Published online Nov 14, 2013. doi: 10.3748/wjg.v19.i42.7461
Revised: August 8, 2013
Accepted: August 20, 2013
Published online: November 14, 2013
Processing time: 168 Days and 15.9 Hours
AIM: To compare the long-term clinical efficacy of chemotherapy plus radiotherapy (CRT) with that of radiotherapy alone (RT) or chemotherapy alone (CT) for locally advanced pancreatic carcinoma (LAPC).
METHODS: Using manual and computer-aided methods, we searched the data through the databases, including PubMed/EmBase/CNKI/CQVIP/China Journals Full Text Database and websites and proceedings of major annual meetings such as ASCO and CSCO. The methodological quality of the included studies was assessed using the Jadad scoring system. Both English and Chinese publications were searched. We collected data from controlled clinical trials on CRT vs RT or CT for LAPC, and conducted a meta-analysis of 15 included studies. Meta-analysis was performed using RevMan4.2 Software according to the method recommended by Cochrane Collaboration.
RESULTS: Fifteen eligible randomized controlled trials including a total of 1128 patients were screened. Jadad score was 2 in only one article, and 3-4 in the remaining 14 studies. The meta-analysis showed that CRT was superior in the 6- and 12-mo survivals to the RT alone group or CT alone group (P = 0.0001 and P = 0.02, respectively), whereas the 18-mo survival showed no significant difference (P = 0.23). Subgroup analysis showed that the 6-, 12-, and 18-mo survivals were not significantly different between the CRT group and CT group (P = 0.07, P = 0.23, and P = 0.91, respectively). Notably, the CRT group had significantly better 6-, 12-, and 18-mo survivals than the RT group (all P < 0.01). CRT group had significantly more grade 3-4 treatment-related hematologic and non-hematologic toxicities than the CT group or RT group (all P < 0.01).
CONCLUSION: Compared with CT or RT, CRT can benefit the long-term survival of LAPC patients, although it may also increase treatment-related toxicities.
Core tip: To compare the long-term clinical efficacy of chemotherapy plus radiotherapy (CRT) with that of radiotherapy alone (RT) or chemotherapy alone (CT) for locally advanced pancreatic carcinoma (LAPC),the authors analyzed the potential impact of CRT, CT or RT on the survival of the patients using meta-analysis methodologies. Meta-analysis showed that compared with CT or RT, CRT can benefit the long-term survival of LAPC patients, although it may also increase treatment-related toxicities.
- Citation: Chen Y, Sun XJ, Jiang TH, Mao AW. Combined radiochemotherapy in patients with locally advanced pancreatic cancer: A meta-analysis. World J Gastroenterol 2013; 19(42): 7461-7471
- URL: https://www.wjgnet.com/1007-9327/full/v19/i42/7461.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i42.7461
Pancreatic cancer is the fourth leading cause of cancer-related death in the United States[1]. Over the past decades, the standard treatment for patients with inoperable locally advanced pancreatic cancer (LAPC) was chemotherapy alone (CT) or chemo-radiation therapy (CRT)[2-4]; however, the median survival time was only 6-9 mo, and less than 10% of patients survived for 2 years[5]. Definitive results of the 2000-01 FFCD/SFRO study, in which patients randomly received CRT (60 Gy + 5-fluorouracil + cisplatin + gemcitabine) or CT, showed that the progression free survival (PFS) and overall survival (OS) were not significantly different between these two groups[6]. On the contrary, the Eastern Cooperative Oncology Group (ECOG) study, which randomly divided the LAPC patients into a gemcitabine chemotherapy group and a gemcitabine + radiotherapy group, showed that the CRT group had significantly superior OS over the CT group; meanwhile, treatment-related toxicity in the CRT group was acceptable. In the present study, we analyzed the potential impact of CRT or CT or radiotherapy alone (RT) on the survival using meta-analysis methodologies in an attempt to obtain more robust evidence.
The search strategy was: exp pancreas/(pancreas.tw.exp); pancreatic neoplasms/(pancreas adj neoplasms, pancreas adj cancers, pancreas adj carcinomas); 30-38 exp drug therapy/exp chemotherapy adjuvant/chemotherapy; chemoradiotherapy (combin adj chemotherapy, concurrent adj chemoradiotherapy; 49-50 exp radiotherapy/exp radiotherapy adjuvant/radiotherapy.
The literature search was conducted in both English and Chinese publications. The data sources included PubMed (1964-2012), EmBase (1964-2012), CNKI (1979-2012), CQVIP(1979-2012), China Journals Full Text Database (1979-2012), websites (e.g., Web of Science) and proceedings of major annual meetings such as ASCO (1995-2012) and CSCO (1995-2012). Internet searches were carried out and bibliographies of included articles were searched. Finally, the Chinese references in the selected articles were reviewed for other relevant studies. The latest updates of serial clinical studies were used. Date of last search was October 15, 2012.
Inclusion criteria: (1) Patients were pathologically confirmed to have locally advanced pancreatic malignant tumors (Tumors were judged as nonresectable due to extension to regional lymph nodes and/or vascular structures such as the superior mesenteric artery or the celiac trunk or the existence of a portal or superior mesenteric-portal venous confluent thrombosis), which were naive to surgical treatment or other anti-tumor therapies before enrollment; (2) the study was a prospective randomized controlled trial; (3) the interventions only included radiotherapy and/or chemotherapy; (4) the main endpoint was survival, and the observation lasted at least 6 mo, along with survival records; and (5) except for the treatment methods, the treatment group was parallel with the control group.
Exclusion criteria: (1) Patients with metastatic pancreatic cancer; individuals who suffered from relapse after anti-tumor treatment; patients who had previously received surgical treatment; patients with non-LAPC; (2) non-prospective and non-randomized/non-controlled studies; (3) other interventions were applied in addition to radiotherapy and chemotherapy; (4) only local efficacy was evaluated and no data on survival was available; and (5) low-quality studies that had a Jadad score of less than 2.
Assessment of the included literature: Two reviewers independently conducted methodological quality assessment and data retrieval. Cross-checking was then performed; any disagreements were resolved by discussion and, if necessary, a third reviewer. The methodological quality of the included studies was assessed using the Jadad scoring system: (1) Was the study described as randomized? Were the patients actually randomized into the treatment group or control group, and both the observers and patients did not know which group would be allocated? (2 = the appropriate randomization method was described; 1 = the author claimed the use of a randomization method); (2) Except for the targeted intervention(s), were the other procedures consistent between these two groups? (3) Was the study described as blinded (2 = both the patients and observers were blinded, and the blinding method was described; 1 = the author claimed that a double-blinding method was applied; 0 = not blinded); and (4) Was there a description of exclusion bias (i.e., systematical difference of the withdrawals and drop-outs between these two groups; scored 0 or 1 for any reason of drop-outs). The final score ranged from 1 to 5. Studies scored 3 or higher were judged as “high-quality”, and those scored 1 or 2 as “poor-quality”. The criteria for methodological quality analysis were developed: (1) randomization; (2) blinding; (3) withdrawal or drop-outs; (4) allocation concealment; and (5) adoption of intentional analysis. The quality of the included literature was presented as weightings in a forest plot.
Assessment of the result quality: Using evidence-based medicine principles, we conducted a quality assessment on the design of evidence sources and finally obtained the quality of the meta-analysis results.
Meta-analysis was performed using RevMan4.2 Software. For clinical trials reporting the summary measures or Kaplan-Meier curves, we applied the published statistical methods to analyze the risk ratio and the variance of the temporal data that cause the events[7,8]. The statistical heterogeneity of the clinical trials was analyzed using χ2 test and I2 test; P > 0.05 during χ2 test indicates low heterogeneity. The primary analysis was completed using a fixed-effects model. If there was a significant statistical heterogeneity, a secondary confirmatory analysis was performed using a random-effects model. Meanwhile, subgroup analysis was performed to determine whether the results were affected by different interventions. By analyzing the difference between combination therapy and CT/RT, we tried to understand their different impacts on the prognosis.
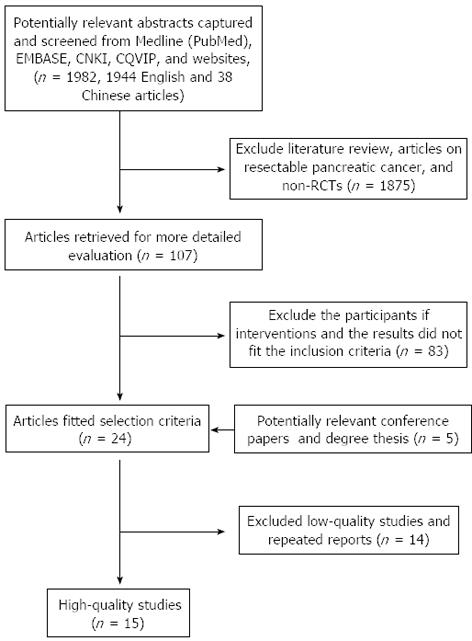
The flow chart of this study is shown in Figure 1. Reviews, articles about resectable pancreatic cancer, non-randomized controlled trials (RCTs), low-quality studies and repeated reports were excluded. Finally, both reviewers agreed to include 15 RCTs involving 1128 patients in the meta-analysis.
All of them were published in peer-reviewed international and domestic journals. The cases had been followed up for 6-36 mo. All the studies were prospective, randomized, and controlled trials. One article was claimed to be randomized, with a Jadad score of only 2, and therefore the quality was low. The remaining 14 studies scored 3-4, and belonged to high-quality literature, among which two articles used the blinding method. The meta-analysis results are listed in Table 1. There were no duplicates or mis-citations.
| Ref. | Treatment arms | No. of participants | Sex (male/female) | Median age (yr) | PS 0-2/KPS≥50 | Pathology (W/M/P/other) | Location of primary tumor(head of pancreas/other) | Jad score |
| Loehrer et al[9] | RT 50.4 Gy + GEM vs GEM | 37 | 18/19 | 67 | 100% | 4/6/5/22 | 12/25 | |
| 34 | 19/15 | 65.3 | 100% | 6/8/7/13 | 20/14 | 3 | ||
| Klaassen et al[10] | RT 40 Gy + 5FU vs 5FU | 44 | 31/13 | 100% | NR | NR | ||
| 47 | 22/25 | NR | 100% | 3 | ||||
| Moertel et al[11] | RT 35-40 Gy + saline vs RT 35-40 Gy + 5FU | 32 | NR | NR | NR | NR | NR | |
| 32 | 4 | |||||||
| GITSG et al[12] | RT 60 Gy vs RT 60Gy + 5FU vs RT 40 Gy + 5FU | 28 | 12/16 | NR | 100% | 8/17/3/0 | 10/18 | |
| 32 | 17/15 | 100% | 6/20/6/0 | 9/23 | 3 | |||
| 29 | 16/13 | 100% | 6/18/5/0 | 6/23 | ||||
| Cohen et al[13] | RT 59.4 Gy vs RT 59.4 Gy + 5FU + MMC | 49 | 27/22 | 62 | 100% | 7/21/11/10 | NR | |
| 55 | 37/18 | 64 | 100% | 12/19/17/7 | 3 | |||
| Chauffert et al[6] | RT 60 Gy + 5FU + DDP + GEM vs GEM | 59 | 31/28 | 60 | 100% | NR | 46/13 | |
| 60 | 34/26 | 62 | 100% | 40/20 | 3 | |||
| Moertel et al[14] | RT 60 Gy vs RT 60 Gy + 5FU vs RT 40 Gy + 5FU | 25 | 54 | 88% | 5/8/2/10 | 8/17 | 3 | |
| 86 | 60 | 95% | 20/39/9/14 | 68/18 | ||||
| 83 | 61 | 95% | 13/30/9/31 | 64/19 | ||||
| Sun et al[15] | RT 45-50 Gy + GEM vs GEM | 25 | 32/22 | NR | 100% | NR | NR | 3 |
| 29 | 100% | |||||||
| Sun et al[16] | RT 50-60 Gy + GEM vs GEM + DDP | 26 | 33/23 | NR | 100% | NR | NR | 3 |
| 30 | 100% | |||||||
| Wu et al[17] | RT 48-56 Gy vs RT 48-60 Gy + GEM + DDP | 31 | 50/14 | 57 | 98% | NR | NR | 3 |
| 33 | 57 | 99% | ||||||
| Wu et al[18] | RT 60 Gy vs RT 50 Gy + GEM | 34 | 43/27 | NR | 87% | NR | NR | 3 |
| 36 | 90% | |||||||
| Ding et al[19] | RT 45-50 Gy + 5FU + GEM vs 5FU + GEM | 25 | 32/22 | NR | 100% | NR | NR | 3 |
| 29 | 100% | |||||||
| Childs et al[20] | RT 35-40 Gy + saline vs RT 35-40 Gy + 5FU | 12 | 11/1 | 58.8 | NR | NR | NR | 4 |
| 13 | 8/5 | 56.3 | ||||||
| GITSG et al[21] | RT 54 Gy + 5FU + SMF vs SMF | 22 | 8/14 | 61 | 100% | NR | 3/18 | 3 |
| 21 | 8/13 | 60 | 100% | 3/19 | ||||
| Hazel et al[22] | RT 46 Gy + 5FU vs 5FU + CCNU | 15 | 10/5 | 62 | NR | NR | NR | 2 |
| 15 | 10/5 | 62 |
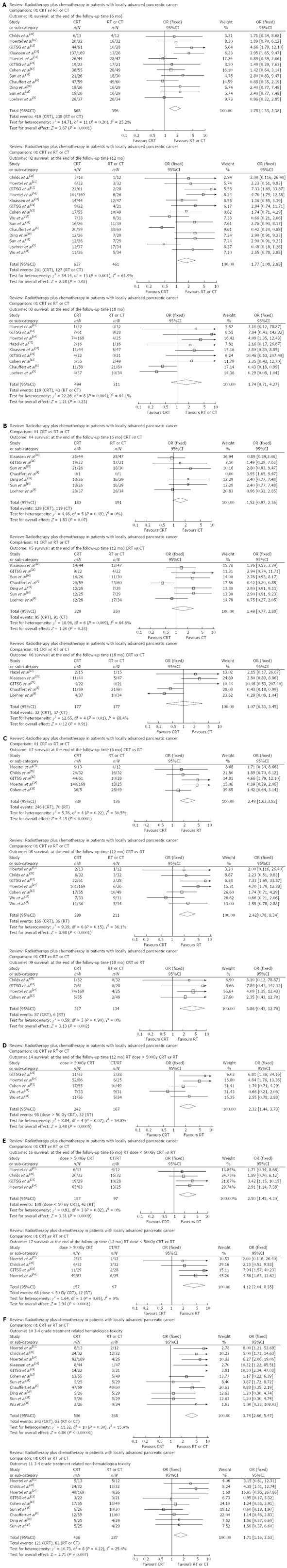
Meta-analysis on radiochemotherapy and CT/RT for LAPC: Of these 15 RCTs (n = 1128), 12 (n = 964) reported the 6-mo survival; 14 (n = 1098) reported the 12-mo survival; 9 (n = 805) reported the 18-mo survival; the 6-, 12-, and 18-mo survival was 419, 261, and 119 cases, respectively, in the CRT group, and 238, 127, and 43 cases, respectively, in the CT group/RT group. P value was larger than 0.05 in the meta-analysis on the heterogeneity of the 6-mo survival, and a fixed-effects model was used to merge the data. P values were lower than 0.05 in the meta-analysis on the heterogeneity of the 12- and 18-mo survivals, and a random-effects model was used to merge the data. The CRT group had superior survival over the CT/RT group, while the 18-mo survival showed no such significant difference (OR = 1.78, 1.77, and 1.74; 95%CI: 1.33-2.38, 1.08-2.88, and 0.71-4.27, P = 0.0001, 0.02, and 0.23) (Table 2, Figure 2A).
| Ref. | Treatment arms | Participants | Overall survival (n) | 3-4 grade treatment-related toxicity (n) | |||
| 6 mo | 12 mo | 18 mo | Hematological | Non-hematological | |||
| Loehrer et al[9] | RT 50.4 Gy + GEM vs GEM | 37 | 28 | 12 | 4 | 29 | |
| 34 | 26 | 17 | 10 | 26 | |||
| Klaassen et al[10] | RT 40 Gy + 5FU vs 5FU | 44 | 25 | 14 | 11 | 8 | NR |
| 47 | 28 | 12 | 5 | 1 | |||
| Moertel et al[11] | RT 35-40 Gy + saline vs RT 35-40 Gy + 5FU | 32 | 15 | 3 | 0 | 12 | 13 |
| 32 | 20 | 6 | 1 | 24 | 24 | ||
| GITSG et al[12] | RT 60 Gy vs RT 60 Gy + 5FU vs RT 40 Gy + 5FU | 28 | 10 | 2 | 0 | ||
| 32 | 25 | 11 | 3 | NR | NR | ||
| 29 | 19 | 11 | 4 | ||||
| Cohen et al[13] | RT 59.4 Gy vs RT 59.4 Gy + 5FU + MMC | 49 | 28 | 10 | 2 | 5 | 13 |
| 55 | 36 | 17 | 5 | 13 | 17 | ||
| Chauffert et al[6] | RT 60 Gy + 5FU + DDP + GEM vs GEM | 59 | 47 | 20 | 11 | 29 | 12 |
| 60 | 49 | 33 | 21 | 12 | 11 | ||
| Moertel et al[14] | RT 60 Gy vs RT 60 Gy + 5FU vs RT 40 Gy + 5FU | 25 | 13 | 6 | 4 | 4 | 0 |
| 86 | 74 | 52 | 34 | 53 | 22 | ||
| 83 | 63 | 49 | 40 | 39 | 18 | ||
| Sun et al[15] | RT 45-50 Gy + GEM vs GEM | 25 | 18 | 12 | NR | 5 | 5 |
| 29 | 15 | 7 | 5 | 4 | |||
| Sun et al[16] | RT 50-60 Gy + GEM vs GEM + DDP | 26 | 21 | 16 | NR | 3 | 6 |
| 30 | 18 | 11 | 3 | 10 | |||
| Wu et al[17] | RT 48-56 Gy vs RT 48-60 Gy + GEM + DDP | 31 | NR | 9 | NR | NR | NR |
| 33 | 7 | ||||||
| Wu et al[18] | RT 60 Gy vs RT 50 Gy + GEM | 34 | NR | 5 | NR | 0 | NR |
| 36 | 11 | 2 | |||||
| Ding et al[19] | RT 45-50 Gy + 5FU + GEM vs 5FU + GEM | 25 | 18 | 12 | NR | 5 | 5 |
| 29 | 15 | 7 | 5 | 4 | |||
| Childs et al[20] | RT 35-40 Gy + saline vs RT 35-40 Gy + 5FU | 12 | 4 | 1 | NR | 2 | 5 |
| 13 | 6 | 2 | 8 | 9 | |||
| GITSG et al[21] | RT 54 Gy + 5FU + SMF vs SMF | 22 | 19 | 9 | 4 | 14 | 3 |
| 21 | 17 | 4 | 0 | 3 | 3 | ||
| Hazel et al[22] | RT 46 Gy + 5FU vs 5FU + CCNU | 15 | NR | NR | 2 | NR | NR |
| 15 | 1 | ||||||
Subgroup analysis on the efficacy of radiochemotherapy and CT/RT for LAPC: Seven RCTs (n = 371) reported the 6-mo survival of patients after combination treatment or CT, 7 (n = 479 ) reported the 12-mo survival, and 5 (n = 354) reported the 18-mo survival. The 6-, 12-, and 18-mo survivals were reported in 129, 95, and 32 cases, respectively, in the CRT group, and 119, 91, and 37 cases, respectively, in the CT group. P value was larger than 0.05 in the meta-analysis on the heterogeneity of 6-mo survival, and a fixed-effects model was used to merge the data. P values were lower than 0.05 in the meta-analysis on the heterogeneity of the 12- and 18-mo survivals, and a random-effects model was used to merge the data. The 6-, 12-, and 18-mo survivals were not significantly different between the CRT group and the CT group (OR = 1.52, 1.49, and 1.07; 95%CI: 0.97-2.36, 0.77-2.88, and 0.33-3.45, P = 0.07, 0.23, and 0.91) (Figure 2B).
Five RCTs (n = 476) reported the 6-mo survival after combination treatment or RT, 7 (n = 610) reported the 12-mo survival, 4 (n = 451) reported the 18-mo survival. The 6-, 12-, and 18-mo survivals were 246, 166, and 87 cases, respectively, in the CRT group, and 70, 36, and 37 cases, respectively, in the RT group. Meta-analysis on the heterogeneity of the 6-, 12-, and 18-mo survivals showed that the P values were larger than 0.05, and a fixed-effects model was used to merge the data. The 6-, 12-, and 18-mo survivals were significantly higher in the CRT group than in the RT group (OR = 2.49, 2.42, and 3.86; 95%CI: 1.62-3.82, 1.57-3.74, 1.66-8.99, all P < 0.01), (Figure 2C).
Subgroup analysis on the efficacy of CRT > 50Gy and RT for LAPC: Due to the limited number of patients and RCTs, this subgroup analysis only covered the 12-mo survival rate. Five RCTs (n = 409) reported the 12-mo survival of patients after CRT at a dose larger than 50Gy or RT, including 32 cases in the CRT group and 98 cases in the RT group. P value was larger than 0.05 in the meta-analysis on the heterogeneity of the 12-mo survival, and a fixed-effects model was used to pool the data. The CRT group had superior 12-mo survival over the RT group (OR = 2.32; 95%CI: 1.44-3.73, P =0.0005) (Figure 2D).
Subgroup analysis on the efficacy of CRT < 50 Gy and RT for LAPC: Due to the limited number of patients and RCTs, this subgroup analysis was only made on the 6- and 12-mo survival rates. Four RCTs (n = 254) reported the 6-mo survival and 4 (n = 254) reported the 12-mo survival. The 6- and 12-mo survival was reported in 108 and 68 cases in the CRT group, and 42 and 12 cases in the RT group, respectively. P value was larger than 0.05 in the meta-analysis on the heterogeneity of 6- and 12-mo survival, and a fixed-effects model was used to pool the data. The CRT group had superior 6- and 12-mo survival over the RT group (OR = 2.5 and 4.12; 95%CI: 1.45-4.30 and 2.04-8.35, P = 0.0009 and < 0.0001) (Figure 2E).
Due to the limited number of cases and RCTs included, subgroup analysis was not performed on the efficacy of different doses of CRT and CT for LAPC.
Meta-analysis on grade 3-4 treatment-related toxicity: In total, 12 RCTs (n = 874) reported grade 3-4 treatment-related hematologic toxicities, and 10 (n = 713) reported grade 3-4 treatment-related non-hematologic toxicities. Meta-analysis on the heterogeneity showed that the P value was larger than 0.05, and a fixed-effects model was used to merge the data. The CRT group had significantly more grade 3-4 treatment-related hematologic and non-hematologic toxicities than the CT group or RT group (OR = 3.74 and 1.71; 95%CI: 2.56-5.47 and 1.16-2.53, both P < 0.01) (Figure 2F).
By re-analyzing the results, the sensitivity analysis was designed to re-combine the available studies to explore the effect of a certain factor on the effect, so as to understand the influences of uncertain factors and study design on the aggregate results. To avoid the effect of different chemotherapy or radiotherapy in the different randomized controlled trials on the overall results, subgroup analyses were performed to re-analyze the above studies, and the results were generally consistent with the meta-analysis findings. As shown in the sensitivity analysis, our current meta-analysis neither increased or decreased the efficacy nor exaggerated the efficacy; after the strength of the articles was changed, the results did not become negative or reversed. Therefore, our results were relatively stable and reliable.
Pancreatic adenocarcinoma is among the most challenging solid malignancies to treat on account of its propensity for late presentation with inoperable disease, aggressive tumor biology and resistance to chemotherapy[23]. About a third of all pancreatic cancers is found to be locally advanced at the time of diagnosis, LAPC refers to local tumors that have invaded the surrounding normal tissues and can not be surgically resected while no distant metastasis occurs[24]. As shown in early clinical practice, conventional radiotherapy for LAPC often can not improve the efficacy. The availability of three-dimensional conformal radiotherapy and intensity-modulated radiotherapy improves the therapeutic effectiveness, although controversies persist. Using the meta-analysis methodologies, we rigorously screened eligible randomized controlled trials for analysis. As shown in our current meta-analysis: (1) the CRT group had higher 6- and 12-mo survival rates than the RT alone and CT alone group; (2) subgroup analysis showed that the CRT group had higher 6-, 12-, and 18-mo survival rates than CT alone group; (3) subgroup analysis showed that the CRT group had higher 6-, 12-, and 18-mo survival rates than the RT alone group; and (4) CRT group had significantly more grade 3-4 treatment-related hematologic and non-hematologic toxicities than the CT group or RT group. By analyzing the results of 2000 FFCD/SFR0[6], we found that concurrent three-dimensional conformal radiotherapy (total dosage: 60 Gy) with chemotherapy (DDP + 5FU), followed by GMZ chemotherapy achieved a median survival of 8.6 mo and a 1-year survival rate of 32%; the GMZ CT group had a survival of 13 mo and 1-year survival rate of 53%, suggesting that the combination therapy did not change the efficacy. Meanwhile, the 60 Gy dosage induced more complications and therefore increased the mortality and decreased the survival. The CRT protocol applied in this trial used high radiotherapy and chemotherapy dosages; furthermore, the radiation fields included not only the cancer foci but also the peripancreatic, hilar and celiac trunk lymph nodes that had high metastatic potential (but not confirmed), which not only remarkably increased the radiotherapy-related toxic reactions but also shortened the survival. Similarly, radiochemotherapy was applied in the ECOG4201 trial, although the radiotherapy dose was lowered to 50.4 Gy. Compared with CT, concurrent radio-chemotherapy prolonged the survival (11 mo vs 9.2 mo) and yielded a higher 1-year survival rate (50% vs 32%)[25]. During concurrent radiochemotherapy, the sequencing of radiotherapy and chemotherapy can also affect the clinical efficacy. In the GERCOR study, 181 patients received 4 cycles of chemotherapy initially, among whom 53 experienced disease progression; the remaining 128 patients without disease progression were divided into a concurrent CRT group (n = 72) and a CT group (n = 56). The results showed that the PFS and OS were improved in the concurrent CRT group (10.8 mo and 15 mo, respectively), and these were significantly longer than those in the CT group (7.4 mo and 11.7 mo, respectively) (P = 0.005, P = 0.0009, respectively), indicating that radiotherapy combined with chemotherapy can remarkably improve the survival[26].
In summary, as shown in this study, the combination of chemotherapy and radiotherapy can prolong the long-term survival although it may also increase the treatment-related toxicity. More reasonably designed randomized controlled trials should be conducted to further elucidate the optimal radiotherapy dosage, the use of gemcitabine or 5-FU, and more specific chemotherapy protocols. Chemotherapy may be started for several cycles; if no disease progression occurs, concurrent radiochemotherapy may be used. By doing so, we may rule out patients with rapid disease progression to avoid the “double whammy” from chemotherapy. Along with the improvement of radiotherapy technology, the optimization of the sequencing of radiochemotherapy, and the definition of target population, the efficacy of radiochemotherapy for pancreatic cancer will be further improved, and multidisciplinary and individualized radiochemotherapy for LAPC will play a more important role.
Pancreatic cancer is the fourth leading cause of cancer-related death in the United States. Over the past decades, the standard treatment for patients with locally advanced pancreatic carcinoma (LAPC) was chemotherapy alone or chemo-radiation therapy. However, the efficacy of the combined radiotherapy and chemotherapy is currently unclear.
Over the past decades, many studies have been performed to understand the role of chemotherapy or radiation therapy alone and chemo-radiation therapy in LAPC. Some trials showed no improvement in local progression or progression-free or overall survival with the addition of radiation therapy to chemotherapy in patients with LAPC; however, some trials demonstrated improved overall survival, with acceptable toxicity. Moreover, several systematic reviews were recently published to investigate the role of chemo-radiation therapy in LAPC. However, these reviews were methodologically insufficient and thus could not achieve a comprehensive conclusion.
Based on this meta-analysis, chemo-radiation therapy was superior in the 6- and 12-mo survivals to the radiation therapy group or chemotherapy group alone. Similar results were indicated in the subgroup analyses. The radiation therapy group had significantly more grade 3-4 treatment-related hematologic and non-hematologic toxicities than the radiation therapy group or chemotherapy group alone. These findings were not presented clearly in previous systematic reviews.
The combination of chemotherapy and radiotherapy can prolong the long-term survival of the patients with LAPC although it may also increase the treatment-related toxicity. Chemotherapy may be started for several cycles; if no disease progression occurs, concurrent radiochemotherapy may be used.
Locally advanced pancreatic malignant tumors were judged as nonresectable due to extension to regional lymph nodes and/or vascular structures such as the superior mesenteric artery or the celiac trunk or the existence of a portal or superior mesenteric-portal venous confluent thrombosis. Over the past decades, the standard treatment for patients with inoperable locally advanced pancreatic cancer was chemotherapy alone or chemo-radiation therapy.
This is a well written manuscript analyzing therapeutic management of pancreatic cancer. In this manuscript, the authors compared the long-term clinical efficacy of chemotherapy plus radiotherapy with that of radiotherapy alone or chemotherapy alone for locally advanced pancreatic carcinoma. The data were well collected, and analyzed.
P- Reviewers: Fietkau R, Lalic H S- Editor: Wen LL L- Editor: A E- Editor: Zhang DN
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 2. | Hu J, Zhao G, Wang HX, Tang L, Xu YC, Ma Y, Zhang FC. A meta-analysis of gemcitabine containing chemotherapy for locally advanced and metastatic pancreatic adenocarcinoma. J Hematol Oncol. 2011;4:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Yip D, Karapetis C, Strickland A, Steer CB, Goldstein D. Chemotherapy and radiotherapy for inoperable advanced pancreatic cancer. Cochrane Database Syst Rev. 2006;CD002093. [PubMed] |
| 4. | Hackert T, Büchler MW. Pancreatic cancer: advances in treatment, results and limitations. Dig Dis. 2013;31:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Niederhuber JE, Brennan MF, Menck HR. The National Cancer Data Base report on pancreatic cancer. Cancer. 1995;76:1671-1677. [PubMed] |
| 6. | Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouché O, Bosset JF, Aparicio T, Mineur L, Azzedine A. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19:1592-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 535] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 7. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [PubMed] |
| 8. | Walter SD. Choice of effect measure for epidemiological data. J Clin Epidemiol. 2000;53:931-939. [PubMed] |
| 9. | Loehrer PJ, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, Flynn P, Ramanathan RK, Crane CH, Alberts SR. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105-4112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 621] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 10. | Klaassen DJ, MacIntyre JM, Catton GE, Engstrom PF, Moertel CG. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil--an Eastern Cooperative Oncology Group study. J Clin Oncol. 1985;3:373-378. [PubMed] |
| 11. | Moertel CG, Childs DS, Reitemeier RJ, Colby MY, Holbrook MA. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet. 1969;2:865-867. [PubMed] |
| 12. | The Gastrointestinal Tumor Study Group. A multi-institutional comparative trial of radiation therapy alone and in combination with 5-fluorouracil for locally unresectable pancreatic carcinoma. Ann Surg. 1979;189:205-208. [PubMed] |
| 13. | Cohen SJ, Dobelbower R, Lipsitz S, Catalano PJ, Sischy B, Smith TJ, Haller DG. A randomized phase III study of radiotherapy alone or with 5-fluorouracil and mitomycin-C in patients with locally advanced adenocarcinoma of the pancreas: Eastern Cooperative Oncology Group study E8282. Int J Radiat Oncol Biol Phys. 2005;62:1345-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 14. | Moertel CG, Frytak S, Hahn RG, O’Connell MJ, Reitemeier RJ, Rubin J, Schutt AJ, Weiland LH, Childs DS, Holbrook MA. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705-1710. [PubMed] |
| 15. | Sun YW, Zhang RH, Zhou JY, Liang J. Effect of gemcitabine plus three dimensionalconformal radiotherapy on locally advanced pancreatic cancer. Zhonghua Shiyong Yixue Zazhi. 2010;37:11-15. |
| 16. | Sun YW, Zhou JY, An YH, Liang J. The Clinical efficiency of gemcitabine plus three dimensional conformal radiotherapy on locally advanced pancreatic cancer-comparison with gencitabine plus cisplatin. Xiandai Zhongliu Yixue. 2007;15:826-828. [DOI] [Full Text] |
| 17. | Wu DH, Chen YQ, Chen LH. Observation of the Effects of 3-dimensional conformal radiotherapy therapy combined with chemotherapy in treatment of pancreatic cancer. Zhongguo Linchuang Zhongliu Zazhi. 2004;31:449-551. [DOI] [Full Text] |
| 18. | Wu F, Meng CY. Radiotherapy alone or plus chemotherapy in patients with locally advanced pancreatic cancer. Zhongguo Shiyong Yiyao Zazhi. 2011;25:117-119. |
| 19. | Ding ZJ, Chen Y, Zhou JY, An YH. The effect of combination chemotherapy of gemcitabine and 5-FU plus three dimensional conformal radiotherapy on locally advanced pancreatic cancer. Zhongguo Linchuang Shiyong Yixue. 2008;2:42-43. |
| 20. | Childs DS, Moertel CG, Holbrook MA, Reitemeier RJ, Colby MY. Treatment of malignant neoplasms of the gastrointestinal tract with a combination of 5-fluorouracil and radiation: a randomized double-blind study. Radiology. 1965;84:843-848. [PubMed] |
| 21. | Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst. 1988;80:751-755. [PubMed] |
| 22. | Hazel JJ, Thirlwell MP, Huggins M, Maksymiuk A, MacFarlane JK. Multi-drug chemotherapy with and without radiation for carcinoma of the stomach and pancreas: a prospective randomized trial. J Can Assoc Radiol. 1981;32:164-165. [PubMed] |
| 23. | Javle M, Hsueh CT. Recent advances in gastrointestinal oncology--updates and insights from the 2009 annual meeting of the American society of clinical oncology. J Hematol Oncol. 2010;3:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Savir G, Huber KE, Saif MW. Locally advanced pancreatic cancer. Looking beyond traditional chemotherapy and radiation. JOP. 2013;14:337-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 25. | Saif MW. New developments in the treatment of pancreatic cancer. Highlights from the “44th ASCO Annual Meeting”. Chicago, IL, USA. May 30 - June 3, 2008. JOP. 2008;9:391-397. [PubMed] |
| 26. | Huguet F, André T, Hammel P, Artru P, Balosso J, Selle F, Deniaud-Alexandre E, Ruszniewski P, Touboul E, Labianca R. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326-331. [PubMed] |