Published online Nov 21, 2013. doi: 10.3748/wjg.v19.i43.7726
Revised: July 24, 2013
Accepted: August 20, 2013
Published online: November 21, 2013
Processing time: 217 Days and 19.2 Hours
AIM: To investigate the events associated with the apoptotic effect of p-Coumaric acid, one of the phenolic components of honey, in human colorectal carcinoma (HCT-15) cells.
METHODS: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tertazolium-bromide assay was performed to determine the antiproliferative effect of p-Coumaric acid against colon cancer cells. Colony forming assay was conducted to quantify the colony inhibition in HCT 15 and HT 29 colon cancer cells after p-Coumaric acid treatment. Propidium Iodide staining of the HCT 15 cells using flow cytometry was done to study the changes in the cell cycle of treated cells. Identification of apoptosis was done using scanning electron microscope and photomicrograph evaluation of HCT 15 cells after exposing to p-Coumaric acid. Levels of reactive oxygen species (ROS) of HCT 15 cells exposed to p-Coumaric acid was evaluated using 2’, 7’-dichlorfluorescein-diacetate. Mitochondrial membrane potential of HCT-15 was assessed using rhodamine-123 with the help of flow cytometry. Lipid layer breaks associated with p-Coumaric acid treatment was quantified using the dye merocyanine 540. Apoptosis was confirmed and quantified using flow cytometric analysis of HCT 15 cells subjected to p-Coumaric acid treatment after staining with YO-PRO-1.
RESULTS: Antiproliferative test showed p-Coumaric acid has an inhibitory effect on HCT 15 and HT 29 cells with an IC50 (concentration for 50% inhibition) value of 1400 and 1600 μmol/L respectively. Colony forming assay revealed the time-dependent inhibition of HCT 15 and HT 29 cells subjected to p-Coumaric acid treatment. Propidium iodide staining of treated HCT 15 cells showed increasing accumulation of apoptotic cells (37.45 ± 1.98 vs 1.07 ± 1.01) at sub-G1 phase of the cell cycle after p-Coumaric acid treatment. HCT-15 cells observed with photomicrograph and scanning electron microscope showed the signs of apoptosis like blebbing and shrinkage after p-Coumaric acid exposure. Evaluation of the lipid layer showed increasing lipid layer breaks was associated with the growth inhibition of p-Coumaric acid. A fall in mitochondrial membrane potential and increasing ROS generation was observed in the p-Coumaric acid treated cells. Further apoptosis evaluated by YO-PRO-1 staining also showed the time-dependent increase of apoptotic cells after treatment.
CONCLUSION: These results depicted that p-Coumaric acid inhibited the growth of colon cancer cells by inducing apoptosis through ROS-mitochondrial pathway.
Core tip: This article describes apoptotic effect of p-Coumaric acid, one of the phenolic components of honey, against colon cancer cells. p-Coumaric acid treatment resulted in the inhibition of proliferation and colony forming ability of human colorectal carcinoma (HCT-15) and HT 29 cells. Major events associated with growth-inhibition are increasing reactive oxygen species generation, increasing lipid layer breaks and a fall in Mitochondrial membrane potential. Further, membrane blebbing and shrinkage of p-Coumaric acid exposed HCT 15 cells insinuated apoptosis. Hence our results depicted that p-Coumaric acid is a prospective candidate for chemoprevention of colon cancer.
-
Citation: Jaganathan SK, Supriyanto E, Mandal M. Events associated with apoptotic effect of
p -Coumaric acid in HCT-15 colon cancer cells. World J Gastroenterol 2013; 19(43): 7726-7734 - URL: https://www.wjgnet.com/1007-9327/full/v19/i43/7726.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i43.7726
Phenolic compounds are present in various dietary agents. Consumption of such agents has been linked to improve various disease conditions like cancer, diabetes and cardiac disorders. Diet is believed to be much influential in explaining the susceptibility to cancer. Most interestingly, colon cancer is more vulnerable to diet because these epithelial cells are chronically exposed to these dietary agents[1,2]. Since, cancer of colon is among the most common malignancy among the Western and Asian nations, research communities explore various new dietary agents rich in phenolic compounds to purge this malignancy.
In our laboratory, experiments in studying the preventive effect of honey against colon cancer had been constantly done. Previous results depicted honey could inhibit the colon cancer cell proliferation. Antiproliferative effect was found to vary with the phenolic content present in the honey[3-5]. Since honey containing higher phenolic content was found to induce apoptosis significantly, the scope of this research was extended to study the apoptosis induced by one of the phenolic components of honey, p-Coumaric acid, against the colon cancer cells.
p-Coumaric acid is the abundant isomer of cinnamic acid and also widely found in edible plants such as peanuts, tomatoes, carrots etc. p-Coumaric acid is reported to have antitumor and anti-mutagenic activities[6,7]. In a study, p-Coumaric acid along with the combination of hydrocaffeic acid found to reduce the UV-B oxidation damage in human conjunctival cells in vitro and in cornea and sclera of rabbits in vivo[8]. In one of the latest studies, the ability of p-Coumaric acid to protect rat’s heart against doxorubicin (DOX)-induced oxidative stress was investigated. It showed that p-Coumaric acid could reduce the DOX-induced high serum levels of lactic dehydrogenase and creatine phosphokinase[9]. In one of the most recent studies, effect of p-Coumaric acid against the colonic epithelial cells (Caco-2) was studied. p-Coumaric acid at a concentration of 1500 μmol/L was found to inhibit the proliferation of Caco-2 cells by 43%-75% after 24-72 h of treatment[10]. However, literature available does not depict the mechanism of p-Coumaric acid induced apoptosis in colon cancer cells.
Apoptosis is the major form of cell death accompanied by morphological changes like membrane blebbing and shrinkage of cells. Further, events like nuclear and chromatin condensation, DNA fragmentation and segregation of apoptotic bodies were the characteristic features of apoptosis. Reactive oxygen species (ROS) is involved in various biochemical functions like cell proliferation and apoptosis. Recent studies reported ROS mediated apoptosis is accompanied with the loss of mitochondrial membrane potential[11,12].
This current study, deals with the growth inhibitory effect of p-Coumaric acid in colon cancer cells. Further, an attempt has been made to explore the ROS and mitochondrial dependent mechanism in the apoptosis induced by the p-Coumaric acid.
DMEM, RPMI-1640, fetal bovine serum (FBS), L-glutamine, sodium pyruvate, nonessential amino acids, vitamin solution, penicillin and streptomycin were obtained from Life Technologies, Inc., Grand Island, NY, United States. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tertazolium-bromide (MTT), propidium iodide, mercury orange, rhodamine-123, RNase and p-Coumaric acid were purchased from Sigma-Aldrich, United States. Merocyanine 540 and YO-PRO-1 were obtained from Invitrogen Inc, United States.
Colon carcinoma cell line HT 29 and human colorectal carcinoma (HCT-15) (Organ: Colon, Disease: Colorectal adenocarcinoma; Organism: Human; procured from National Centre for Cell science, Pune, India) was grown in DMEM and RPMI medium respectively, supplemented with 10% FBS, L-glutamine, penicillin, sodium pyruvate, nonessential amino acids and vitamin solution Adherent monolayer cultures of HCT 15 were maintained in T-25 flasks and incubated at 37 °C in 5% carbon dioxide (CO2). The cultures were free of mycoplasma and maintained no longer than 12 wk after recovery from frozen stocks.
Thiazolyl blue tetrazolium bromide (MTT) assay was carried out as follows: Cells were trypsinized, counted and 1000 cells were seeded per well in 96-well plate. The following day, 100 μL of medium containing the desired concentration of p-Coumaric acid was added to the appropriate wells. The cells were then kept at 37 °C in 5% CO2 for the desired length of time. Control used in these experiments was untreated cells kept for 48 h. For all the experiments performed below, control cells remained untreated and kept for the same duration as the longest time-point of the respective experiment. At this point, 100 μL of (5 mg/mL) MTT reagent was added to each well, and the plate was placed at 37 °C in the incubator for 2 h. 200 μL of dimethyl sulfoxide was added to each well, after aspirating the supernatant. Colored formazan product was assayed spectrophotometrically at 570 nm using enzyme-linked immunosorbent assay plate reader[12].
HCT 15 and HT 29 cells were treated with p-Coumaric acid at a concentration of 1400 and 1600 μmol/L respectively for definite time periods (12, 24 and 48 h) and collected by trypsinization. The cells were counted and seeded again in triplicate on a 6-well tissue culture plate with 3000 cells/well. The cells were cultured for 15 d with growth media replaced after every two days. The cells were stained with 0.5% crystal violet (in methanol) and colonies were counted[12].
After the appropriate treatment with p-Coumaric acid, HCT 15 cells were washed with phosphate-buffered saline, then resuspended in 50 μg/mL propidium iodide containing 0.1% sodium citrate with 0.1% Triton X-100 for 20 min at 4 °C. Cells were then analyzed by flow cytometry (FACScan; Becton Dickinson Immunocytometry Systems), and the sub-G1 fraction was used as a measure of the apoptotic cells. Control used in the experiments was untreated cells kept for 48 h. Analysis was performed in linear amplification mode in case of cell cycle analysis. Remaining experiments of flow cytometry were performed in logarithmic amplification mode unless otherwise stated[13].
Dichlorofluorescein-diacetate (DCFH-DA) was cleaved by the intracellular nonspecific esterase to form DCFH. DCFH are oxidized by ROS to form the fluorescent compound DCF. p-Coumaric acid treated cells (1400 μmol/L) were harvested using trypsin/EDTA and resuspended in PBS. Working solution (20 μmol/L) of DCFH-DA was directly added cells and then it was incubated at 37 °C for 15 min. Cells were washed and resuspended in PBS and kept on ice immediately before analyzing by flow cytometry[12]. This fluorescent intensity of DCF was measured and correlated with the ROS generated in the cells.
HCT 15 colon cancer cells were treated with p-Coumaric acid (1400 μmol/L) for different time points. Afterwards, cells were harvested and resuspended in 1 mL of rhodamine-123 (5 μg/mL) for 1 h at 37 °C. The intensity of fluorescence from rhodamine-123 was measured by flow cytometry[12].
Colon cancer cells (HCT 15) were treated with p-Coumaric acid (1400 μmol/L) for different time points. Cells were harvested and re-suspended in 1 mL of merocyanine 540 (10 μg/mL) for 15 min at 37 °C. The intensity of fluorescence was measured by flow cytometry[13].
YO-PRO-1 permits analysis of apoptotic cells without interfering cell viability. After treatment with p-Coumaric acid (1400 μmol/L), the cell pellets were mixed in 1 μmol/L YO-PRO-1 for 20 min at room temperature. After incubation intensity was measured using flow cytometry[13].
Fixed amount of HCT 15 cells were seeded in a sterilized glass slide and incubated for 24 h. p-Coumaric acid at a concentration of 1400 μmol/L was added for 48 h time interval. After incubation, cells were harvested by using trypsin/EDTA and centrifuged for 5 min at room temperature. Then the supernatant was decanted and pellet was dried. Pellet was treated with 2.5% glutaraldehyde in distilled water for 45 min in hybrid oven shaker at 37 °C. Cells were washed thrice with PBS for 5 min and then dehydrated by ethyl alcohol of different concentration (30%, 50%, 70%, 95% and 100%) for 5-10 min. Fixing of cells was done with hexamethyl disilazane and the sample was taken for scanning electron microscope analysis. Photomicrograph images of HCT 15 and HT 29 cells were acquired using microscope.
All values are expressed as the mean ± SE. Figures were plotted using Graphpad Prism software. All experiments were performed three times independently (biological triplicates). One-way ANOVA was performed to find statistical significance.
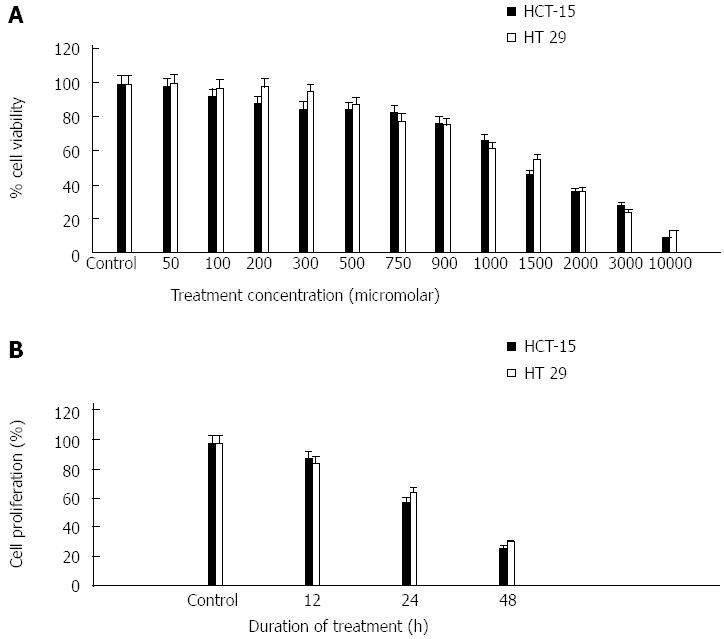
MTT assay of treated cells was performed after 48 h of treatment. Colon cancer cells (HCT 15 and HT 29) growth was inhibited in a dose-dependent manner. Both HCT-15 and HT-29 cell growth were inhibited significantly with an IC50 of around 1400 μmol/L and 1600 μmol/L respectively (Figure 1A). HCT 15 cells were found more sensitive to p-Coumaric acid, however at higher concentrations both cell lines were found to be equally affected. Statistical analysis showed that p-Coumaric acid treatment results in significant inhibition (P < 0.05) compared with untreated control cells at 200 μmol/L and 500 μmol/L for HCT 15 and HT 29 cells respectively (Figure 1A).
p-Coumaric acid treated HCT 15 cells showed a maximum of 94, 67, 32 colonies after 12, 24 and 48 h of treatment. Untreated HCT 15 cells produced a maximum of 105 colonies. Similar experiment with HT29 cells displayed a maximum of 131, 101, 51 colonies after 12, 24 and 48 h treatment whereas the control HT 29 cells produced 154. A time-dependent inhibition of colony formation was clearly evident from this experiment (Figure 1B). There was a significant reduction (P < 0.05) in the number of colonies formed under the various time intervals examined (both HCT 15 and HT 29 cells) when compared with corresponding untreated cells (Figure 1B).
Cell populations were tabulated among the sub-G1, G0/G1, S and G2/M phases of the cell cycle. It showed an increasing sub-G1 arrest from 1.00% (control) to 37.45% after 48 h (Table 1). Statistical analysis of the sub-G1 column indicated significant increase (P < 0.05) of cells in the sub-G1 phase insinuating apoptosis increases with the time-dependency.
| Time in h | Sub G11 | G0/G1 | S | G2/M |
| Control | 1.07 ± 1.01 | 42.82 ± 1.92 | 8.03 ± 1.23 | 40.07 ± 2.85 |
| 12 h | 5.98 ± 1.17 | 23.06 ± 3.15 | 10.29 ± 4.01 | 46.67 ± 1.89 |
| 24 h | 16.46 ± 2.03 | 23.92 ± 1.74 | 9.91 ± 3.29 | 39.03 ± 1.58 |
| 48 h | 37.45 ± 1.98 | 12.79 ± 4.45 | 4.9 ± 3.82 | 17.12 ± 4.65 |
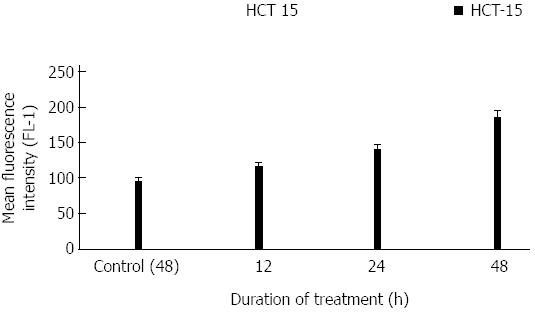
ROS levels were increased significantly after treatment. The increasing mean fluorescent intensity was found to be 116, 141, and 185 during 12, 24 and 48 h respectively. Untreated control cells showed an intensity of 96 after 48 h. ROS intensity after 48 h treatment was almost double the intensity of the control cells. Moreover, the differences in the ROS levels at various h examined were significant, compared to control with a P value of less than 0.05 (Figure 2).
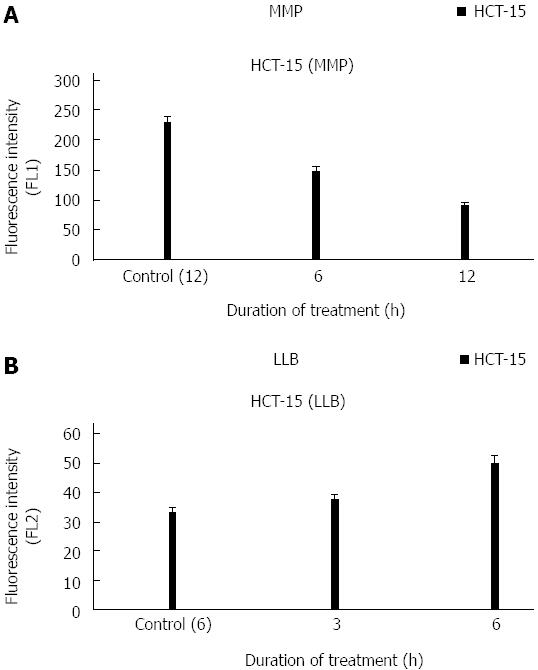
The decreasing mean fluorescent intensity was found to be 147, 91 during 6 and 12 h of treatment respectively. Untreated control cells showed an average intensity of 229 after 12 h. From the results, it was observed that p-Coumaric acid treatment reduced the potential by 2.5 fold after 12 h. There was also statistically significant reduction (P < 0.05) of potential at the estimated intervals compared to untreated cells (Figure 3A).
Untreated cells displayed a mean intensity of 33 after 6 h. Treated cells showed 37 and 50 after 3 and 6 h respectively (Figure 3B). It is evident from the above results that treated cells displayed an increase in the lipid layer breaks.
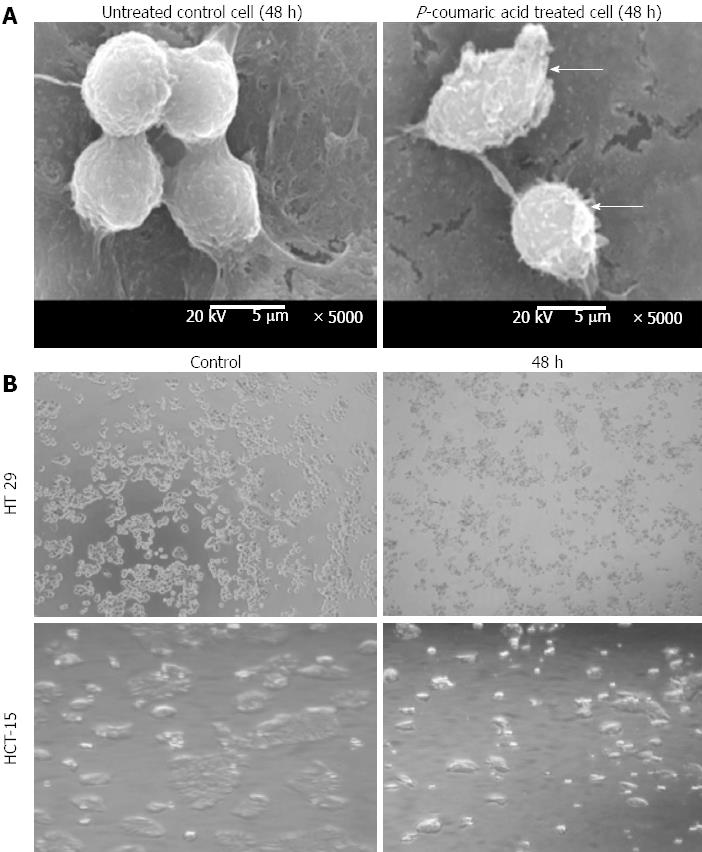
Scanning electron microscope (SEM) images of p-Coumaric acid treated cells (48 h) showed typical signs of apoptosis like membrane blebbing and shrinkage as indicated by arrow marks. Normal cells were found almost spherical without marked shrinkage (Figure 4A). This was further corroborated with the photomicrograph images (Figure 4B).
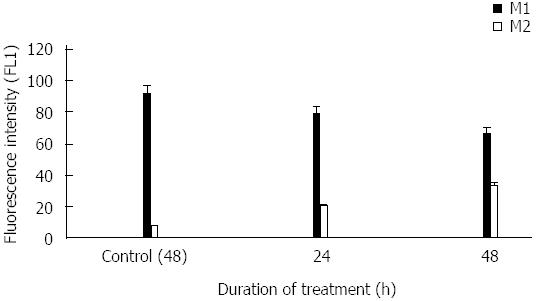
The percentage of cells distributed in M2 population signifying apoptosis increased depending upon the duration of treatment. It was found to be 20, 33 after 24 and 48 h of p-Coumaric acid treatment. M2 phase population of untreated control cells was found to be 8% after 48 h (Figure 5).
Diet consumption and cancer have been linked by various studies[14,15]. They postulated that consistent pattern of consumption of diets which are rich in vegetables and fruits may reduce the risk of cancer. Phenolic compounds, one of the classes of non-nutritive phytochemicals, are widely distributed in our foods and suggested to have preventive effect against various disease conditions like cancer, diabetes and several cardiac disorders[16,17]. From our laboratory, it was showed that honey rich in phenolic content was able to induce apoptosis significantly in colon cancer cells. Hence, in this research the effect of p-Coumaric acid, one of the phenolic constituents of honey, induced apoptosis in colon cancer cells was studied.
p-Coumaric acid inhibited the proliferation of colon cancer cells. Both HCT-15 and HT-29 cell growth were inhibited significantly with an IC50 of around 1400 μmol/L and 1600 μmol/L respectively. This was similar to the previously published report on the antiproliferative effect of p-Coumaric acid against Caco-2 cells[10]. Bioavailability of phenolic constituents is a major factor when we would like to examine the effect of p-coumaric acid in in vivo. In one of the researches, it was showed that bioavailability of coumaric acid after consumption of 200 g plum is in the range of 28-230 mg/serving[18]. In a colonic volume of 200 mL, this would yield a concentration in the range of 850 to 7000 μmol/L. Hence, it is believed that estimated IC50 values against these colon cancer cells are achievable internally. Human diet is complex and the supply of coumaric acid from different diets has to be evaluated simultaneously to have an idea about its bioavailability. To add further, bioavailability varies among the individuals and this makes estimation of intakes and prediction of physiological range of phenolics in body fluids is a mammoth task. The biggest drawback is that bioavailability of p-Coumaric acid will be in pulses depending upon the food intake whereas in cell culture environments it is constant[10].
p-Coumaric acid significantly inhibited the colony formation in vitro. This is indispensable, since most of the chemotherapeutic drugs were shown to inhibit the colony formation[12]. The effect of p-coumaric acid against intestinal epithelial cells (IEC) isolated from the mouse was evaluated. It was found that p-Coumaric acid was not toxic to these cells. Even at a higher concentration of 5.1 mmol/L nearly 80% cells were viable (results not shown). Sparing nature of p-Coumaric acid against mouse IEC was interesting and would warrant further study with normal human colonic cells.
Mitochondrial malfunction is one of the key events occurring at the initial stages of apoptosis. Studies reported a fall in the mitochondrial membrane potential during apoptosis induced by various chemotherapeutic drugs. Mitochondrial membrane potential of p-Coumaric acid treated cells using rhodamine-123 showed decreasing intensity, confirming the mitochondrial malfunction. ROS is involved in various biochemical functions like cell proliferation and apoptosis. Generally, ROS stress is oncogenic and it is found to increase the metabolic activity. It also stimulates further ROS generation through mitochondrial respiratory chain and maintains the cancer phenotype. On the other hand, high dose of ROS for prolonged duration could induce cellular damage and apoptosis[19,20]. Hence by utilizing time and dose-dependent ROS generation, we can trigger cell death by using exogenous ROS-generating agents. Our experiment involving DCFDH-DA staining indicated increasing ROS generation in the p-Coumaric acid treated cells. Hence, p-Coumaric acid may be considered as a potential exogenous candidate (generating ROS) to induce apoptosis in colon cancer cells.
The most notable property of phenolic phytochemicals is that they have antioxidant activity. This is due to the ability of phenolic hydroxyl groups which can provide hydrogen atoms in scavenging the ROS. Hence it is suggested that phenolic phytochemicals could scavenge the ROS molecules and inhibit the mitogen activated protein kinase (MAPK) signaling and blocking the nuclear factor kappaB and activator protein 1 activation which eventually lead to inhibit cancer cell proliferation. Although antioxidant properties of phenolic phytochemicals were explained for its mechanism of inhibiting cancer cells, they also show pro-oxidant activity under certain experimental conditions[21]. ROS generation was observed in the cell culture media containing EGCG, quercetin and gallic acid in both time and concentration-dependent manner[22]. In our case, p-Coumaric acid was also found to increase ROS generation in a time-dependent manner. Hence, treating the cancer cells with p-Coumaric acid can produce significant ROS resulting stressful or cytotoxic effects. Excess of ROS generation by phenolic phytochemicals induces apoptosis through MAPK activation. Simultaneously, increased p53 activation was mediated by Ras/MAPK kinase/MAPK pathway as observed in the apoptosis of EGCG and reseveratrol[23,24]. Hence, we hypothesize that the increased ROS generation may result in the activation of p53 in the p-Coumaric acid treated cells. This may in-turn would have caused the up-regulation of Bax and down-regulation of Bcl2 which are the down-stream targets of p53 resulting in apoptosis.
Apoptosis, a distinguished form of cell death, is characterized by membrane blebbing and DNA fragmentation. Electron Microscopy is used as a golden standard in detecting apoptosis[25-27]. In our case, both scanning electron microscope and photomicrograph images of p-Coumaric acid treated cells showed typical membrane blebbing and shrinkage portraying apoptosis. Sub-G1 arrest of cell cycle is considered as a sign of apoptosis[28-30]. p-Coumaric acid treatment showed increasing accumulation of cells in the sub-G1 phase confirming apoptosis. This was similar to the most anticancer drugs which inducted apoptosis by arresting the cells at sub-G1 phase[31-33]. At an early stage of apoptosis, there will be considerable damage to plasma membrane and the lipid layer will be disorganized. Nowadays in addition to the nuclear and morphological assessment, lipid layer perturbations in plasma membrane can also insinuate apoptosis. Merocyanine staining of treated cells for lipid layer organization showed increasing fluorescence intensity depicting apoptosis. This observation was similar to eugenol induced apoptosis shown recently[13].
In conclusion, p-Coumaric acid exerted antiproliferative activity against colon cancer cells like HT 29 and HCT 15. Both the cell lines growth was repressed significantly by inducing apoptosis. Apoptosis induced by p-Coumaric acid involved various physical and biochemical changes. To enumerate, cells showed membrane blebbing and shrinkage as depicted by SEM and photomicrograph images. Earlier lipid layer breaks were associated with the p-Coumaric acid induced apoptosis. Cell cycle progression was arrested at sub-G1 phase by p-Coumaric acid treatment. Mitochondrial membrane potential of treated cells also showed a decrease after p-Coumaric acid treatment. Moreover, there was increase in the ROS generation and lipid layer breaks after treatment. These results insinuate that p-Coumaric acid inhibited the growth of colon cancer cells by inducing apoptosis through ROS-mitochondrial pathway. However, further experiments in preclinical and clinical settings will promote this as a likely candidate for chemoprevention of colon cancer.
Jaganathan SK acknowledges the directors’ (Mr. Raguram, Dr. Lakshmana Prabhu, and Mr. Sugumaran) invaluable support and their constant encouragement. Authors acknowledge the efforts of Dr. Joseph Thomas for providing his valuable comments for English-editing service. Thanks to Ms. Bhuvaneswari S and Mr. Joseph Raja for checking this paper.
Consumption of phenolic components has been linked to improve various disease conditions like cancer, diabetes and cardiac disorders. Diet is believed to be much influential in explaining the susceptibility to cancer. Most interestingly, colon cancer is more vulnerable to diet because these epithelial cells are chronically exposed to these dietary agents. Honey has been reported to possess protective effect in many inflammatory diseases and oxidative stress-related injuries. Recent works from the laboratory showed phenolic components of honey were attributed with inherent potential to inhibit colon cancer cells. In this article p-Coumaric acid, one of the phenolic components of honey, has been examined for its growth inhibitory effects.
Chemotherapy utilizes antineoplastic or dietary agents for treating colon cancer. However, there is still a continuous search for novel agents with improved efficiency. To their knowledge p-Coumaric acid, one of the phenolic components of honey, have never been examined for its inhibitory mechanism against colon cancer.
Events associated with the inhibitory nature of p-Coumaric acid are clearly highlighted in this manuscript. Authors have shown that p-Coumaric acid inhibited the colon cancer cells in dose-dependent manner. Further it was deciphered that p-Coumaric acid induced apoptosis is accompanied with increasing reactive oxygen species (ROS) levels, a fall in the mitochondrial membrane potential and increased lipid layer breaks. Hence authors concluded that p-Coumaric acid inhibited the growth of colon cancer cells by inducing apoptosis through ROS-mitochondrial pathway.
p-Coumaric acid induced apoptosis in colon cancer cells through ROS-mitochondrial pathway. Hence, further experiments in preclinical and clinical settings will promote p-Coumaric acid as a plausible candidate for chemoprevention of colon cancer.
This work describes the events associated with the growth-inhibitory effect of p-Coumaric acid in colon cancer cells. Since p-Coumaric acid is one of the phenolic components of honey, the study has a clear interest in the field of chemoprevention of colon cancer. The results of this study are interesting and demonstrate that p-Coumaric acid has antiproliferative activity against colon cancer cells inducing apoptosis and causing physical and biochemical changes.
P- Reviewers: Cadena MP, De Nardi P S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
| 1. | Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 866] [Cited by in RCA: 779] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 2. | Johnson IT. Anticarcinogenic effects of diet-related apoptosis in the colorectal mucosa. Food Chem Toxicol. 2002;40:1171-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Jaganathan SK, Mandal M. Honey constituents and its apoptotic effect in colon cancer cells. JAAS. 2009;1:29-36. [RCA] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Jaganathan SK, Mandal SM, Jana SK, Das S, Mandal M. Studies on the phenolic profiling, anti-oxidant and cytotoxic activity of Indian honey: in vitro evaluation. Nat Prod Res. 2010;24:1295-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Jaganathan SK, Mondhe D, Wani ZA, Pal HC, Mandal M. Effect of honey and eugenol on Ehrlich ascites and solid carcinoma. J Biomed Biotechnol. 2010;2010:989163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Ferguson LR, Lim IF, Pearson AE, Ralph J, Harris PJ. Bacterial antimutagenesis by hydroxycinnamic acids from plant cell walls. Mutat Res. 2003;542:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Kroon PA, Williamson G. Hydroxycinnamates in plants and food: current and future perspectives. J Sci Food Agric. 1999;79:355-361. [DOI] [Full Text] |
| 8. | Larrosa M, Lodovici M, Morbidelli L, Dolara P. Hydrocaffeic and p-coumaric acids, natural phenolic compounds, inhibit UV-B damage in WKD human conjunctival cells in vitro and rabbit eye in vivo. Free Radic Res. 2008;42:903-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Abdel-Wahab MH, El-Mahdy MA, Abd-Ellah MF, Helal GK, Khalifa F, Hamada FM. Influence of p-coumaric acid on doxorubicin-induced oxidative stress in rat’s heart. Pharmacol Res. 2003;48:461-465. [PubMed] |
| 10. | Janicke B, Onning G, Oredsson SM. Differential effects of ferulic acid and p-coumaric acid on S phase distribution and length of S phase in the human colonic cell line Caco-2. J Agric Food Chem. 2005;53:6658-6665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Jaganathan SK, Mandal M. Involvement of non-protein thiols, mitochondrial dysfunction, reactive oxygen species and p53 in honey-induced apoptosis. Invest New Drugs. 2010;28:624-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Jaganathan SK. Growth inhibition by caffeic acid, one of the phenolic constituents of honey, in HCT 15 colon cancer cells. ScientificWorldJournal. 2012;2012:372345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Jaganathan SK, Mazumdar A, Mondhe D, Mandal M. Apoptotic effect of eugenol in human colon cancer cell lines. Cell Biol Int. 2011;35:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Key TJ. Fruit and vegetables and cancer risk. Br J Cancer. 2011;104:6-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 198] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 15. | Boffetta P, Couto E, Wichmann J, Ferrari P, Trichopoulos D, Bueno-de-Mesquita HB, van Duijnhoven FJ, Büchner FL, Key T, Boeing H. Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2010;102:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 16. | Wollgast J, Anklam E. Review on polyphenols in Theobroma cacao: changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res Int. 2000;33:423-447. [RCA] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 364] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 17. | Madhavi DV, Despande SS, Salunkhe DK. In: Food Antioxidants. New York: Marcel Dekker Publisher 1996; 267-311. |
| 18. | Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727-747. [PubMed] |
| 19. | Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241-252. [PubMed] |
| 20. | Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97-110. [PubMed] |
| 21. | Loo G. Redox-sensitive mechanisms of phytochemical-mediated inhibition of cancer cell proliferation (review). J Nutr Biochem. 2003;14:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Long LH, Clement MV, Halliwell B. Artifacts in cell culture: rapid generation of hydrogen peroxide on addition of (-)-epigallocatechin, (-)-epigallocatechin gallate, (+)-catechin, and quercetin to commonly used cell culture media. Biochem Biophys Res Commun. 2000;273:50-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Chen C, Yu R, Owuor ED, Kong AN. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch Pharm Res. 2000;23:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 281] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 24. | Shih A, Davis FB, Lin HY, Davis PJ. Resveratrol induces apoptosis in thyroid cancer cell lines via a MAPK- and p53-dependent mechanism. J Clin Endocrinol Metab. 2002;87:1223-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 25. | Taatjes DJ, Sobel BE, Budd RC. Morphological and cytochemical determination of cell death by apoptosis. Histochem Cell Biol. 2008;129:33-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 26. | Yasuhara S, Zhu Y, Matsui T, Tipirneni N, Yasuhara Y, Kaneki M, Rosenzweig A, Martyn JA. Comparison of comet assay, electron microscopy, and flow cytometry for detection of apoptosis. J Histochem Cytochem. 2003;51:873-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Martinez MM, Randall DR, Pappas D. Detection of apoptosis: A review of conventional and novel techniques. Anal Methods. 2010;2:996-1004. [DOI] [Full Text] |
| 28. | Sun PC, Tzao C, Chen BH, Liu CW, Yu CP, Jin JS. Suberoylanilide hydroxamic acid induces apoptosis and sub-G1 arrest of 320 HSR colon cancer cells. J Biomed Sci. 2010;17:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Zheng PW, Chiang LC, Lin CC. Apigenin induced apoptosis through p53-dependent pathway in human cervical carcinoma cells. Life Sci. 2005;76:1367-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Chiruvella KK, Raghavan SC. A natural compound, methyl angolensate, induces mitochondrial pathway of apoptosis in Daudi cells. Invest New Drugs. 2011;29:583-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Wang YF, Shyu HW, Chang YC, Tseng WC, Huang YL, Lin KH, Chou MC, Liu HL, Chen CY. Nickel (II)-induced cytotoxicity and apoptosis in human proximal tubule cells through a ROS- and mitochondria-mediated pathway. Toxicol Appl Pharmacol. 2012;259:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Bartholomeusz C, Yamasaki F, Saso H, Kurisu K, Hortobagyi GN, Ueno NT. Gemcitabine Overcomes Erlotinib Resistance in EGFR-Overexpressing Cancer Cells through Downregulation of Akt. J Cancer. 2011;2:435-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Kumar VB, Yuan TC, Liou JW, Yang CJ, Sung PJ, Weng CF. Antroquinonol inhibits NSCLC proliferation by altering PI3K/mTOR proteins and miRNA expression profiles. Mutat Res. 2011;707:42-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |