Published online Nov 21, 2013. doi: 10.3748/wjg.v19.i43.7620
Revised: August 12, 2013
Accepted: September 15, 2013
Published online: November 21, 2013
Processing time: 176 Days and 13.5 Hours
Branched chain amino acids (BCAAs) have been shown to affect gene expression, protein metabolism, apoptosis and regeneration of hepatocytes, and insulin resistance. They have also been shown to inhibit the proliferation of liver cancer cells in vitro, and are essential for lymphocyte proliferation and dendritic cell maturation. In patients with advanced chronic liver disease, BCAA concentrations are low, whereas the concentrations of aromatic amino acids such as phenylalanine and tyrosine are high, conditions that may be closely associated with hepatic encephalopathy and the prognosis of these patients. Based on these basic observations, patients with advanced chronic liver disease have been treated clinically with BCAA-rich medicines, with positive effects.
Core tip: Advanced liver diseases are commonly accompanied by nutritional disturbances, which worsen the prognosis of the patients. Serum levels of branched-chain amino acids (BCAAs) are decreased in patients with liver cirrhosis, and the amino acids imbalance could affect the clinical picture of the disease and the prognosis of the patients. However, there are few comprehensive reviews on the biological activities of BCAAs. In this review, we summarize the biological activities of BCAAs, and discuss possible applications of BCAAs for the management of patients with advanced liver diseases with a list of clinical trials of BCAA administration.
- Citation: Tajiri K, Shimizu Y. Branched-chain amino acids in liver diseases. World J Gastroenterol 2013; 19(43): 7620-7629
- URL: https://www.wjgnet.com/1007-9327/full/v19/i43/7620.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i43.7620
The three branched chain amino acids (BCAAs), leucine, isoleucine and valine, are among the nine essential amino acids for humans. Recent studies have revealed the functions of these BCAAs, and they have been administered for the treatment of advanced liver diseases. In this review, we summarize current understanding of the biological properties of BCAAs and review the results of clinical application of BCAAs to treat patients with liver diseases.
Serum concentrations of BCAAs are decreased, while the concentrations of the aromatic amino acids (AAAs) phenylalanine and tyrosine are increased, in patients with advanced liver diseases, resulting in a low ratio of BCAAs to AAAs, a ratio called the Fischer ratio[1]. A low Fischer ratio has been associated with hepatic encephalopathy (HE). The imbalance of amino acids tends to become more marked with the progression of liver diseases, and aminograms are useful for assessing the prognosis of cirrhotic patients with or without hepatocellular carcinoma (HCC)[2,3]. Moreover, a simplified Fischer ratio, the BCAA to tyrosine ratio (BTR), has been reported useful for predicting serum albumin concentration one year later[4]. These data indicate that amino acid imbalance, either low Fischer ratio or BTR, is a marker for progression of liver diseases, and that correcting this ratio may have therapeutic potential, not only for nutritional improvement, but also for HE, in patients with advanced liver diseases.
In mice, BCAA-rich diets have shown to up-regulate the expression of peroxisome proliferator-activated receptor (PPAR) γ coactivator-1α (PGC-1α), a master regulator of mitochondrial biogenesis and the defense system against reactive oxygen species (ROS), and of sirtuin-1, a member of the sirtuin family linked to life span extension, enhanced mitochondrial biogenesis, and decreased ROS production, leading to the prolongation of the lifespan of male mice[5]. BCAAs have also been shown to induce the activation of genes involved in antioxidant defenses and inhibition of ROS production, as well as to induce the hepatic expression of mRNA encoding 8-oxyoguanine DNA glycosilase 1, an enzyme involved in repair of oxidative DNA damage, in a rat model of liver injury, indicating that BCAAs are involved in the induction of antioxidant DNA repair[6].
In various cell lines, BCAAs, especially leucine, have been shown to activate the mammalian target of rapamycin (mTOR) signals, stimulating the synthesis of proteins, including albumin, and of glycogen[7]. The ability of leucine to enhance glucose metabolism was confirmed in normal rats and in a rat cirrhosis model. BCAA activation of mTORC1 has also been associated with cell growth[8] and PGC-1α-mediated mitochondrial gene expression[9]. BCAAs have been shown to up-regulate PPAR-γ and uncouple (UCP) 2, reducing triglyceride concentrations in mouse livers[10]. These findings suggest that BCAAs may have a therapeutic effect on metabolic disorders and/or obesity.
BCAA supplementation was shown to delay the progression of CCl4-induced chronic liver injury in a rat model by reducing hepatic apoptosis[11]. On the other hand, BCAAs promoted hepatocyte regeneration in a rat model of hepatectomy[12]. Moreover, BCAAs were reported to stimulate the production of hepatocyte growth factor[13]. Taken together, these findings indicate that supplementation with BCAAs, by reducing hepatocyte apoptosis and promoting liver regeneration, may result in rapid recovery from liver injury.
BCAAs activate mTOR and subsequently increase the production of eukaryotic initiation factor 4E-binding protein-1 and ribosomal protein S6 kinase, which upregulate the synthesis of albumin[14-16]. Furthermore, leucine stimulates the nuclear importation of polypyrimidine-tract-binding protein, which binds to albumin mRNA and increases its translation[17].
BCAAs were shown to improve homeostasis model assessment scores for insulin resistance (HOMA-IR) and beta cell function (HOMA-%B) in patients with chronic liver disease, indicating that BCAAs can ameliorate insulin resistance[18]. In mice lacking the gene encoding mitochondrial BCAA aminotransferase, an enzyme that catalyzes BCAAs, serum BCAA concentrations were elevated. In those mice, fasting blood glucose and insulin concentrations were decreased and HOMA-IR was significantly lower than in wild-type mice[19]. Furthermore, administration of leucine or isoleucine improved insulin sensitivity in mice with high-fat diets[20,21]. BCAAs were also shown to temporarily increase plasma insulin concentrations in healthy young men, although plasma glucose concentrations were not altered[22].
Several organs are involved in the mechanism by which BCAAs improve insulin resistance. In the liver, BCAAs increase the liver X receptor/sterol regulatory element binding protein-1c pathway and subsequently activate liver-type glucokinase and glucose transporter. Furthermore, BCAAs suppress hepatic expression of glucose-6-phosphatase[23]. In adipose tissue, leucine increases insulin-induced phosphorylation of Akt and mTOR, increasing glucose uptake[24]. In skeletal muscle, BCAAs promote glucose uptake through activation of phosphatidylinositol 3-kinase (PI3K) and protein kinase C and subsequent translocation of glucose transporter to the plasma membrane[25]. In addition, BCAAs increase PPAR-γ and subsequent UCP2 in liver and UCP3 in muscle, stimulating oxidation of free fatty acids. Thus, BCAAs improve insulin resistance through interactions in organs targeted by insulin.
The direct effects of BCAAs on liver cancer cells have been analyzed in culture systems. Increased concentrations of BCAAs in culture medium were reported to suppress the proliferation of HCC cell lines[26]. Moreover, all three BCAAs were found to accelerate insulin-induced vascular endothelial growth factor (VEGF) mRNA degradation at the post transcriptional level, downregulating VEGF expression during the development of HCCs[27]. BCAAs were also shown to induce apoptosis of liver cancer cell lines by inhibiting insulin-induced PI3K/Akt and NFκB pathways through mTORC1- and mTORC2-dependent mechanisms[28]. Moreover, BCAAs may inhibit obesity-related hepatocarcinogenesis by suppressing the stimulatory effect of visfatin, an adipokine with a critical role in HCC proliferation[29].
Insulin was found to induce cell proliferation through activation of the mitogen-activated protein kinase pathway[30], and BCAAs inhibit insulin signals by suppressing the expression of insulin-like growth factor[31]. BCAAs have been reported to decrease insulin resistance-induced expression of endothelial growth factor and to subsequently suppress tumor angiogenesis[32]. Collectively, these data suggest that BCAAs inhibit the proliferation of HCC cells or hepatocarcinogenesis through multiple mechanisms.
Immunity and nutrition are closely associated, and several studies have indicated the importance of BCAAs during lymphocyte proliferation or dendritic cell maturation. Depletion of any of the three BCAAs from the culture medium was shown to markedly inhibit phytohemagglutinin-induced lymphocyte proliferation[33], with removal of valine from the culture medium completely abolishing lymphocyte proliferation. In contrast, increased concentrations of BCAAs in the culture medium did not significantly affect lymphocyte proliferation, indicating that, although the BCAAs are requisite for lymphocyte proliferation, there are optimal concentrations. On the other hand, BCAAs have little effect on macrophage functions.
In vivo studies have also shown the importance of BCAAs for immunity. We previously analyzed the effects of a BCAA-rich diet on immune system functions in the spleen and liver of rats[34]. We found that addition of BCAAs to the diet increased the numbers of intrahepatic lymphocytes and stimulated natural killer (NK) cell activity and lectin-dependent cytotoxic activities in the liver. Interestingly, the number of intrahepatic lymphocytes was positively correlated with valine concentrations in plasma and the liver. BCAAs, especially valine, are also involved in the maturation of dendritic cells. For example, valine was found to dose-dependently increase the allostimulatory capacity of IL-12 production by monocyte-derived dendritic cells (DCs) obtained from both healthy volunteers and cirrhotic patients with chronic hepatitis C virus (HCV) infection[35]. These findings suggest that valine may have therapeutic potential in HCV-infected cirrhotic patients by restoring immune system activities, which may lead to inhibit hepatocarcinogenesis[35,36]. In patients with cirrhosis, BCAA administration increases the numbers of hepatic lymphocytes and restores the phagocytic activity of neutrophils and the NK activity of lymphocytes[37]. In addition, BCAAs increased the number of blood lymphocytes in postsurgical patients[38,39], and significant correlations were observed between the serum concentration of BCAAs and the survival rates of the patients with sepsis[40]. These data indicate that BCAAs are closely associated with the maturation and function of various immune cells.
The liver is a central organ for nutrient metabolism, and patients with chronic liver diseases may develop various metabolic and nutrition disorders[41]. Patients with cirrhosis frequently show protein and energy deficiency. Protein deficiency leads to hypoalbuminemia, inducing ascites and edema, whereas energy deficiency decreases fat and muscle mass and causes muscle weakness, decreasing the quality of life of patients with cirrhosis[42]. Several clinical trials have suggested that BCAA supplementation improves the prognosis of cirrhotic patients[43,44]. For example, a multicenter randomized trial from Italy showed that oral BCAA supplementation in patients with advanced cirrhosis prevented progressive hepatic failure and improved surrogate markers and perceived health status[44]. Furthermore, a large scale post marketing clinical study in Japan showed that oral BCAA administration significantly reduced the occurrence of complications associated with poor prognosis, such as liver failure, ruptured esophageal varices, HCC, and death, compared with patients who received diet therapy with defined daily food intake (HR = 0.67, 95%CI: 0.49-0.93)[43]. Furthermore, BCAA supplementation in patients with advanced cirrhosis may improve abnormal glucose tolerance in addition to improving serum albumin concentration[45], and a randomized study showed that oral BCAA was effective in patients with both compensated and decompensated cirrhosis, maintaining or increasing serum albumin concentrations[46]. Oral BCAA treatment has also been reported to improve protein malnutrition in patients, especially during the early stages of liver cirrhosis, increasing serum albumin level to 3.5-3.9 g/dL and increasing total hepatic parenchymal cell mass[47-49]. BCAA treatment also improved nutritional status and reduced the frequency of albumin infusion in children with end-stage liver disease[50]. Taken together, these findings indicate that BCAA supplementation is effective in improving nutritional status in cirrhotic patients, regardless of patient age or disease stage.
Furthermore, BCAA supplementation was reported to improve the quality of life in cirrhotic patients. Two randomized trials showed that BCAA supplementation improved the Short Form-36 scores of general health perception compared with control groups[43,44]. Another randomized study showed that BCAA-enriched supplements improved weakness and fatigue compared with ordinary foods[51]. BCAA-enriched supplementation has also been reported to improve sleep disturbance[52].
Accelerated fat oxidation and a catabolic state after fasting, represented as a decreased respiratory quotient (RQ), are frequently observed in patients with cirrhosis[53]. Late evening snack supplementation with a BCAA mixture was found to improve RQ, nutritional state and glucose intolerance[53,54]. The energy efficiency of BCAAs is higher than that of glucose or fatty acids, suggesting that BCAAs may be the preferred energy substrate for patients with cirrhosis[55]. Others also reported that late evening snacks with BCAAs were useful in improving protein metabolism and lipolysis in cirrhotic patients[56].
Thus, BCAA supplementation for advanced cirrhotic patients improves nutritional status and quality of life. The guidelines of the European Society for Clinical Nutrition and Metabolism and the Study Group for the Standardization of Treatment of Viral Hepatitis Including Cirrhosis of the Ministry of Health, Labour and Welfare of Japan recommend BCAA supplementation in the treatment of patients with advanced cirrhosis[57,58].
HE is a major complication of cirrhosis associated with poor prognosis and quality of life, and often occurs repeatedly. Elevated blood ammonia is seen in patients with HE, and ammonia is one of the pathogenic factors for the development of HE[59]. Unfortunately, infusion of BCAAs was reported to increase venous blood ammonia in most patients with liver failure[60]. Thus, the effects of BCAAs on HE may not be associated with blood ammonia levels, especially when administered intravenously. HE may also be caused by a decreased plasma ratio of BCAAs to AAAs. In patients with advanced cirrhosis, HE frequently occurs after gastrointestinal bleeding, perhaps due to an absence of isoleucine and an abundance of leucine in hemoglobin molecules, leading to HE by way of BCAA antagonism[61]. Treatment with BCAAs may therefore have a beneficial effect on patients with hepatic encephalopathy mainly by compensating decreased ratio of BCAAs to AAAs, but not by reducing serum ammonia levels. A systematic review reported that BCAAs appeared to have a modest effect in improving encephalopathy without adverse events, although convincing evidence was not supplied[62]. Two randomized studies also showed that BCAAs did not clearly prevent HE in patients with advanced cirrhosis, although BCAAs prevented the progression of hepatic failure[43,44]. Furthermore, postoperative BCAA treatment could not prevent postoperative hepatic encephalopathy[63]. A recent randomized, double-blind, multicenter study evaluating the effect of BCAAs on HE found that BCAAs did not decrease the recurrence of HE but improved minimal HE and muscle mass[64]. Moreover, a systematic review showed that oral (RR = 1.44; 95%CI: 1.07-1.94) but not intravenous (RR=1.12; 95%CI: 0.91-1.39) administration of BCAAs improved HE manifestations[65]. Non-absorbable disaccharides such as lactulose or lactitol also improved the manifestations of HE (RR = 1.99; 95%CI: 1.14-3.48) and prevented clinically overt HE (RR = 0.26; 95%CI: 0.17-0.41), suggesting that non-absorbable disaccharides be used as the first line treatment of HE and BCAAs may be considered as a second line treatment[65].
Recently, a systematic review with meta-analyses on the effect of oral BCAAs for the treatment of HE was published[66]. The review has revealed that supplementation of oral BCAAs in cirrhotic patients inhibits the manifestation of HE, especially in patients with overt HE rather than those with minimal HE, but showed no effect on the survival of those patients[66]. Thus, oral administration of BCAAs is the treatment of choice in cirrhotic patients with HE, especially in combination with non-absorbable disaccharides.
Clinical studies have suggested that BCAA supplementation can help in the management of HCC. Prolonged surgical stress and advanced malignancy can result in systemic catabolism and muscle wasting, with BCAA supplementation having the potential to improve these conditions[67].
A randomized control trial in obese, HCV-infected patients with cirrhosis showed that BCAA supplementation reduced the frequency of development of HCC, by approximately 30% over 3 years[68]. In addition, a second randomized trial in patients with compensated liver cirrhosis due to HCV showed that oral BCAAs reduced the incidence of HCC (15.8% vs 25.0%)[69]. A retrospective analysis in patients with cirrhosis showed that the incidence of HCC was significantly lower in patients who did than did not receive BCAAs (HR = 0.416, 95%CI: 0.216-0.800, P = 0.0085) [70]. Furthermore, combinations of BCAAs and angiotensin-converting enzyme inhibitors may prevent the development of HCC in patients with insulin resistance[71].
Perioperative nutritional support, especially enteral rather than parental nutrition, was found to improve the prognosis of cirrhotic patients by reducing complications following hepatectomy[72,73]. Recently, a randomized trial showed that BCAA supplementation after hepatectomy promoted rapid improvement in protein metabolism and inhibited progression to liver cirrhosis[74]. Furthermore, another randomized trial showed that oral BCAA supplementation after hepatectomy for HCC significantly reduced the 30 month recurrence of HCC (28.5% vs 55.7%, P = 0.044)[75]. Perioperative BCAA treatment in patients undergoing hepatectomy was also shown to contribute to shorter hospital stay and quicker improvement of liver function during the early postoperative period[76] and to improve postoperative quality of life by restoring and maintaining nutritional status and whole-body kinetics[77].
The effect of BCAAs on HCC recurrence after radiofrequency ablation (RFA) remains unclear. Two prospective studies showed that BCAA supplementation improved nutritional state and liver function, but its effect on HCC recurrence was not determined[78,79]. However, a recent retrospective study showed that oral BCAA supplementation after RFA improved 1 year (61.8% vs 52.0%) and 3-year (28.0% vs 12.0%) progression-free survival rates compared with a control group after RFA (P = 0.013) [80].
Oral BCAA supplementation after chemoembolization also prevents the decrease of liver function after treatment and improves the quality of life, although its ability to prevent HCC recurrence was not determined[81,82]. Oral BCAA treatment before chemoembolization was found useful in maintaining hepatic functional reserve[83]. A randomized trial also found that oral BCAA supplementation improved nutritional status by increasing BCAA concentration during radiotherapy for HCC[84].
Thus, BCAA supplementation for patients with HCC is of clinical importance in the preservation of liver function and quality of life during treatment, although it is unclear whether BCCAs directly prevent HCC.
Although BCAAs have no proven benefit in patients with acute liver injury, enteric nutritional support is essential[85]. Several animal studies have shown that BCAAs may prevent acute liver injury[86-88], although its effects in humans are as yet undetermined. BCAA concentrations have been reported to be increased, unaltered or decreased following acute liver injury[89,90]. In alcoholic hepatitis, parentally or enterally administered hyperalimentation with or without BCAAs did not show survival benefits[91].
Insulin resistance occurs frequently in patients infected with HCV and is associated with various complications, such as steatosis, disturbances in glucose metabolism, and carcinogenesis[92]. BCAAs, especially leucine or isoleucine, have been shown to have beneficial effects on glucose metabolism[93]. A randomized study showed that BCAA treatment of patients with chronic hepatitis C and insulin resistance improved HbA1c concentrations in patients with marked peripheral insulin resistance, although BCAA did not significantly affect parameters of glucose metabolism or lipid profiles[94]. A multicenter randomized control trial showed that BCAAs prevented the development of HCC in obese, HCV-infected patients[68]. Furthermore, BCAA treatment can restore impaired interferon signaling caused by malnutrition through the mTOR and FoxO pathways in patients with chronic hepatitis C[95]. Interestingly, valine was reported to reduce HCV viral load, possibly by enhancing DC function or interferon signaling[96]. Thus, BCAA supplementation may be useful for adherence to interferon therapy in patients with chronic hepatitis C and may enhance the effects of interferon in these patients[97].
Protein-energy malnutrition is commonly found in patients with end-stage liver disease requiring liver transplantation and is a risk factor for posttransplant morbidity. A report of 50 recipients undergoing living donor liver transplantation (LDLT) showed that absence of preoperative BCAA treatment was an independent risk factor for postoperative severe infection and in-hospital death[98]. Kawamura et al[99] reported that early interventional oral BCAAs might prolong the liver transplant waiting period by preserving hepatic reserve in patients with cirrhosis. A retrospective analysis also showed that BCAA treatment before LDLT may reduce the incidence of posttransplant bacteremia[100].
Insulin resistance: Increased insulin resistance is found in patients with chronic liver diseases and is a therapeutic target associated with malnutrition and hepatocarcinogenesis. BCAAs are thought to act on insulin target organs, such as skeletal muscles, adipose tissue, and the liver[101]. BCAA infusion was reported to decrease plasma glucose concentrations in patients with advanced liver cirrhosis[102], and oral BCAA administration was recently shown to reduce both blood glucose concentrations[103,104] and insulin resistance in patients with chronic liver diseases, especially in men[19,105]. More recently, long-term BCAA supplementation was shown to improve glucose tolerance in patients with nonalcoholic steatohepatitis (NASH)-related cirrhosis, and may be an alternative treatment for NASH[106].
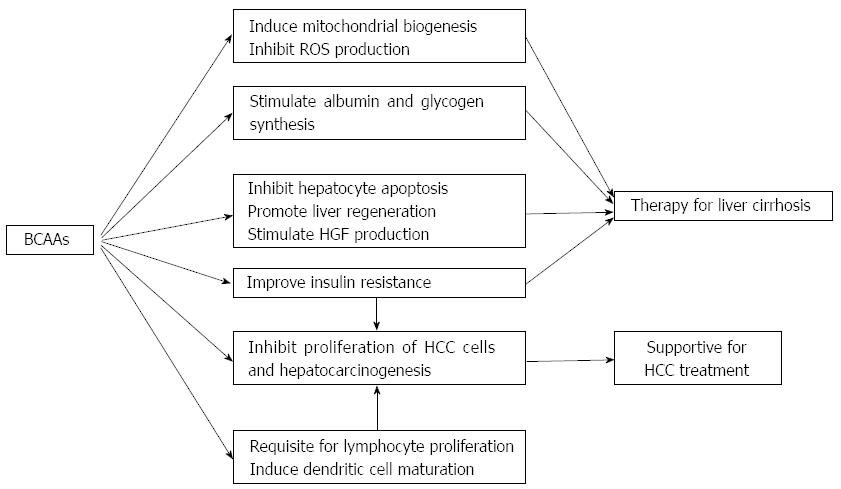
BCAAs are involved in various biological activities (Figure 1), and prospective randomized clinical trials showing possible effectiveness of BCAAs in the management of chronic liver diseases are summarized in Table 1. Supplementation with BCAAs may be a promising therapeutic option for patients with chronic liver diseases, although more analyses are needed to determine their basic mechanisms of action.
| Object | Time | No. | Major outcome | Ref. |
| Cirrhosis | 2 yr | 646 | Improve event-free survival and QOL. Increase serum albumin levels. | [43] |
| Cirrhosis (advanced) | 1 yr | 174 | Improve event-free survival. Lower hospital admission. Improve the Child-Pugh score and QOL. | [44] |
| Cirrhosis (decompensated) | 24 wk | 281 | Increase serum albumin levels. | [45] |
| Cirrhosis | 2 yr | 65 | Maintain serum albumin levels. | [46] |
| Cirrhosis (early) | 2 yr | 65 | Maintain serum albumin levels. | [49] |
| Cirrhosis | 3 mo | 48 | Increase serum albumin levels. Improve energy metabolism. | [51] |
| Cirrhosis (HCV) | 168 wk | 39 | Reduce hepatic carcinogenesis in patients with compensated cirrhosis with a serum albumin level of < 4.0 g/dL. | [69] |
| Cirrhosis (HCV, obese) | 2 yr | 622 | Reduce hepatic carcinogenesis in patients with BMI of 25 or higher and with an alpha-fetoprotein level of 20 ng/mL or higher. | [68] |
| Cirrhosis (pre liver transplant) | 3.3 yr | 50 | Preserve hepatic reserve functions. Lower complications associated with cirrhosis. | [99] |
| Cirrhosis after an episode of HE | 56 wk | 116 | Not decrease recurrence of HE. Improve minimal HE and muscle mass. | [64] |
| Cirrhosis after hepatectomy | 1 yr | 43 | Improve hepatic metabolism after hepatectomy. Inhibit progression to cirrhosis. | [74] |
| HCC after hepatectomy | 6.5 mo | 56 | Reduce early recurrence of HCC. | [75] |
| HCC after hepatectomy | 12 wk | 44 | Shorten hospital stay. Quicker improvement of liver functions. | [76] |
| After hepatectomy | 12 mo | 76 | Improve post operative QOL. | [77] |
| HCC after RFA | 12 mo | 35 | Improve nutritional state and QOL. | [78] |
| HCC after RFA | 12 wk | 30 | Improve liver functions. | [79] |
| HCC undergoing chemoembolization | 12 mo | 84 | Increase serum albumin levels, reduce morbidity, and improve QOL. | [81] |
| HCC undergoing chemoembolization | 2 wk | 56 | Prevent reduction of liver functions. | [82] |
| HCC during radiotherapy | 10 wk | 30 | Increase serum albumin levels. | [84] |
P- Reviewers: Faintuch J, Loguercio C, Ramsay M S- Editor: Zhai HH L- Editor: A E- Editor: Wang CH
| 1. | Campollo O, Sprengers D, McIntyre N. The BCAA/AAA ratio of plasma amino acids in three different groups of cirrhotics. Rev Invest Clin. 1992;44:513-518. [PubMed] |
| 2. | Steigmann F, Szanto PB, Poulos A, Lim PE, Dubin A. Significance of serum aminograms in diagnosis and prognosis of liver diseases. J Clin Gastroenterol. 1984;6:453-460. [PubMed] |
| 3. | Watanabe A, Higashi T, Sakata T, Nagashima H. Serum amino acid levels in patients with hepatocellular carcinoma. Cancer. 1984;54:1875-1882. [PubMed] |
| 4. | Suzuki K, Suzuki K, Koizumi K, Ichimura H, Oka S, Takada H, Kuwayama H. Measurement of serum branched-chain amino acids to tyrosine ratio level is useful in a prediction of a change of serum albumin level in chronic liver disease. Hepatol Res. 2008;38:267-272. [PubMed] |
| 5. | D’Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, Caliaro F, Corsetti G, Bottinelli R, Carruba MO. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010;12:362-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 424] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 6. | Ichikawa K, Okabayashi T, Shima Y, Iiyama T, Takezaki Y, Munekage M, Namikawa T, Sugimoto T, Kobayashi M, Mimura T. Branched-chain amino acid-enriched nutrients stimulate antioxidant DNA repair in a rat model of liver injury induced by carbon tetrachloride. Mol Biol Rep. 2012;39:10803-10810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Nishitani S, Ijichi C, Takehana K, Fujitani S, Sonaka I. Pharmacological activities of branched-chain amino acids: specificity of tissue and signal transduction. Biochem Biophys Res Commun. 2004;313:387-389. [PubMed] |
| 8. | Schieke SM, Phillips D, McCoy JP, Aponte AM, Shen RF, Balaban RS, Finkel T. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem. 2006;281:27643-27652. [PubMed] |
| 9. | Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736-740. [PubMed] |
| 10. | Arakawa M, Masaki T, Nishimura J, Seike M, Yoshimatsu H. The effects of branched-chain amino acid granules on the accumulation of tissue triglycerides and uncoupling proteins in diet-induced obese mice. Endocr J. 2011;58:161-170. [PubMed] |
| 11. | Kuwahata M, Kubota H, Kanouchi H, Ito S, Ogawa A, Kobayashi Y, Kido Y. Supplementation with branched-chain amino acids attenuates hepatic apoptosis in rats with chronic liver disease. Nutr Res. 2012;32:522-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Kim SJ, Kim DG, Lee MD. Effects of branched-chain amino acid infusions on liver regeneration and plasma amino acid patterns in partially hepatectomized rats. Hepatogastroenterology. 2011;58:1280-1285. [PubMed] |
| 13. | Tomiya T, Omata M, Fujiwara K. Significance of branched chain amino acids as possible stimulators of hepatocyte growth factor. Biochem Biophys Res Commun. 2004;313:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Okuno M, Moriwaki H, Kato M, Muto Y, Kojima S. Changes in the ratio of branched-chain to aromatic amino acids affect the secretion of albumin in cultured rat hepatocytes. Biochem Biophys Res Commun. 1995;214:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Ijichi C, Matsumura T, Tsuji T, Eto Y. Branched-chain amino acids promote albumin synthesis in rat primary hepatocytes through the mTOR signal transduction system. Biochem Biophys Res Commun. 2003;303:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Montoya A, Gómez-Lechón MJ, Castell JV. Influence of branched-chain amino acid composition of culture media on the synthesis of plasma proteins by serum-free cultured rat hepatocytes. In Vitro Cell Dev Biol. 1989;25:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Kuwahata M, Yoshimura T, Sawai Y, Amano S, Tomoe Y, Segawa H, Tatsumi S, Ito M, Ishizaki S, Ijichi C. Localization of polypyrimidine-tract-binding protein is involved in the regulation of albumin synthesis by branched-chain amino acids in HepG2 cells. J Nutr Biochem. 2008;19:438-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Kawaguchi T, Nagao Y, Matsuoka H, Ide T, Sata M. Branched-chain amino acid-enriched supplementation improves insulin resistance in patients with chronic liver disease. Int J Mol Med. 2008;22:105-112. [PubMed] |
| 19. | She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007;6:181-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 294] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 20. | Ikehara O, Kawasaki N, Maezono K, Komatsu M, Konishi A. Acute and chronic treatment of L-isoleucine ameliorates glucose metabolism in glucose-intolerant and diabetic mice. Biol Pharm Bull. 2008;31:469-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56:1647-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 409] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 22. | Zhang Y, Kobayashi H, Mawatari K, Sato J, Bajotto G, Kitaura Y, Shimomura Y. Effects of branched-chain amino acid supplementation on plasma concentrations of free amino acids, insulin, and energy substrates in young men. J Nutr Sci Vitaminol (Tokyo). 2011;57:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Higuchi N, Kato M, Miyazaki M, Tanaka M, Kohjima M, Ito T, Nakamuta M, Enjoji M, Kotoh K, Takayanagi R. Potential role of branched-chain amino acids in glucose metabolism through the accelerated induction of the glucose-sensing apparatus in the liver. J Cell Biochem. 2011;112:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Hinault C, Mothe-Satney I, Gautier N, Lawrence JC, Van Obberghen E. Amino acids and leucine allow insulin activation of the PKB/mTOR pathway in normal adipocytes treated with wortmannin and in adipocytes from db/db mice. FASEB J. 2004;18:1894-1896. [PubMed] |
| 25. | Nishitani S, Matsumura T, Fujitani S, Sonaka I, Miura Y, Yagasaki K. Leucine promotes glucose uptake in skeletal muscles of rats. Biochem Biophys Res Commun. 2002;299:693-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Sugiyama K, Yu L, Nagasue N. Direct effect of branched-chain amino acids on the growth and metabolism of cultured human hepatocellular carcinoma cells. Nutr Cancer. 1998;31:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Miuma S, Ichikawa T, Arima K, Takeshita S, Muraoka T, Matsuzaki T, Ootani M, Shibata H, Akiyama M, Ozawa E. Branched-chain amino acid deficiency stabilizes insulin-induced vascular endothelial growth factor mRNA in hepatocellular carcinoma cells. J Cell Biochem. 2012;113:3113-3121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Hagiwara A, Nishiyama M, Ishizaki S. Branched-chain amino acids prevent insulin-induced hepatic tumor cell proliferation by inducing apoptosis through mTORC1 and mTORC2-dependent mechanisms. J Cell Physiol. 2012;227:2097-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Ninomiya S, Shimizu M, Imai K, Takai K, Shiraki M, Hara T, Tsurumi H, Ishizaki S, Moriwaki H. Possible role of visfatin in hepatoma progression and the effects of branched-chain amino acids on visfatin-induced proliferation in human hepatoma cells. Cancer Prev Res (Phila). 2011;4:2092-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Formisano P, Oriente F, Fiory F, Caruso M, Miele C, Maitan MA, Andreozzi F, Vigliotta G, Condorelli G, Beguinot F. Insulin-activated protein kinase Cbeta bypasses Ras and stimulates mitogen-activated protein kinase activity and cell proliferation in muscle cells. Mol Cell Biol. 2000;20:6323-6333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Iwasa J, Shimizu M, Shiraki M, Shirakami Y, Sakai H, Terakura Y, Takai K, Tsurumi H, Tanaka T, Moriwaki H. Dietary supplementation with branched-chain amino acids suppresses diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BL/KsJ-db/db mice. Cancer Sci. 2010;101:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Yoshiji H, Noguchi R, Kaji K, Ikenaka Y, Shirai Y, Namisaki T, Kitade M, Tsujimoto T, Kawaratani H, Fukui H. Attenuation of insulin-resistance-based hepatocarcinogenesis and angiogenesis by combined treatment with branched-chain amino acids and angiotensin-converting enzyme inhibitor in obese diabetic rats. J Gastroenterol. 2010;45:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Chuang JC, Yu CL, Wang SR. Modulation of lymphocyte proliferation by enzymes that degrade amino acids. Clin Exp Immunol. 1990;82:469-472. [PubMed] |
| 34. | Tsukishiro T, Shimizu Y, Higuchi K, Watanabe A. Effect of branched-chain amino acids on the composition and cytolytic activity of liver-associated lymphocytes in rats. J Gastroenterol Hepatol. 2000;15:849-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Kakazu E, Kanno N, Ueno Y, Shimosegawa T. Extracellular branched-chain amino acids, especially valine, regulate maturation and function of monocyte-derived dendritic cells. J Immunol. 2007;179:7137-7146. [PubMed] |
| 36. | Kakazu E, Ueno Y, Kondo Y, Fukushima K, Shiina M, Inoue J, Tamai K, Ninomiya M, Shimosegawa T. Branched chain amino acids enhance the maturation and function of myeloid dendritic cells ex vivo in patients with advanced cirrhosis. Hepatology. 2009;50:1936-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Nakamura I, Ochiai K, Imai Y, Moriyasu F, Imawari M. Restoration of innate host defense responses by oral supplementation of branched-chain amino acids in decompensated cirrhotic patients. Hepatol Res. 2007;37:1062-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Nuwer N, Cerra FB, Shronts EP, Lysne J, Teasley KM, Konstantinides FN. Does modified amino acid total parenteral nutrition alter immune-response in high level surgical stress. JPEN J Parenter Enteral Nutr. 1983;7:521-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Cerra FB, Mazuski JE, Chute E, Nuwer N, Teasley K, Lysne J, Shronts EP, Konstantinides FN. Branched chain metabolic support. A prospective, randomized, double-blind trial in surgical stress. Ann Surg. 1984;199:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 112] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | De Bandt JP, Cynober L. Therapeutic use of branched-chain amino acids in burn, trauma, and sepsis. J Nutr. 2006;136:308S-313S. [PubMed] |
| 41. | Charlton MR. Protein metabolism and liver disease. Baillieres Clin Endocrinol Metab. 1996;10:617-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Miwa Y, Moriwaki H. Nocturnal energy and BCAA supplementation in patients with liver cirrhosis. Hepatol Res. 2004;30S:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 364] [Article Influence: 18.2] [Reference Citation Analysis (1)] |
| 44. | Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, Rossi Fanelli F, Abbiati R. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 392] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 45. | Sato S, Watanabe A, Muto Y, Suzuki K, Kato A, Moriwaki H, Kato M, Nakamura T. Clinical comparison of branched-chain amino acid (l-Leucine, l-Isoleucine, l-Valine) granules and oral nutrition for hepatic insufficiency in patients with decompensated liver cirrhosis (LIV-EN study). Hepatol Res. 2005;31:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Habu D, Nishiguchi S, Nakatani S, Lee C, Enomoto M, Tamori A, Takeda T, Ohfuji S, Fukushima W, Tanaka T. Comparison of the effect of BCAA granules on between decompensated and compensated cirrhosis. Hepatogastroenterology. 2009;56:1719-1723. [PubMed] |
| 47. | Koreeda C, Seki T, Okazaki K, Ha-Kawa SK, Sawada S. Effects of late evening snack including branched-chain amino acid on the function of hepatic parenchymal cells in patients with liver cirrhosis. Hepatol Res. 2011;41:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Habu D, Nishiguchi S, Nakatani S, Kawamura E, Lee C, Enomoto M, Tamori A, Takeda T, Tanaka T, Shiomi S. Effect of oral supplementation with branched-chain amino acid granules on serum albumin level in the early stage of cirrhosis: a randomized pilot trial. Hepatol Res. 2003;25:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Nishiguchi S, Habu D. Effect of oral supplementation with branched-chain amino acid granules in the early stage of cirrhosis. Hepatol Res. 2004;30S:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Chin SE, Shepherd RW, Thomas BJ, Cleghorn GJ, Patrick MK, Wilcox JA, Ong TH, Lynch SV, Strong R. Nutritional support in children with end-stage liver disease: a randomized crossover trial of a branched-chain amino acid supplement. Am J Clin Nutr. 1992;56:158-163. [PubMed] |
| 51. | Nakaya Y, Okita K, Suzuki K, Moriwaki H, Kato A, Miwa Y, Shiraishi K, Okuda H, Onji M, Kanazawa H. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition. 2007;23:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 52. | Ichikawa T, Naota T, Miyaaki H, Miuma S, Isomoto H, Takeshima F, Nakao K. Effect of an oral branched chain amino acid-enriched snack in cirrhotic patients with sleep disturbance. Hepatol Res. 2010;40:971-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Nakaya Y, Harada N, Kakui S, Okada K, Takahashi A, Inoi J, Ito S. Severe catabolic state after prolonged fasting in cirrhotic patients: effect of oral branched-chain amino-acid-enriched nutrient mixture. J Gastroenterol. 2002;37:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 54. | Tsuchiya M, Sakaida I, Okamoto M, Okita K. The effect of a late evening snack in patients with liver cirrhosis. Hepatol Res. 2005;31:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Kato M, Miwa Y, Tajika M, Hiraoka T, Muto Y, Moriwaki H. Preferential use of branched-chain amino acids as an energy substrate in patients with liver cirrhosis. Intern Med. 1998;37:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Yamauchi M, Takeda K, Sakamoto K, Ohata M, Toda G. Effect of oral branched chain amino acid supplementation in the late evening on the nutritional state of patients with liver cirrhosis. Hepatol Res. 2001;21:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J, Ferenci P, Holm E, Vom Dahl S, Müller MJ. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr. 2006;25:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 404] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 58. | Kumada H, Okanoue T, Onji M, Moriwaki H, Izumi N, Tanaka E, Chayama K, Sakisaka S, Takehara T, Oketani M. Guidelines for the treatment of chronic hepatitis and cirrhosis due to hepatitis C virus infection for the fiscal year 2008 in Japan. Hepatol Res. 2010;40:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 59. | Bak LK, Iversen P, Sørensen M, Keiding S, Vilstrup H, Ott P, Waagepetersen HS, Schousboe A. Metabolic fate of isoleucine in a rat model of hepatic encephalopathy and in cultured neural cells exposed to ammonia. Metab Brain Dis. 2009;24:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Nishikawa Y, Ukida M, Matsuo R, Morimoto Y, Omori N, Mikami M, Tsuji T. Administration of a branched-chain amino acid preparation during hepatic failure: a study emphasizing ammonia metabolism. Acta Med Okayama. 1994;48:25-30. [PubMed] |
| 61. | Plauth M, Schütz T. Branched-chain amino acids in liver disease: new aspects of long known phenomena. Curr Opin Clin Nutr Metab Care. 2011;14:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Als-Nielsen B, Koretz RL, Kjaergard LL, Gluud C. Branched-chain amino acids for hepatic encephalopathy. Cochrane Database Syst Rev. 2003;CD001939. [PubMed] |
| 63. | Kanematsu T, Koyanagi N, Matsumata T, Kitano S, Takenaka K, Sugimachi K. Lack of preventive effect of branched-chain amino acid solution on postoperative hepatic encephalopathy in patients with cirrhosis: a randomized, prospective trial. Surgery. 1988;104:482-488. [PubMed] |
| 64. | Les I, Doval E, García-Martínez R, Planas M, Cárdenas G, Gómez P, Flavià M, Jacas C, Mínguez B, Vergara M. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol. 2011;106:1081-1088. [PubMed] |
| 65. | Gluud LL, Dam G, Borre M, Les I, Cordoba J, Marchesini G, Aagaard NK, Vilstrup H. Lactulose, rifaximin or branched chain amino acids for hepatic encephalopathy: what is the evidence? Metab Brain Dis. 2013;28:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 66. | Gluud LL, Dam G, Borre M, Les I, Cordoba J, Marchesini G, Aagaard NK, Risum N, Vilstrup H. Oral branched-chain amino acids have a beneficial effect on manifestations of hepatic encephalopathy in a systematic review with meta-analyses of randomized controlled trials. J Nutr. 2013;143:1263-1268. [PubMed] |
| 67. | Choudry HA, Pan M, Karinch AM, Souba WW. Branched-chain amino acid-enriched nutritional support in surgical and cancer patients. J Nutr. 2006;136:314S-318S. [PubMed] |
| 68. | Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol Res. 2006;35:204-214. [PubMed] |
| 69. | Kobayashi M, Ikeda K, Arase Y, Suzuki Y, Suzuki F, Akuta N, Hosaka T, Murashima N, Saitoh S, Someya T. Inhibitory effect of branched-chain amino acid granules on progression of compensated liver cirrhosis due to hepatitis C virus. J Gastroenterol. 2008;43:63-70. [PubMed] |
| 70. | Hayaishi S, Chung H, Kudo M, Ishikawa E, Takita M, Ueda T, Kitai S, Inoue T, Yada N, Hagiwara S. Oral branched-chain amino acid granules reduce the incidence of hepatocellular carcinoma and improve event-free survival in patients with liver cirrhosis. Dig Dis. 2011;29:326-332. [PubMed] |
| 71. | Yoshiji H, Noguchi R, Ikenaka Y, Kaji K, Aihara Y, Yamazaki M, Yamao J, Toyohara M, Mitoro A, Sawai M. Combination of branched-chain amino acids and angiotensin-converting enzyme inhibitor suppresses the cumulative recurrence of hepatocellular carcinoma: a randomized control trial. Oncol Rep. 2011;26:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Fan ST, Lo CM, Lai EC, Chu KM, Liu CL, Wong J. Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. N Engl J Med. 1994;331:1547-1552. [PubMed] |
| 73. | Richter B, Schmandra TC, Golling M, Bechstein WO. Nutritional support after open liver resection: a systematic review. Dig Surg. 2006;23:139-145. [PubMed] |
| 74. | Togo S, Tanaka K, Morioka D, Sugita M, Ueda M, Miura Y, Kubota T, Nagano Y, Matsuo K, Endo I. Usefulness of granular BCAA after hepatectomy for liver cancer complicated with liver cirrhosis. Nutrition. 2005;21:480-486. [PubMed] |
| 75. | Ichikawa K, Okabayashi T, Maeda H, Namikawa T, Iiyama T, Sugimoto T, Kobayashi M, Mimura T, Hanazaki K. Oral supplementation of branched-chain amino acids reduces early recurrence after hepatic resection in patients with hepatocellular carcinoma: a prospective study. Surg Today. 2013;43:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 76. | Meng WC, Leung KL, Ho RL, Leung TW, Lau WY. Prospective randomized control study on the effect of branched-chain amino acids in patients with liver resection for hepatocellular carcinoma. Aust N Z J Surg. 1999;69:811-815. [PubMed] |
| 77. | Okabayashi T, Iyoki M, Sugimoto T, Kobayashi M, Hanazaki K. Oral supplementation with carbohydrate- and branched-chain amino acid-enriched nutrients improves postoperative quality of life in patients undergoing hepatic resection. Amino Acids. 2011;40:1213-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 78. | Kuroda H, Ushio A, Miyamoto Y, Sawara K, Oikawa K, Kasai K, Endo R, Takikawa Y, Kato A, Suzuki K. Effects of branched-chain amino acid-enriched nutrient for patients with hepatocellular carcinoma following radiofrequency ablation: a one-year prospective trial. J Gastroenterol Hepatol. 2010;25:1550-1555. [PubMed] |
| 79. | Morihara D, Iwata K, Hanano T, Kunimoto H, Kuno S, Fukunaga A, Yotsumoto K, Takata K, Tanaka T, Sakurai K. Late-evening snack with branched-chain amino acids improves liver function after radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2012;42:658-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 80. | Nishikawa H, Osaki Y, Iguchi E, Koshikawa Y, Ako S, Inuzuka T, Takeda H, Nakajima J, Matsuda F, Sakamoto A. The effect of long-term supplementation with branched-chain amino acid granules in patients with hepatitis C virus-related hepatocellular carcinoma after radiofrequency thermal ablation. J Clin Gastroenterol. 2013;47:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 81. | Poon RT, Yu WC, Fan ST, Wong J. Long-term oral branched chain amino acids in patients undergoing chemoembolization for hepatocellular carcinoma: a randomized trial. Aliment Pharmacol Ther. 2004;19:779-788. [PubMed] |
| 82. | Takeshita S, Ichikawa T, Nakao K, Miyaaki H, Shibata H, Matsuzaki T, Muraoka T, Honda T, Otani M, Akiyama M. A snack enriched with oral branched-chain amino acids prevents a fall in albumin in patients with liver cirrhosis undergoing chemoembolization for hepatocellular carcinoma. Nutr Res. 2009;29:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 83. | Nishikawa H, Osaki Y, Inuzuka T, Takeda H, Nakajima J, Matsuda F, Henmi S, Sakamoto A, Ishikawa T, Saito S. Branched-chain amino acid treatment before transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2012;18:1379-1384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (2)] |
| 84. | Lee IJ, Seong J, Bae JI, You SH, Rhee Y, Lee JH. Effect of Oral Supplementation with Branched-chain Amino Acid (BCAA) during Radiotherapy in Patients with Hepatocellular Carcinoma: A Double-Blind Randomized Study. Cancer Res Treat. 2011;43:24-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 85. | Charlton M. Branched-chain amino acid enriched supplements as therapy for liver disease. J Nutr. 2006;136:295S-298S. [PubMed] |
| 86. | Kitagawa T, Yokoyama Y, Kokuryo T, Nagino M. Protective effects of branched-chain amino acids on hepatic ischemia-reperfusion-induced liver injury in rats: a direct attenuation of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2013;304:G346-G355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 87. | Deshpande G, Adachi N, Liu K, Motoki A, Mitsuyo T, Nagaro T, Arai T. Recovery of brain dopamine metabolism by branched-chain amino acids in rats with acute hepatic failure. J Neurosurg Anesthesiol. 2007;19:243-248. [PubMed] |
| 88. | Kuwahata M, Kuramoto Y, Tomoe Y, Sugata E, Segawa H, Ito M, Oka T, Miyamoto K. Posttranscriptional regulation of albumin gene expression by branched-chain amino acids in rats with acute liver injury. Biochim Biophys Acta. 2004;1739:62-69. [PubMed] |
| 89. | Record CO, Buxton B, Chase RA, Curzon G, Murray-Lyon IM, Williams R. Plasma and brain amino acids in fulminant hepatic failure and their relationship to hepatic encephalopathy. Eur J Clin Invest. 1976;6:387-394. [PubMed] |
| 90. | Rosen HM, Yoshimura N, Hodgman JM, Fischer JE. Plasma amino acid patterns in hepatic encephalopathy of differing etiology. Gastroenterology. 1977;72:483-487. [PubMed] |
| 91. | O’Shea RS, McCullough AJ. Treatment of alcoholic hepatitis. Clin Liver Dis. 2005;9:103-134. [PubMed] |
| 92. | Arrese M, Riquelme A, Soza A. Insulin resistance, hepatic steatosis and hepatitis C: a complex relationship with relevant clinical implications. Ann Hepatol. 2010;9 Suppl:112-118. [PubMed] |
| 93. | Kawaguchi T, Yamagishi S, Sata M. Branched-chain amino acids and pigment epithelium-derived factor: novel therapeutic agents for hepatitis c virus-associated insulin resistance. Curr Med Chem. 2009;16:4843-4857. [PubMed] |
| 94. | Takeshita Y, Takamura T, Kita Y, Ando H, Ueda T, Kato K, Misu H, Sunagozaka H, Sakai Y, Yamashita T. Beneficial effect of branched-chain amino acid supplementation on glycemic control in chronic hepatitis C patients with insulin resistance: implications for type 2 diabetes. Metabolism. 2012;61:1388-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 95. | Honda M, Takehana K, Sakai A, Tagata Y, Shirasaki T, Nishitani S, Muramatsu T, Yamashita T, Nakamoto Y, Mizukoshi E. Malnutrition impairs interferon signaling through mTOR and FoxO pathways in patients with chronic hepatitis C. Gastroenterology. 2011;141:128-140, 140.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 96. | Kawaguchi T, Torimura T, Takata A, Satomi S, Sata M. Valine, a branched-chain amino Acid, reduced HCV viral load and led to eradication of HCV by interferon therapy in a decompensated cirrhotic patient. Case Rep Gastroenterol. 2012;6:660-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 97. | Nagao Y, Kawaguchi T, Ide T, Sata M. Effect of branched-chain amino acid-enriched nutritional supplementation on interferon therapy in Japanese patients with chronic hepatitis C virus infection: a retrospective study. Virol J. 2012;9:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 98. | Kaido T, Mori A, Oike F, Mizumoto M, Ogura Y, Hata K, Yoshizawa A, Iida T, Uemoto S. Impact of pretransplant nutritional status in patients undergoing liver transplantation. Hepatogastroenterology. 2010;57:1489-1492. [PubMed] |
| 99. | Kawamura E, Habu D, Morikawa H, Enomoto M, Kawabe J, Tamori A, Sakaguchi H, Saeki S, Kawada N, Shiomi S. A randomized pilot trial of oral branched-chain amino acids in early cirrhosis: validation using prognostic markers for pre-liver transplant status. Liver Transpl. 2009;15:790-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Shirabe K, Yoshimatsu M, Motomura T, Takeishi K, Toshima T, Muto J, Matono R, Taketomi A, Uchiyama H, Maehara Y. Beneficial effects of supplementation with branched-chain amino acids on postoperative bacteremia in living donor liver transplant recipients. Liver Transpl. 2011;17:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 101. | Kawaguchi T, Izumi N, Charlton MR, Sata M. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology. 2011;54:1063-1070. [PubMed] |
| 102. | Tabaru A, Shirohara H, Moriyama A, Otsuki M. Effects of branched-chain-enriched amino acid solution on insulin and glucagon secretion and blood glucose level in liver cirrhosis. Scand J Gastroenterol. 1998;33:853-859. [PubMed] |
| 103. | Korenaga K, Korenaga M, Uchida K, Yamasaki T, Sakaida I. Effects of a late evening snack combined with alpha-glucosidase inhibitor on liver cirrhosis. Hepatol Res. 2008;38:1087-1097. [PubMed] |
| 104. | Sakaida I, Tsuchiya M, Okamoto M, Okita K. Late evening snack and the change of blood glucose level in patients with liver cirrhosis. Hepatol Res. 2004;30S:67-72. [PubMed] |
| 105. | Kawaguchi T, Taniguchi E, Itou M, Sumie S, Oriishi T, Matsuoka H, Nagao Y, Sata M. Branched-chain amino acids improve insulin resistance in patients with hepatitis C virus-related liver disease: report of two cases. Liver Int. 2007;27:1287-1292. [PubMed] |
| 106. | Miyake T, Abe M, Furukawa S, Tokumoto Y, Toshimitsu K, Ueda T, Yamamoto S, Hirooka M, Kumagi T, Hiasa Y. Long-term branched-chain amino acid supplementation improves glucose tolerance in patients with nonalcoholic steatohepatitis-related cirrhosis. Intern Med. 2012;51:2151-2155. [PubMed] |