Published online Nov 21, 2013. doi: 10.3748/wjg.v19.i43.7816
Revised: August 26, 2013
Accepted: September 15, 2013
Published online: November 21, 2013
Processing time: 148 Days and 15.2 Hours
Gastrointestinal stromal tumor (GIST) is a rare mesenchymal tumor of the gastrointestinal tract that has been associated with the formation of fistulas to adjacent organs in few case reports. However, GIST with enterohepatic fistula has not been reported. Here we report the case of an enterohepatic fistula that occurred after embolization of a liver mass originating in the distal ileum. An 87-year-old woman was hospitalized for melena. On initial conventional endoscopy, a bleeding focus in the gastrointestinal tract was not found. Because of massive hematochezia, enteroscopy was performed through the anus. A protruding, ulcerative mass was found in the distal ileum that was suspected to be the source of the bleeding; a biopsy sample was taken. Electrocoagulation was not successful in controlling the bleeding; therefore, embolization was performed. After embolization, the patient developed a high fever and severe abdominal tenderness with rebound tenderness. Follow-up abdominopelvic computed tomography revealed an enterohepatic fistula between the liver and distal ileum. The fistula was treated surgically by segmental resection of the distal ileum and unlooping of the liver mass.
Core tip: Gastrointestinal stromal tumor (GIST) with fistula is a rare condition, however, it can be seen during treatment. Herein we report a case of an enterohepatic fistula that occurred after therapeutic embolization of liver mass originated from ileal GIST.
- Citation: Lee YH, Koo JS, Jung CH, Chung SY, Lee JJ, Kim SY, Hyun JJ, Jung SW, Choung RS, Lee SW, Choi JH. Development of enterohepatic fistula after embolization in ileal gastrointestinal stromal tumor: A case report. World J Gastroenterol 2013; 19(43): 7816-7819
- URL: https://www.wjgnet.com/1007-9327/full/v19/i43/7816.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i43.7816
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor originating in the digestive tract. GISTs are thought to arise from the interstitial cells of Cajal in the normal myenteric plexus[1]. GISTs most commonly occur in the stomach (60%), followed by the jejunum and ileum (30%), duodenum (5%), colon and rectum (5%), and, rarely, the esophagus and appendix[2]. However, they account for less than 1% of all gastrointestinal (GI) tumors[3]. It is estimated that up to 6000 new cases are diagnosed in the United States every year[4,5].
Before the late 1990s, these mesenchymal tumors arising in the GI tract were most often classified as smooth muscle tumors or neural tumors. In the 1990s, investigators noted similarities between GIST cells and the interstitial cells of Cajal, a group of cells located in the muscularis propria and around the myenteric plexus throughout the GI tract. These tumors may not cause any symptoms unless they are in a certain location or grow to a certain size. GISTs are often found because they cause bleeding into the GI tract. Other symptoms can result from the mass effect of the tumor causing abdominal discomfort, nausea, vomiting or early satiety. The critical determinants of GIST behavior include tumor size, mitotic rate, and location[6]. The liver is the most common site of metastasis from GISTs, and liver metastases are a major determinant of patient survival[7,8]. Complications such as abscess formation, fistulae or perforation are rare but can be seen during treatment[9,10].
Here we describe the case of a patient with ileal GIST with an enterohepatic fistula caused by tumor necrosis after therapeutic embolization for the control of active bleeding.
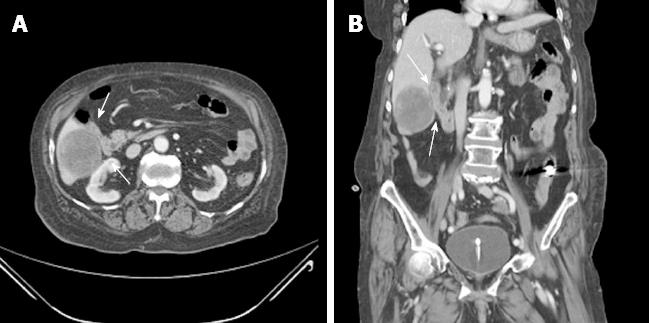
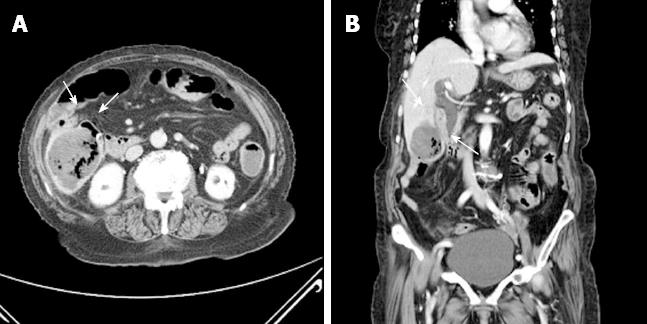
An 87-year-old woman was admitted to the emergency department because of melena that occurred the day before. She had been taking antihypertensive medication for 7 years and was not taking aspirin or other antiplatelet agents. She had a history of cholecystectomy, total abdominal hysterectomy, and right hemicolectomy due to GI bleeding. At admission, her vital signs were stable; laboratory findings showed anemia with a serum hemoglobin concentration of 7.8 g/dL and leukocytosis with a white blood cell count of 14000/mm3. A definite bleeding focus was not found on upper endoscopy and total colonoscopy. On a subsequent capsule endoscopy, a small amount of fresh blood was seen in the small bowel but the exact bleeding site was not identified. Abdominopelvic computed tomography (CT) showed a 5.7 cm × 5.2 cm heterogeneous enhancing mass in segments V and VI of the liver (Figure 1) adjacent to the distal ileum and multiple ill-defined low-attenuation lesions that were thought to represent metastases.
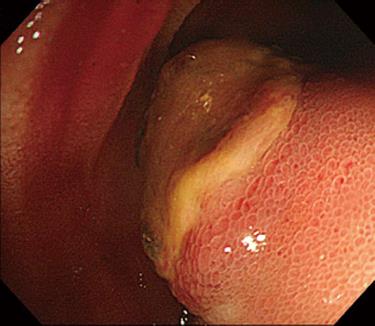
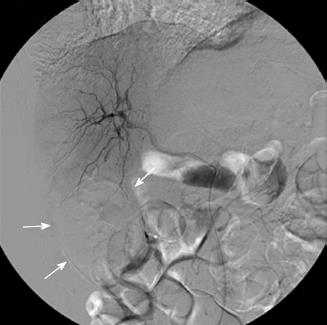
On the 9th day of hospitalization, she showed massive hematochezia. Single-balloon enteroscopy was performed through the anus, and a large protruding mass with central ulceration, which was suspected to be the bleeding focus, was found on the distal ileum (Figure 2). After an enteroscopic biopsy of the ileal mass was taken, active bleeding occurred that was not controlled by enteroscopic electrocoagulation. The patient refused surgical treatment for the active ileal bleeding. Therapeutic angiography was performed; however, no extravasation of the contrast was found in the inferior mesenteric or superior mesenteric arteries. Because a liver mass adjacent to the small bowel had been seen on the abdominopelvic CT, it was suspected that the protruding mass in the distal ileum originated from the suspected malignant liver mass. Based on the assumptions that the bleeding lesion originated from the hepatic mass and was supplied by the hepatic artery, a branch of the right hepatic artery was embolized, and there was no evidence of additional bleeding in the GI tract (Figure 3).
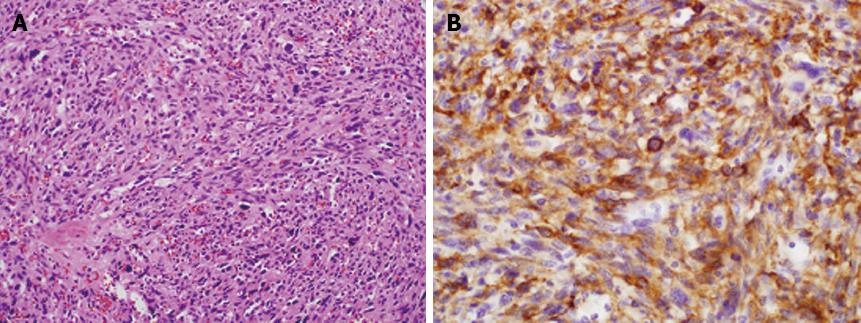
The biopsy specimen showed that the ileal mass was a GIST with more than 20 cells undergoing mitosis per 20 high-power fields (Figure 4). After the embolization, the patient developed fever and right upper quadrant pain. On repeated abdominopelvic CT 5 d after embolization, newly developed internal necrosis and a direct communication with distal ileum were identified in the previously seen liver mass (Figure 5).
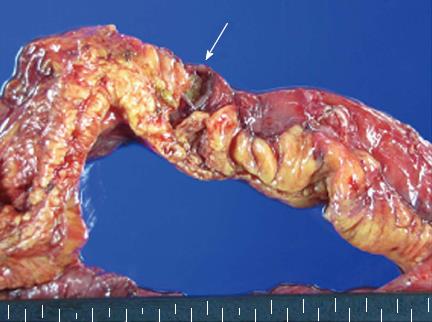
Segmental resection of the distal ileum and unlooping of the liver mass were undertaken, and fistula formation between the liver mass and the distal ileum was found intraoperatively. The gross specimen of the resected ileum showed a large fistula orifice that connected with the adjacent liver (Figure 6). Histopathologic examination of the surgical specimen obtained from the resected distal ileum and adjacent liver mass showed that both specimens were identical with GIST on immunohistochemical staining. After the operation, the patient refused chemotherapy and was transferred to a convalescent hospital. She died 6 mo later of multiple organ failure after recurrent GI sepsis.
GISTs with intraperitoneal rupture or organ invasion have rarely been reported[11]. Nonetheless, an enterocolic fistula can be caused by prior surgery, malignancy, and infection[12,13]. In similar situations, albeit very rarely, enterohepatic fistulas can develop with GISTs.
We presented a case of an enterohepatic fistula that occurred after embolization of a metastatic liver mass from a malignant GIST. An active bleeding site was not found on initial upper endoscopy and colonoscopy. Abdominopelvic CT demonstrated a 5.7 cm liver mass adjacent to the small bowel.
On initial workup, there was no evidence suggesting that the hepatic mass was the source of the GI bleeding. After enteroscopy by the anal approach because of the patient’s massive hematochezia, we found an exophytic mass that was thought to be connected with the hepatic mass and the cause of the bleeding. Therefore, embolization of the right hepatic artery supplying the liver mass was performed. However, within 24 h of arterial embolization, the patient developed fever and right upper quadrant abdominal pain, indicating that fistula formation between the liver mass and distal ileum occurred.
As the patient had undergone several previous abdominal operations, we assumed there was a strong possibility that intraabdominal adhesions were present, specifically between the small bowel and liver. High mitotic rates (> 20 per 20 high power field) also suggested that the tumor was likely to be invasive. Therefore, it was highly probable that the primary ileal tumor directly invaded the adjacent liver and then developed multiple hepatic metastases. Embolization of the right hepatic artery was performed to control the patient’s massive hematochezia, and necrosis of the hepatic tumor gradually organized into a fistula.
There have been several cases of GIST with abscess formations, perforations, or various fistulas[9,14-17]. However, GIST with a hepatic fistula is a unique presentation that has not been reported to our knowledge.
Watanabe et al[9] presented a similar case of GIST with a vesicocutaneous fistula during treatment with sunitinib. Sunitinib has anti-tumor and anti-angiogenic effects that cause necrosis, similar to our case in which arterial embolization induced visceral necrosis, with a resulting fistula in both cases.
In conclusion, we have reported a case of an enterohepatic fistula that occurred after embolization of a metastatic liver mass in an ileal GIST. It is important that physicians consider the possible complications, such as fistula, perforation and abscess formation, during treatment of highly invasive GISTs.
P- Reviewer: Kozarek R S- Editor: Ma YJ L- Editor: A E- Editor: Wu HL
| 1. | Demetri G, DeMatteo RP. NCCN Task Force report: gastrointestinal stromal tumor (GIST). J Natl Compr Canc Netw. 2004;2 Suppl 3:S-25-S-28. [PubMed] |
| 2. | Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30:477-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 437] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 3. | Stamatakos M, Douzinas E, Stefanaki C, Safioleas P, Polyzou E, Levidou G, Safioleas M. Gastrointestinal stromal tumor. World J Surg Oncol. 2009;7:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (2)] |
| 4. | Judson I, Demetri G. Advances in the treatment of gastrointestinal stromal tumours. Ann Oncol. 2007;18 Suppl 10:x20-x24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Corless CL, Heinrich MC. Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol. 2008;3:557-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 228] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 6. | Dematteo RP, Gold JS, Saran L, Gönen M, Liau KH, Maki RG, Singer S, Besmer P, Brennan MF, Antonescu CR. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer. 2008;112:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 345] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 7. | Bayraktar UD, Bayraktar S, Rocha-Lima CM. Molecular basis and management of gastrointestinal stromal tumors. World J Gastroenterol. 2010;16:2726-2734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 8. | Cao G, Li J, Shen L, Zhu X. Transcatheter arterial chemoembolization for gastrointestinal stromal tumors with liver metastases. World J Gastroenterol. 2012;18:6134-6140. [PubMed] [DOI] [Full Text] |
| 9. | Watanabe K, Otsu S, Morinaga R, Kawano S, Hirashima Y, Sakashita H, Shirao K. Vesicocutaneous fistula formation during treatment with sunitinib malate: Case report. BMC Gastroenterol. 2010;10:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Hur H, Park AR, Jee SB, Jung SE, Kim W, Jeon HM. Perforation of the colon by invading recurrent gastrointestinal stromal tumors during sunitinib treatment. World J Gastroenterol. 2008;14:6096-6099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Judson I. Gastrointestinal stromal tumours (GIST): biology and treatment. Ann Oncol. 2002;13 Suppl 4:287-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Chun HB, Baek IH, Lee MS, Kim JB, Shin SR, Kim BC, Jung SY, Kim JW. Jejunocolic fistula associated with an intestinal T cell lymphoma. Gut Liver. 2011;5:387-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Filipovic B, Randjelovic T, Nikolić G. Gastrojejunocolic fistula as a complication of Billroth II gastrectomy: a case report. Acta Chir Belg. 2008;108:592-594. [PubMed] |
| 14. | Kim BH, Lee JH, Baik du S, Yun SW, Kim JH, Kong JH, Kim SB. [A case of malignant gastrointestinal stromal tumor of ileum with liver abscess]. Korean J Gastroenterol. 2007;50:393-397. [PubMed] |
| 15. | Okamoto N, Yamafuji K, Takeshima K, Hayashi N, Baba H. [A case of gastrointestinal stromal tumor of the duodenum with a huge abscess]. Nihon Shokakibyo Gakkai Zasshi. 2011;108:1886-1891. [PubMed] |
| 16. | Shamsaeefar A, Hosseini SM, Motazedian N, Razmi T. Enterocolic fistula associated with a gastrointestinal stromal tumor. Indian J Cancer. 2009;46:246-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Memmi N, Cipe G, Bektasoglu H, Toydemir T, Kadioglu H, Bozkurt S, Buyukpinarbasili N, Karatepe O, Muslumanoglu M. Perforated gastrointestinal stromal tumor in the jejunum: A rare cause of acute abdomen. Oncol Lett. 2012;4:1244-1246. [PubMed] |