered interferon-alpha-signaling in natural killer cells from patients with chronic hepatitis C virus infection. J Hepatol. 2010;53:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 102. | Bozzano F, Picciotto A, Costa P, Marras F, Fazio V, Hirsch I, Olive D, Moretta L, De Maria A. Activating NK cell receptor expression/function (NKp30, NKp46, DNAM-1) during chronic viraemic HCV infection is associated with the outcome of combined treatment. Eur J Immunol. 2011;41:2905-2914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 103. | Oliviero B, Mele D, Degasperi E, Aghemo A, Cremonesi E, Rumi MG, Tinelli C, Varchetta S, Mantovani S, Colombo M. Natural killer cell dynamic profile is associated with treatment outcome in patients with chronic HCV infection. J Hepatol. 2013;59:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 104. | De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P, Congia S, Mingari MC, Moretta L. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37:445-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 105. | Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 106. | Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, De Filippi F, Bruno S, Mondelli MU. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137:1151-1160, 1160.e1-7. [PubMed] |
| 107. | Varchetta S, Mele D, Mantovani S, Oliviero B, Cremonesi E, Ludovisi S, Michelone G, Alessiani M, Rosati R, Montorsi M. Impaired intrahepatic natural killer cell cytotoxic function in chronic hepatitis C virus infection. Hepatology. 2012;56:841-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 108. | Crotta S, Brazzoli M, Piccioli D, Valiante NM, Wack A. Hepatitis C virions subvert natural killer cell activation to generate a cytokine environment permissive for infection. J Hepatol. 2010;52:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 109. | Crotta S, Stilla A, Wack A, D’Andrea A, Nuti S, D’Oro U, Mosca M, Filliponi F, Brunetto RM, Bonino F. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J Exp Med. 2002;195:35-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 326] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 110. | Krämer B, Schulte D, Körner C, Zwank C, Hartmann A, Michalk M, Söhne J, Langhans B, Nischalke HD, Coenen M. Regulation of NK cell trafficking by CD81. Eur J Immunol. 2009;39:3447-3458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 111. | Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002;195:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 322] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 112. | Yoon JC, Shiina M, Ahlenstiel G, Rehermann B. Natural killer cell function is intact after direct exposure to infectious hepatitis C virions. Hepatology. 2009;49:12-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 113. | Golden-Mason L, Stone AE, Bambha KM, Cheng L, Rosen HR. Race- and gender-related variation in natural killer p46 expression associated with differential anti-hepatitis C virus immunity. Hepatology. 2012;56:1214-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 114. | Krämer B, Körner C, Kebschull M, Glässner A, Eisenhardt M, Nischalke HD, Alexander M, Sauerbruch T, Spengler U, Nattermann J. Natural killer p46High expression defines a natural killer cell subset that is potentially involved in control of hepatitis C virus replication and modulation of liver fibrosis. Hepatology. 2012;56:1201-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 115. | Pembroke T, Christian A, Jones E, Hills RK, Wang EC, Gallimore AM, Godkin A. The paradox of NKp46+ natural killer cells: drivers of severe hepatitis C virus-induced pathology but in vivo resistance to interferon α treatment. Gut. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 116. | Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2244] [Cited by in RCA: 2201] [Article Influence: 129.5] [Reference Citation Analysis (0)] |
| 117. | Melhem A, Muhanna N, Bishara A, Alvarez CE, Ilan Y, Bishara T, Horani A, Nassar M, Friedman SL, Safadi R. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J Hepatol. 2006;45:60-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 216] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 118. | Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 473] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 119. | Glässner A, Eisenhardt M, Krämer B, Körner C, Coenen M, Sauerbruch T, Spengler U, Nattermann J. NK cells from HCV-infected patients effectively induce apoptosis of activated primary human hepatic stellate cells in a TRAIL-, FasL- and NKG2D-dependent manner. Lab Invest. 2012;92:967-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 120. | Eisenhardt M, Glässner A, Krämer B, Körner C, Sibbing B, Kokordelis P, Nischalke HD, Sauerbruch T, Spengler U, Nattermann J. The CXCR3(+)CD56Bright phenotype characterizes a distinct NK cell subset with anti-fibrotic potential that shows dys-regulated activity in hepatitis C. PLoS One. 2012;7:e38846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 121. | Glässner A, Eisenhardt M, Kokordelis P, Krämer B, Wolter F, Nischalke HD, Boesecke C, Sauerbruch T, Rockstroh JK, Spengler U. Impaired CD4⁺ T cell stimulation of NK cell anti-fibrotic activity may contribute to accelerated liver fibrosis progression in HIV/HCV patients. J Hepatol. 2013;59:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 122. | Pestka JM, Zeisel MB, Bläser E, Schürmann P, Bartosch B, Cosset FL, Patel AH, Meisel H, Baumert J, Viazov S. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci USA. 2007;104:6025-6030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 435] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 123. | Logvinoff C, Major ME, Oldach D, Heyward S, Talal A, Balfe P, Feinstone SM, Alter H, Rice CM, McKeating JA. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci USA. 2004;101:10149-10154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 335] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 124. | von Hahn T, Yoon JC, Alter H, Rice CM, Rehermann B, Balfe P, McKeating JA. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;132:667-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 308] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 125. | Law M, Maruyama T, Lewis J, Giang E, Tarr AW, Stamataki Z, Gastaminza P, Chisari FV, Jones IM, Fox RI. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008;14:25-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 493] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 126. | Meunier JC, Russell RS, Goossens V, Priem S, Walter H, Depla E, Union A, Faulk KN, Bukh J, Emerson SU, Purcell RH. Isolation and characterization of broadly neutralizing human monoclonal antibodies to the e1 glycoprotein of hepatitis C virus. J Virol. 2008;82:966-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 127. | Perotti M, Mancini N, Diotti RA, Tarr AW, Ball JK, Owsianka A, Adair R, Patel AH, Clementi M, Burioni R. Identification of a broadly cross-reacting and neutralizing human monoclonal antibody directed against the hepatitis C virus E2 protein. J Virol. 2008;82:1047-1052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 128. | Semmo N, Lucas M, Krashias G, Lauer G, Chapel H, Klenerman P. Maintenance of HCV-specific T-cell responses in antibody-deficient patients a decade after early therapy. Blood. 2006;107:4570-4571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 129. | Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, Miller JL, Manns MP, Rehermann B. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 559] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 130. | Abdelwahab SF, Zakaria Z, Sobhy M, Rewisha E, Mahmoud MA, Amer MA, Del Sorbo M, Capone S, Nicosia A, Folgori A. Hepatitis C virus-multispecific T-cell responses without viremia or seroconversion among Egyptian health care workers at high risk of infection. Clin Vaccine Immunol. 2012;19:780-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 131. | Post JJ, Pan Y, Freeman AJ, Harvey CE, White PA, Palladinetti P, Haber PS, Marinos G, Levy MH, Kaldor JM. Clearance of hepatitis C viremia associated with cellular immunity in the absence of seroconversion in the hepatitis C incidence and transmission in prisons study cohort. J Infect Dis. 2004;189:1846-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 132. | Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 578] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 133. | Dazert E, Neumann-Haefelin C, Bressanelli S, Fitzmaurice K, Kort J, Timm J, McKiernan S, Kelleher D, Gruener N, Tavis JE. Loss of viral fitness and cross-recognition by CD8+ T cells limit HCV escape from a protective HLA-B27-restricted human immune response. J Clin Invest. 2009;119:376-386. [PubMed] |
| 134. | Fitzmaurice K, Petrovic D, Ramamurthy N, Simmons R, Merani S, Gaudieri S, Sims S, Dempsey E, Freitas E, Lea S. Molecular footprints reveal the impact of the protective HLA-A*03 allele in hepatitis C virus infection. Gut. 2011;60:1563-1571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 135. | Kim AY, Kuntzen T, Timm J, Nolan BE, Baca MA, Reyor LL, Berical AC, Feller AJ, Johnson KL, Schulze zur Wiesch J. Spontaneous control of HCV is associated with expression of HLA-B 57 and preservation of targeted epitopes. Gastroenterology. 2011;140:686-696.e1. [PubMed] |
| 136. | Neumann-Haefelin C, Thimme R. Impact of the genetic restriction of virus-specific T-cell responses in hepatitis C virus infection. Genes Immun. 2007;8:181-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 137. | Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, Walker CM. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645-1655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 496] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 138. | Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 635] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 139. | Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499-1512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1003] [Cited by in RCA: 1001] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 140. | Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395-1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 925] [Cited by in RCA: 901] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 141. | Diepolder HM, Zachoval R, Hoffmann RM, Wierenga EA, Santantonio T, Jung MC, Eichenlaub D, Pape GR. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346:1006-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 522] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 142. | Hiroishi K, Kita H, Kojima M, Okamoto H, Moriyama T, Kaneko T, Ishikawa T, Ohnishi S, Aikawa T, Tanaka N. Cytotoxic T lymphocyte response and viral load in hepatitis C virus infection. Hepatology. 1997;25:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 143. | Nelson DR, Marousis CG, Davis GL, Rice CM, Wong J, Houghton M, Lau JY. The role of hepatitis C virus-specific cytotoxic T lymphocytes in chronic hepatitis C. J Immunol. 1997;158:1473-1481. [PubMed] |
| 144. | Rehermann B, Chang KM, McHutchinson J, Kokka R, Houghton M, Rice CM, Chisari FV. Differential cytotoxic T-lymphocyte responsiveness to the hepatitis B and C viruses in chronically infected patients. J Virol. 1996;70:7092-7102. [PubMed] |
| 145. | Koziel MJ, Wong DK, Dudley D, Houghton M, Walker BD. Hepatitis C virus-specific cytolytic T lymphocyte and T helper cell responses in seronegative persons. J Infect Dis. 1997;176:859-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 146. | Cox AL, Mosbruger T, Lauer GM, Pardoll D, Thomas DL, Ray SC. Comprehensive analyses of CD8+ T cell responses during longitudinal study of acute human hepatitis C. Hepatology. 2005;42:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 147. | Folgori A, Spada E, Pezzanera M, Ruggeri L, Mele A, Garbuglia AR, Perrone MP, Del Porto P, Piccolella E, Cortese R. Early impairment of hepatitis C virus specific T cell proliferation during acute infection leads to failure of viral clearance. Gut. 2006;55:1012-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 148. | Gerlach JT, Diepolder HM, Jung MC, Gruener NH, Schraut WW, Zachoval R, Hoffmann R, Schirren CA, Santantonio T, Pape GR. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 1999;117:933-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 526] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 149. | Lechner F, Gruener NH, Urbani S, Uggeri J, Santantonio T, Kammer AR, Cerny A, Phillips R, Ferrari C, Pape GR. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J Immunol. 2000;30:2479-2487. [PubMed] |
| 150. | Grabowska AM, Lechner F, Klenerman P, Tighe PJ, Ryder S, Ball JK, Thomson BJ, Irving WL, Robins RA. Direct ex vivo comparison of the breadth and specificity of the T cells in the liver and peripheral blood of patients with chronic HCV infection. Eur J Immunol. 2001;31:2388-2394. [PubMed] |
| 151. | He XS, Rehermann B, López-Labrador FX, Boisvert J, Cheung R, Mumm J, Wedemeyer H, Berenguer M, Wright TL, Davis MM. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc Natl Acad Sci USA. 1999;96:5692-5697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 308] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 152. | Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, Missale G, Ferrari C. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 231] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 153. | Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, Alter H, Rehermann B. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447-3458. [PubMed] |
| 154. | Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, Pircher H, Thimme R. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 305] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 155. | Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1703] [Cited by in RCA: 1642] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 156. | McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120:4546-4557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 260] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 157. | Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 299] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 158. | Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545-2553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 380] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 159. | Schlaphoff V, Lunemann S, Suneetha PV, Jaroszewicz J, Grabowski J, Dietz J, Helfritz F, Bektas H, Sarrazin C, Manns MP. Dual function of the NK cell receptor 2B4 (CD244) in the regulation of HCV-specific CD8+ T cells. PLoS Pathog. 2011;7:e1002045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 160. | Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, Shaked A, Olthoff K, Gostick E, Price DA. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927-1937, 1937.e1-2. [PubMed] |
| 161. | Cox AL, Mosbruger T, Mao Q, Liu Z, Wang XH, Yang HC, Sidney J, Sette A, Pardoll D, Thomas DL. Cellular immune selection with hepatitis C virus persistence in humans. J Exp Med. 2005;201:1741-1752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 232] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 162. | Ray SC, Fanning L, Wang XH, Netski DM, Kenny-Walsh E, Thomas DL. Divergent and convergent evolution after a common-source outbreak of hepatitis C virus. J Exp Med. 2005;201:1753-1759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 163. | Timm J, Lauer GM, Kavanagh DG, Sheridan I, Kim AY, Lucas M, Pillay T, Ouchi K, Reyor LL, Schulze zur Wiesch J. CD8 epitope escape and reversion in acute HCV infection. J Exp Med. 2004;200:1593-1604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 242] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 164. | Callendret B, Bukh J, Eccleston HB, Heksch R, Hasselschwert DL, Purcell RH, Hughes AL, Walker CM. Transmission of clonal hepatitis C virus genomes reveals the dominant but transitory role of CD8⁺ T cells in early viral evolution. J Virol. 2011;85:11833-11845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 165. | Meyer-Olson D, Shoukry NH, Brady KW, Kim H, Olson DP, Hartman K, Shintani AK, Walker CM, Kalams SA. Limited T cell receptor diversity of HCV-specific T cell responses is associated with CTL escape. J Exp Med. 2004;200:307-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 166. | Rutebemberwa A, Ray SC, Astemborski J, Levine J, Liu L, Dowd KA, Clute S, Wang C, Korman A, Sette A. High-programmed death-1 levels on hepatitis C virus-specific T cells during acute infection are associated with viral persistence and require preservation of cognate antigen during chronic infection. J Immunol. 2008;181:8215-8225. [PubMed] |
| 167. | Moskophidis D, Laine E, Zinkernagel RM. Peripheral clonal deletion of antiviral memory CD8+ T cells. Eur J Immunol. 1993;23:3306-3311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 168. | Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2481] [Cited by in RCA: 3052] [Article Influence: 218.0] [Reference Citation Analysis (0)] |
| 169. | Larrubia JR, Lokhande MU, García-Garzón S, Miquel J, González-Praetorius A, Parra-Cid T, Sanz-de-Villalobos E. Persistent hepatitis C virus (HCV) infection impairs HCV-specific cytotoxic T cell reactivity through Mcl-1/Bim imbalance due to CD127 down-regulation. J Viral Hepat. 2013;20:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 170. | Accapezzato D, Francavilla V, Paroli M, Casciaro M, Chircu LV, Cividini A, Abrignani S, Mondelli MU, Barnaba V. Hepatic expansion of a virus-specific regulatory CD8(+) T cell population in chronic hepatitis C virus infection. J Clin Invest. 2004;113:963-972. [PubMed] |
| 171. | Heeg MH, Ulsenheimer A, Grüner NH, Zachoval R, Jung MC, Gerlach JT, Raziorrouh B, Schraut W, Horster S, Kauke T. FOXP3 expression in hepatitis C virus-specific CD4+ T cells during acute hepatitis C. Gastroenterology. 2009;137:1280-8.e1-6. [PubMed] |
| 172. | Langhans B, Braunschweiger I, Arndt S, Schulte W, Satoguina J, Layland LE, Vidovic N, Hoerauf A, Oldenburg J, Sauerbruch T. Core-specific adaptive regulatory T-cells in different outcomes of hepatitis C. Clin Sci (Lond). 2010;119:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 173. | Boettler T, Spangenberg HC, Neumann-Haefelin C, Panther E, Urbani S, Ferrari C, Blum HE, von Weizsäcker F, Thimme R. T cells with a CD4+ CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79:7860-7867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 329] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 174. | Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, Nelson DR. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 430] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 175. | Rushbrook SM, Ward SM, Unitt E, Vowler SL, Lucas M, Klenerman P, Alexander GJ. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol. 2005;79:7852-7859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 227] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 176. | Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 2003;38:1437-1448. [PubMed] |
| 177. | Langhans B, Krämer B, Louis M, Nischalke HD, Hüneburg R, Staratschek-Jox A, Odenthal M, Manekeller S, Schepke M, Kalff J. Intrahepatic IL-8 producing Foxp3⁺CD4⁺ regulatory T cells and fibrogenesis in chronic hepatitis C. J Hepatol. 2013;59:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 178. | Mahmood S, Sho M, Yasuhara Y, Kawanaka M, Niiyama G, Togawa K, Ito T, Takahashi N, Kinoshita M, Yamada G. Clinical significance of intrahepatic interleukin-8 in chronic hepatitis C patients. Hepatol Res. 2002;24:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 179. | Asselah T, Bièche I, Laurendeau I, Paradis V, Vidaud D, Degott C, Martinot M, Bedossa P, Valla D, Vidaud M. Liver gene expression signature of mild fibrosis in patients with chronic hepatitis C. Gastroenterology. 2005;129:2064-2075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 180. | Claassen MA, de Knegt RJ, Tilanus HW, Janssen HL, Boonstra A. Abundant numbers of regulatory T cells localize to the liver of chronic hepatitis C infected patients and limit the extent of fibrosis. J Hepatol. 2010;52:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 181. | Sturm N, Thélu MA, Camous X, Dimitrov G, Ramzan M, Dufeu-Duchesne T, Bonorino P, Guillermet C, Brambilla E, Arvers P. Characterization and role of intra-hepatic regulatory T cells in chronic hepatitis C pathogenesis. J Hepatol. 2010;53:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 182. | Ward SM, Fox BC, Brown PJ, Worthington J, Fox SB, Chapman RW, Fleming KA, Banham AH, Klenerman P. Quantification and localisation of FOXP3+ T lymphocytes and relation to hepatic inflammation during chronic HCV infection. J Hepatol. 2007;47:316-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 183. | Girard S, Shalhoub P, Lescure P, Sabile A, Misek DE, Hanash S, Bréchot C, Beretta L. An altered cellular response to interferon and up-regulation of interleukin-8 induced by the hepatitis C viral protein NS5A uncovered by microarray analysis. Virology. 2002;295:272-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 184. | Jia Y, Wei L, Jiang D, Wang J, Cong X, Fei R. Antiviral action of interferon-alpha against hepatitis C virus replicon and its modulation by interferon-gamma and interleukin-8. J Gastroenterol Hepatol. 2007;22:1278-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 185. | Langhans B, Nischalke HD, Arndt S, Braunschweiger I, Nattermann J, Sauerbruch T, Spengler U. Ribavirin exerts differential effects on functions of Cd4+ Th1, Th2, and regulatory T cell clones in hepatitis C. PLoS One. 2012;7:e42094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |