Published online Nov 14, 2013. doi: 10.3748/wjg.v19.i42.7389
Revised: September 10, 2013
Accepted: September 29, 2013
Published online: November 14, 2013
Processing time: 142 Days and 4.2 Hours
AIM: To investigate whether an ablative margin (AM) > 1.0 cm might reduce chance of recurrence for patients with hepatocellular carcinoma (HCC) tumors 3.1 to 5.0 cm in size, compared with an AM of 0.5-1.0 cm.
METHODS: From October 2005 to December 2012, 936 consecutive patients with HCC who received radiofrequency ablation were screened. Of these, 281 patients, each with a single primary HCC tumor of 3.1 to 5.0 cm in size on its greatest diameter, were included in the study. Based on the AM width, we categorized patients into the 0.5-1.0 cm group and the > 1.0 cm group. Local tumor progression (LTP)-free survival, intrahepatic distant recurrence (IDR)-free survival and overall survival (OS) rates were obtained using the Kaplan-Meier method.
RESULTS: The 1-, 2-, 3-, 4-, and 5-year LTP-free survival rates and IDR-free survival rates were significantly higher in the > 1.0 cm group compared with the 0.5-1.0 cm group (97.5%, 86.3%, 73.6%, 49.5% and 26.4% vs 91.3%, 78.4%, 49.5%, 27.8%, and 12.8%; 95.1%, 90.3%, 77.0%, 61.0% and 48.3% vs 95.2%, 85.9%, 62.6%, 47.2% and 28.5%; P < 0.05). The 1-, 2-, 3-, 4-, and 5-year OS rates were 98.6%, 91.5%, 69.2%, 56.0% and 42.2%, respectively, in the 0.5-1.0 cm group and 100%, 98.9%, 90.1%, 68.7% and 57.4%, respectively, in the > 1.0 cm group (P = 0.010). There were no significant differences in complication rates between the two groups. Both univariate and multivariate analyses identified AM as an independent prognostic factor linked to LTP, IDR, and OS.
CONCLUSION: For HCC tumors > 3.0 cm and ≤ 5.0 cm, AM > 1.0 cm could reduce chances of recurrence compared with AM of 0.5-1.0 cm, emphasizing the need for a more defensive strategy using AMs > 1.0 cm for ablating HCC tumors of 3.1 to 5.0 cm.
Core tip: Recurrence is the most important factor for prognosis of hepatocellular carcinoma (HCC) after radiofrequency ablation. Although a sufficient ablative margin (AM) is an essential way to minimize recurrence risk, the optimal AM for HCC tumors 3.1 to 5.0 cm remains controversial. This study provides evidence that, for HCC tumors 3.1 to 5.0 cm, AMs > 1.0 cm could reduce chance of recurrence compared to AMs of 0.5-1.0 cm, which emphasizes the need for more strategic AMs that are > 1.0 cm for ablation of HCC tumors of 3.1 to 5.0 cm.
- Citation: Ke S, Ding XM, Qian XJ, Zhou YM, Cao BX, Gao K, Sun WB. Radiofrequency ablation of hepatocellular carcinoma sized > 3 and ≤ 5 cm: Is ablative margin of more than 1 cm justified? World J Gastroenterol 2013; 19(42): 7389-7398
- URL: https://www.wjgnet.com/1007-9327/full/v19/i42/7389.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i42.7389
Radiofrequency (RF) ablation is accepted as a potentially curative treatment modality for hepatocellular carcinoma (HCC) at an early stage when transplantation and resection are precluded[1,2]. Local tumor progression (LTP) and intrahepatic distant recurrence (IDR) have been repeatedly found to be the most important prognostic factors, with the incidence rates of 2%-53% and 43%-53%, respectively[1,3-5]. LTP that occurs after complete RF ablation is widely considered to result from residual tumor cells in the peritumoral area, which contains microvascular invasion and satellite micronodules due to an insufficient ablative margin (AM), although there is a possibility of de novo occurrence at the site. Pathogenesis of IDR is thought to result from intrahepatic metastasis of the primary tumor, viable residual tumor or an HCC of multicentric origin[6,7]. Therefore, a sufficient AM that encompasses both the main tumor and the area of adjacent parenchyma containing microvascular invasion or satellite micronodules, should theoretically ensure pathologically complete ablation, and would thus be an essential way to minimize risk of LTP and IDR[8,9].
The optimal safe AM for HCC is controversial[3,10-12]. Accumulating data have demonstrated that recurrence rates, such as LTP rates, differ greatly for tumors of various sizes but similar AMs[3,13]. For HCCs ≤ 3.0 cm, the 3-year LTP rates for patients treated by RF ablation with AMs of 0.5-1.0 cm are reportedly 10%-20%[3,14-17]. However, for HCC tumors 3.1 to 5.0 cm treated with AMs of 0.5-1.0 cm, the 3-year LTP rates were as high as 39%[18]. This can be well explained by the data from histopathological investigations on both the scope of peritumoral microvascular invasion and satellite micronodules and the incidence rate between HCCs ≤ 3.0 cm and those > 3.0 cm[19,20]. Only 14.5%-19% of single small HCC (≤ 3.0 cm) reportedly have satellite micronodules, located within 1.0 cm from the main tumor[21]. In contrast, for HCC tumors 3.1 to 5.0 cm, 26.3%-36.9% had peritumoral satellite micronodules, located more than 1.0 cm from the main tumor in most cases[9]. These data indicate that for HCCs ≤ 3.0 cm, AMs of 0.5-1.0 cm are likely to remove most peritumoral lesions. However, for HCC tumors 3.1 to 5.0 cm, AMs of ≤ 1.0 cm seem insufficient to ensure pathological complete tumor clearance in most cases.
Although the idea that AMs > 1.0 cm would be more likely to completely delete tumor tissues seems intuitively logical, no study to date has been conducted to determine the optimal AM for HCC tumors 3.1 to 5.0 cm. Based on the experience of the surgical requirement of a tumor-free margin ≥ 1.0 cm wide[9], we supposed that for HCC tumors 3.1 to 5.0 cm, AMs > 1.0 cm might reduce chance of recurrence compared with AMs of 0.5-1.0 cm. Therefore, the purpose of this study was to elucidate the survival benefit of AMs wider than 1.0 cm for HCC tumors 3.1 to 5.0 cm.
To determine whether AMs >1.0 cm in RF ablation for HCC tumors 3.1 to 5.0 cm might greatly reduce chance of recurrence compared with AMs of 0.5-1.0 cm, a prospective cohort study was performed. From October 2005 to December 2012, 936 consecutive patients with HCC received RF ablation at the Department of Hepatobiliary Surgery, Beijing Chao-yang Hospital Affiliated to Capital Medical University, China. Among them, 327 patients suffered from a single primary HCC tumor each, 3.1 to 5.0 cm in diameter. Written informed consent was obtained from all patients. The study was approved by the investigation and ethics committee of Beijing Chao-yang Hospital, Capital Medical University according to the standards of the Declaration of Helsinki.
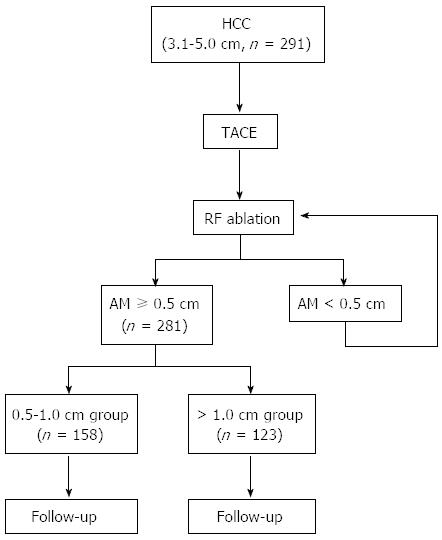
Among the 327 HCC patients, 291 who met the inclusion criteria were enrolled in this study. The inclusion criteria were (1) a single primary HCC tumor 3.1 to 5.0 cm at its greatest diameter on preoperative investigations; (2) no other therapy prior to RF ablation except for trans-arterial chemoembolization (TACE); (3) getting imaging-complete ablation with AM ≥ 0.5 cm after RF ablation; (4) no other therapy in the period of follow-up except for RF ablation or TACE for LTP or IDR; and (5) complete follow-up data. The exclusion criteria were (1) extrahepatic metastasis before LTP or IDR; and (2) follow-up period less than 6 mo. A total of 10 patients were excluded from the study for developing extrahepatic metastasis before LTP or IDR (n = 7), or follow-up period less than 6 mo (n = 3). The remaining 281 patients were included in this study (Table 1). There were 195 men and 86 women. Their median age was 52 years (range, 24-88 years). Of these, 231 (82.2%) were positive for serum hepatitis B virus surface antigen, 18 (6.4%) positive for serum HCV antibody, and 2 (0.7%) positive for both. Liver cirrhosis was observed in 92 patients (32.4%). In 126 of the 281 patients, preoperative diagnosis of HCC was histologically confirmed by needle biopsy under CT guidance. In the remaining 155 patients, HCC was established on the basis of compatible radiological features in contrast-enhanced multiphase helical CT scan and dynamic contrast-enhanced MRI. The median diameter of the HCC nodules was 4.1 cm (range: 3.1-5.0 cm). Tumor location was described according to Couinaud segmental anatomic classification. Liver function was classified according to the Child-Pugh classification. In all, 136 (48.4%) and 145 (51.6%) showed Child-Pugh classes A and B, respectively. Randomization was not performed in this study. The aim of this study was explained to all of the approved patients in advance, and safe AMs > 1.0 cm were tried in all patients, although AMs of ≥ 0.5 cm are routinely considered adequate. On the basis of their AMs, we categorized patients into 2 groups: the 0.5-1.0 cm group and the > 1.0 cm group. Their demographic characteristics are shown in Table 1. The treatment algorithm of the present study is depicted in Figure 1.
| Variable | 0.5-1.0 cm group (n = 158) | > 1.0 cm group (n = 123) | P value1 |
| Age (yr) | 52 (24-88) | 51 (27-84) | 0.467 |
| Gender | |||
| Male/female | 109 (69.0)/49 (31.0) | 86 (69.9)/37 (30.1) | 0.873 |
| Pre-existing hepatitis | |||
| Hepatitis B | 125 (79.1) | 106 (86.2) | 0.133 |
| Hepatitis C | 13 (8.2) | 5 (4.1) | 0.161 |
| Hepatitis B and C | 1 (0.6) | 1 (0.8) | 0.859 |
| Child-Pugh grade | |||
| Class A/class B | 78 (49.4)/80 (50.6) | 58 (47.2)/65 (52.8) | 0.754 |
| Liver cirrhosis | |||
| Yes/No | 55 (34.8)/103 (65.2) | 37 (30.1)/86 (69.9) | 0.417 |
| Serum a-fetoprotein level (ng/mL) | |||
| < 20 | 17 (10.8) | 11 (8.9) | 0.622 |
| 20-200 | 116 (73.4) | 93 (75.6) | 0.681 |
| > 200 | 25 (15.8) | 19 (15.4) | 0.938 |
| Tumor location | |||
| S2,S3,S4,S6,S7/S5,S8 | 73 (46.2)/85 (53.8) | 56 (45.5)/67 (54.5) | 0.923 |
| Biochemical analysis | |||
| AST (IU/L) | 46.5 (11.3-235.2) | 46.1 (12.1-202.7) | 0.793 |
| ALT (IU/L) | 48.4 (15.6-240.0) | 47.9 (13.8-212.4) | 0.560 |
| Alb (g/dL) | 3.7 (2.9-4.2) | 3.6 (2.8-4.2) | 0.460 |
| T-Bil (mg/dL) | 0.9 (0.7-1.4) | 0.9 (0.6-1.4) | 0.594 |
| ALP (IU/L) | 83.5 (7.7-376.6) | 84.2 (8.1-284.8) | 0.744 |
| PT (%) | 80.5 (58-100) | 83.0 (59-100) | 0.262 |
| AFP (ng/mL) | 83.2 (6.3-1301.9) | 82.5 (5.4-750.7) | 0.849 |
| Tumor diameter(cm) | 4.1 (3.1-5.0) | 4.1 (3.3-5.0) | 0.652 |
| No. of ablation sessions before getting AM ≥ 0.5 cm | |||
| 1 session/2 sessions | 115 (72.8)/43 (27.2) | 99 (80.5)/24 (19.5) | 0.143 |
| Approaches of the first ablation session | 0.978 | ||
| Percutaneous | 121 (76.6) | 94 (76.4) | |
| Laparoscopic | 37 (23.4) | 29 (23.6) | |
| Open | 0 (0) | 0 (0) | |
In the study, TACE was performed in all patients, both for radiological assessment of AMs, and for oncological purposes, 2-3 wk before RF ablation by two interventional radiologists (YM Zhou and K Gao) with a standard regimen. TACE was performed through the femoral artery using the technique of Seldinger under local anesthesia[22] by injecting 6-10 mL of an emulsion of iodized oil (Lipiodol; Aulnay-Sous-Bois, France) and 20-40 mg of epirubicin hydrochloride (Zhejiang Haizheng, China) into the tumor feeding arteries. The selected doses of iodized oil and anticancer drug were individually based on the patient’s liver function and tumor size. Injection was discontinued upon full accumulation of iodized oil into the tumor vessels. No gelatin sponge was used after TACE.
A percutaneous approach was most commonly preferred in this study. Laparoscopic RF ablation was considered in the presence of the following: (1) tumors on the liver edge or surface, protruding out of the liver; (2) tumors located in the left lobe, under the bottom of the heart, for which percutaneous RF ablation might cause heart injury; or (3) tumors close to visceral organs, such as the gallbladder, small and large bowels, and stomach. If other intra-abdominal procedures were planned, then open RF ablation was used.
Percutaneous RF ablation was performed under CT guidance (GE Yokogawa Medical Systems Ltd, Tokyo, Japan). To ensure the safety and tolerance of patients, each patient had respiratory control with a tracheal tube or a laryngeal mask airway under intravenous anesthesia during RF ablation procedure. This relatively more invasive protocol also helped improve targeting accuracy by providing a transient stop of respiration at end expiratory status during the procedures of planning and targeting. Laparoscopic RF ablation and open RF ablation were performed with laparoscopic and open surgery techniques as usual with laparoscopic and intraoperative ultrasonographic assistance. All RF procedures in this study were performed using either a 15-gauge multitined electrode (Starburst XL; RITA Medical Systems, Manchester, GA, United States) or Cool-tip ACTC2025 or ACTC1525 electrodes, and an RF generator (RITA 1500; RITA Medical Systems Inc, Manchester, GA, United States or Covidien Healthcare, Ireland), according to their respective manufacturers’ protocols. For patients treated with multitined expandable electrodes, when needle arrays are introduced into the tumor and positioned satisfactorily, the RF generator produces RF energy and maintains an average temperature of 105°C. A series of arrays, radiating from the central hollow probe, are pushed forward and unfolded gradually to 3, 4, or 5 cm until they reach the borders of the tumor. RF energy is delivered at 5-min intervals until the output power drops below 30 W in the final step of the procedure. For patients treated with Cool-tip electrodes, the RF generator (Covidien Healthcare, Ireland) was used. Unlike the RITA electrode, the Cool-tip electrode is straight, without arrays. With a 2.5 cm exposed tip, the Cool-tip electrodes can produce ablation zones of 4.5 cm with a single placement of electrodes and a maximum power of 200 W. Also, the Cool-tip RF generator continuously monitors tissue impedance throughout the procedure and adjusts the output accordingly. For this application, the ablation protocol was preset to the automatic mode, and ablation usually was carried out for 15 to 20 min, which was a little bit longer than that suggested in the manufacturer’s protocol, with the intent of attaining a satisfactory degree of tumor collapse. Cold saline continuous irrigation of the needle was provided with an external pump. In the clinical settings, we selected the type of electrode depending on the size, geometry and location of the index tumor. A multiple-overlapping ablation technique was adopted for pursuing AMs ≥ 0.5 cm. Electrode track ablation technique was also performed to minimize post-procedural bleeding and tumor seeding.
For post-treatment evaluation, contrast-enhanced CT scans were performed 1 mo after the procedure in all cases. The initial unenhanced CT acquisition was followed by enhanced acquisitions obtained at 30 and 70 s after the bolus administration of intravenous contrast material. The contrast agent used was 100 mL iopamidol (Iopromide Injection, Bayer Schering Pharma, Guangzhou, China). Any contrast-enhancing areas beyond the margin of the ablation zone on post-ablation CT indicated incomplete tumor ablation. Additional sessions were scheduled for ablation of residual tumors. The diagnosis and treatment procedures were repeated until imaging complete ablation was achieved (Figure 1).
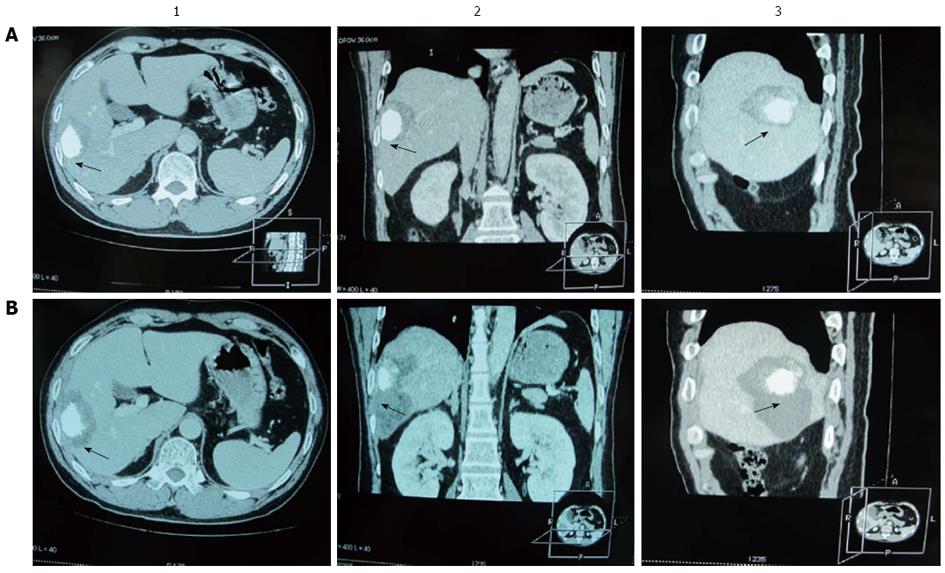
Three-dimensional reconstructions of CT images were made before and after RF ablation (Figure 2). To define the AM as accurate as possible, we performed qualitative side-by-side comparison of CT scans obtained before and after RF ablation. Two radiologists (Cao BX and Qian XJ) who were blind to the results of quantitative analysis assessed in consensus whether an AM of 0.5-1.0 cm or > 1.0 cm was achieved in each case. For this analysis, the adjacent hepatic vessels or the hepatic capsule were used to facilitate comparison. The variation between the two radiologists’ findings was < 5%. In the evaluation, the AM was defined as the narrowest width of the area of low density outside the iodine stain (Figure 2). When the iodine stain in the tumor was not uniform, measuring AM was more difficult. In such instances, when the radiologists did not concur (n = 11), we compared carefully the imaging data before and after RF ablation, outlined the contours of the tumor in this area and measured AM according to the tumor contour rather than the edge of the iodine stain.
The follow-up protocol mainly included routine physical examination, laboratory tests, and measurement of AFP levels every month, as well as dynamic CT studies every 2 or 3 mo. Definitions are based on the standardization by the International Working Group on Image-Guided Tumor Ablation[23]. LTP was defined by the presence of a nodular lesion that was enhanced during the hepatic arterial phase and washed out by the delayed phase, and was found along the peripheral margin of the low-attenuated ablative zone. IDR was defined by a lesion with similar characteristics but not in contact with the original ablation zone in the liver. Overall survival (OS), defined as the interval between date of initial therapy and date of death or the last follow-up examination for living patients, was also evaluated.
In cases of LTP or IDR, other supplemental examinations like hepatic DSA, Lipiodol CT of the liver, CT of the chest and lower abdomen, and bone scintigraphy were performed for other potential tumor nodules. When LTP or IDR was confirmed, patients were hospitalized as soon as possible. Basically, repeat RF ablation treatment cycles were administered for LTP and IDR of ≤ 4 nodules. Five or more IDR nodules, or nodules in unsuitable locations for RF ablation, were treated with TACE. TACE was performed through the femoral artery using the aforementioned technique.
Major complications were assessed on the basis of the previously described guideline for image-guided tumor ablation[23]. Complication rates were evaluated for the total number of ablation sessions. We defined a major complication as an event that led to substantial morbidity and disability, increased the level of care required, resulted in hospital admission, or substantially lengthened the hospital stay. All other complications were considered minor.
Continuous data are expressed as median and range. Comparisons were made with the Mann-Whitney U test. Categorical data were compared using the Fisher’s exact test. Rates of LTP-free survival, IDR-free survival and OS rates were calculated by the Kaplan-Meier method and compared using the log-rank test. Risk factors for LTP, IDR and overall survival were evaluated by univariate analyses using Cox regression tests. If multiple risk factors were shown to be significant by this test, we performed multivariate analysis using Cox regression tests to identify independent prognostic factors for LTP, IDR and OS. All statistical analyses were performed using the SPSS 15.0 statistical software (SPSS Inc., Chicago, Illinois, United States). All reported P values were 2-sided. P < 0.05 was considered statistically significant.
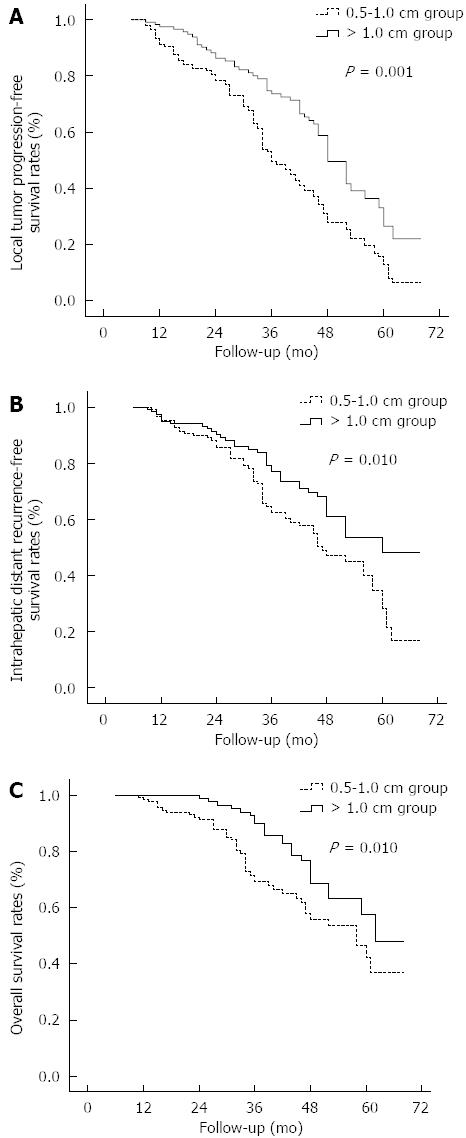
During the follow-up, LTP was found in 112 (70.9%) of 158 patients in the 0.5-1.0 cm group and in 55 (44.7%) of 123 patients in the > 1.0 cm group (P < 0.001) (Table 2). The rates of LTP-only and total LTP in the 0.5-1.0 cm group (46.8% and 70.9%, respectively) were significantly higher than those in the > 1.0 cm group (31.7% and 44.7%, respectively, P = 0.010 and < 0.001, respectively). The 1-, 2-, 3-, 4-, and 5-year LTP-free survival rates were 91.3%, 78.4%, 49.5%, 27.8% and 12.8%, respectively, in the 0.5-1.0 cm group and 97.5%, 86.3%, 73.6%, 49.5% and 26.4%, respectively, in the > 1.0 cm group (Figure 3A), and differed significantly between the two groups (P = 0.001).
| Recurrence pattern | 0.5-1.0 cm group (n = 158) | > 1.0 cm group (n = 123) | P value1 |
| LTP only | 74 (46.8) | 39 (31.7) | 0.01 |
| IDR only | 34 (21.5) | 22 (17.9) | 0.45 |
| LTP + IDR | 38 (24.1) | 16 (13.0) | 0.02 |
| Total LTP | 112 (70.9) | 55 (44.7) | < 0.001 |
| Total IDR | 72 (45.6) | 38 (30.9) | 0.012 |
During the follow-up, IDR was found in 72 (45.6%) of 158 patients in the 0.5-1.0 cm group and 38 (30.9%) of 123 patients in the > 1.0 cm group (Table 2). There was no significant difference in the rates of IDR-only between the 0.5-1.0 cm group and the > 1.0 cm group (21.5% vs 17.9%, P = 0.450). However, the rates of total IDR in the 0.5-1.0 cm group was significantly higher than those in the > 1.0 cm group (45.6% vs 30.9%, P = 0.012). The 1-, 2-, 3-, 4-, and 5-year IDR-free survival rates were 95.2%, 85.9%, 62.6%, 47.2% and 28.5%, respectively, in the 0.5-1.0 cm group and 95.1%, 90.3%, 77.0%, 61.0% and 48.3%, respectively, in the > 1.0 cm group (Figure 3B); the two groups differed statistically (P = 0.010).
As of December 2012 (with a median follow-up of 38.2 mo), 203 patients (72.2%) remained alive, and 78 (27.8%) had died, including 55 patients in the 0.5-1.0 cm group and 23 patients in the > 1.0 cm group. The cause of death was HCC in 58 patients (74.4%), liver failure in 7 (9.0%), upper gastrointestinal bleeding in 3 (3.8%), causes unrelated to liver disease in 4 (including 3 patients who died of cardiovascular disease and 1 of cerebral hemorrhage; 5.1%), and undetermined causes in 6 patients (who died in emergency situations at other hospitals without definite diagnoses related to death; 7.7%). The 1-, 2-, 3-, 4-, and 5-year OS rates were 98.6%, 91.5%, 69.2%, 56.0% and 42.2%, respectively, in the 0.5-1.0 cm group and 100%, 98.9%, 90.1%, 68.7% and 57.4%, respectively, in the > 1.0 cm group (Figure 3C); the two groups differed significantly (P = 0.010, log-rank test).
No procedure-related death was observed. Major complications were observed in 5 (1.8%) of 281 patients. Of these, 2 patients in the 0.5-1.0 cm group and 1 patient in the > 1.0 cm group developed pneumothorax when laparoscopic RF ablation was performed. During the laparoscopic surgery, the presence of pneumothorax was confirmed immediately after the puncture of the RF probe. All of the pneumothorax was successfully treated by chest tube placement at the end of the operation. One patient in the 0.5-1.0 cm group suffered an intra-abdominal hemorrhage. He responded to transfusion of 2 units of packed red blood cells and required no other intervention. Two patients in the > 1.0 cm group developed hemopneumothorax and recovered from chest tightness and chest tube placement. Minor complications, including asymptomatic right pleural effusion, were noted within 3 d of the procedures in 13 patients of the 0.5-1.0 cm group and 15 patients of the > 1.0 cm group (0.5-1.0 cm group vs > 1.0 cm group, P = 0.271); however, none of these patients required interventional-drainage procedures.
Using univariate analysis, the tumor size (4.1-5.0 cm, P = 0.005), AM (> 1.0 cm, P = 0.003), AFP (> 200 ng/mL, P = 0.028), and number of ablation sessions for imaging complete ablation (CA, 2 sessions, P = 0.031) were found to be significant factors for predicting LTP, IDR, and OS (Table 3). In multivariate analyses of the 4 factors that were found to be significant in univariate analysis, the hazard ratios (HRs) for tumor size, AM, AFP, and number of ablation sessions for imaging CA are detailed in Table 4. Only the AM was found to be a significant independent factor linked to LTP, IDR, and OS.
| Significant variable | n | P value1 | ||
| LTP | IDR | OS | ||
| Age (> 65 yr), yes/no | 123/158 | 0.376 | 0.282 | 0.103 |
| Gender (male), yes/no | 195/76 | 0.571 | 0.436 | 0.854 |
| Liver cirrhosis, yes/no | 92/189 | 0.469 | 0.645 | 0.912 |
| Child–Pugh grade (Class B), yes/no | 145/136 | 0.173 | 0.742 | 0.811 |
| Tumor location (S5,S8), yes/no | 152/129 | 0.537 | 0.488 | 0.635 |
| Tumor size (4.1-5.0 cm), yes/no | 154/127 | 0.007 | 0.011 | 0.005 |
| AM (> 1.0 cm), yes/no | 123/158 | 0.001 | 0.024 | 0.003 |
| AST (> 40 IU/L), yes/no | 166/115 | 0.658 | 0.586 | 0.879 |
| ALT (> 40 IU/L), yes/no | 157/124 | 0.672 | 0.460 | 0.734 |
| ALP (> 110 IU/L), yes/no | 120/161 | 0.385 | 0.473 | 0.581 |
| Alb (> 3.5 g/dL), yes/no | 202/79 | 0.622 | 0.564 | 0.838 |
| T-Bil (> 1 mg/dL), yes/no | 93/188 | 0.541 | 0.502 | 0.796 |
| PT (> 70%), yes/no | 216/65 | 0.336 | 0.573 | 0.636 |
| AFP (> 200 ng/mL), yes/no | 44/237 | 0.016 | 0.032 | 0.028 |
| Post-RF ablation antiviral therapy, yes/no | 86/195 | 0.275 | 0.547 | 0.201 |
| Body mass index (> 25 kg/m2), yes/no | 83/198 | 0.611 | 0.582 | 0.913 |
| No. of ablation sessions before getting AM ≥ 0.5 cm (2 sessions), yes/no | 67/214 | 0.034 | 0.041 | 0.031 |
| Approaches of the first ablation session (Laparoscopic), yes/no | 66/215 | 0.459 | 0.383 | 0.728 |
| Significant variable | LTP | IDR | OS | ||||||
| HR | 95%CI | P value1 | HR | 95%CI | P value1 | HR ratio | 95%CI | P value1 | |
| Tumor size (4.1-5.0 cm), yes/no | 1.032 | 0.521-1.376 | 0.475 | 0.891 | 0.452-1.602 | 0.744 | 0.882 | 0.673-1.572 | 0.084 |
| AM (> 1.0 cm), yes/no | 1.484 | 0.101-1.812 | 0.001 | 1.278 | 1.137-1.729 | 0.025 | 1.604 | 0.881-2.753 | 0.002 |
| AFP (> 200 ng/mL), yes/no | 0.947 | 0.540-1.050 | 0.531 | 0.509 | 0.370-1.215 | 0.546 | 1.007 | 0.639-1.158 | 0.748 |
| No. of ablation sessions before getting AM ≥ 0.5 cm (2 sessions), yes/no | 1.012 | 0.683-1.772 | 0.663 | 0.923 | 0.562-1.218 | 0.347 | 0.745 | 0.321-.431 | 0.325 |
Over the past years, the rapid development and refinement of RF ablation technology has led to increasing use of this treatment modality in HCC patients[24]. Extensive clinical studies support RF ablation as a preferred treatment for very early HCC[25]. However, tumor recurrence, including LTP and IDR, frequently occurs, affecting the prognosis. Furthermore, rapid tumor progression after RF ablation, which may mostly be associated with the progression of residual HCC, has been gaining increasing attention[26-29]. These experimental data indicated that any residual tumors might be a disaster for individual HCC patients who received RF ablation. Thus, we should try to remove microvascular invasions and satellite micronodules around the main tumor of HCC to decrease the likelihood of residual tumor, the incidence rates of LTP, IDR and the rapid tumor progression.
From perspective of pathological clearance of the tumor tissue, AMs should be as wide as possible. However, correctly obtaining a sufficient AM around all sides of a tumor of medium size is not easy. Specifically, to get a 1.0-cm AM for lesions sized 3.0, 4.0 and 5.0 cm in diameters, the volumes of peritumoral tissue to ablate are 3.6, 2.4 and 1.7 times, respectively, the volume of the main tumor[30]. A little increase of the dimension of the ablation zone means a tremendous increase of the amount of ablation tissue. For example, if we increase the AM from 1.0 to 1.5 cm for a lesion of 4.0 or 5.0 cm in diameter, respectively, the amounts of tissue to ablate will be theoretically increased by as much as 58.8% and 49.3% respectively, and this does not take into account the increased difficulty and time to accomplish it. So we should balance the perfect AM standard against its feasibility in clinical practice. As a compromise settlement, we take an AM of more than 1.0 cm as a minimum requirement for HCC nodules of 3.1 to 5.0 cm.
Although an AM > 1.0 cm cannot guarantee that all peritumoral tumor tissues are ablated in all cases, 2 ways can be used to compensate for the conservativeness of this standard. First, there may be some decrease in the size of the ablation zone due to tissue healing and scar formation when measured 1 mo after ablation and the real AM might be underestimated in some extent. Second, in our performance of RF ablation treatment, we took an AM of more than 1 cm thick as the least requirement and tried to make it as wide as possible.
The precise evaluation of AM after RF ablation is rather difficult, especially for HCC lesions of medium size or larger which did not get TACE/trans-arterial embolization (TAE) pretreatment[3]. First, AM area is not clearly visualized on images because both the ablated tumor and AM appear as areas that lack contrast enhancement. Second, AMs around tumors are usually not symmetrical and the measurement of AM by subtracting the diameter of the index tumor from that of ablation zone is not as precise as expected. So in the present study, all enrolled patients received TACE pretreatment, aiming to facilitate the evaluation of AM as precise as possible.
We demonstrated that tumor size, AM, AFP, and number of ablation sessions before getting an AM > 1.0 cm were significant risk factors for LTP, IDR, and OS of HCC after RF ablation, using univariate analysis. Our results were in line with those of previous studies on risk factors related to recurrence of HCC after RF ablation[11,31]. However, only AM was found to be a significant independent factor linked to LTP, IDR, and OS of HCC after RF ablation using multivariate analysis. These findings confirmed that AMs > 1.0 cm are an important predictive factor for recurrence of HCC tumors 3.1 to 5.0 cm after RF ablation. It is easy to understand that an AM >1.0 cm can reduce rates of LTP and IDR and eventually increase OS rate. An AM > 1.0 cm can lead to a further clearance of possible residues of microvascular invasion and satellite micronodules by ablating more viable tumor containing liver parenchyma and decrease probability of metastasis of the residual tumor cell by intrahepatic portal vein.
We are aware of the limitations of this analysis. This study was not randomized. Moreover, the AM standard of 1.0 cm referred only to the safety margin for liver resection advocated by most surgeons. Also, the biological nature of HCC as a commonly accepted important risk factor was not demonstrated in this study due to the limited number of cases with pathological examination. One further limitation is the fact that this was a single-center study; these results might not be reproducible consistently in other settings. The results may be influenced by the physicians’ expertise and the institution’s volume of care. Nevertheless, our data may be helpful for clinicians who treat HCC with RF ablation and may also be useful as a basis for the design of future trials. Again, more prospective, large randomized studies are needed to assess the benefit of AMs > 1.0 cm for medium-sized HCC lesions in patients who receive RF ablation.
Notwithstanding its preliminary character, this study does provide evidence that, for HCC tumors 3.1 to 5.0 cm, AMs > 1.0 cm could reduce chance of recurrence compared with AMs of 0.5-1.0 cm, which emphasizes the need for more defensive strategies in using AMs wider than 1.0 cm for ablation of HCC tumors 3.1 to 5.0 cm. However, confirmation in a prospective multicenter randomized trial is required.
Radiofrequency (RF) ablation is becoming accepted as a promising technique for treatment of patients with hepatocellular carcinoma (HCC). Recurrence of HCC after RF ablation occurs frequently and is associated with poor prognosis. A sufficient ablative ablative margin (AM) is an essential way to minimize the risk of recurrence. However, the optimal AM for HCC tumors 3.1 to 5.0 cm in size is unclear.
Theoretically, AMs that encompass both the main tumor and an area of adjacent parenchyma that contains microvascular invasion or satellite micronodules, ensuring a pathological complete ablation, will minimize risk of tumor recurrence. A current research hotspot is to affirm that, for HCC tumors 3.1 to 5.0 cm, AMs > 1.0 cm reduce recurrence risk compared with AMs of 0.5-1.0 cm.
Accumulating data show that the recurrence rates after RF ablation, such as local tumor progression (LTP) or intrahepatic distant recurrence (IDR) rate, are quite different in varied-sized tumors with similar AMs. For HCC tumors ≤ 3.0 cm, an AM of 0.5-1.0 cm may remove most peritumoral lesions. However, for HCC tumors 3.1 to 5.0 cm, AMs ≤ 1.0 cm seem insufficient to ensure pathological complete tumor clearance in most cases. In this study, we confirmed that AMs > 1.0 cm are an important predictor for recurrence of HCC tumors 3.1 to 5.0 cm after RF ablation.
The study results provide evidence that, for HCC tumors 3.1 to 5.0 cm, AMs > 1.0 cm could reduce chances of recurrence compared with AMs of 0.5-1.0 cm, emphasizing the need for a more defensive strategy that used AMs > 1.0 cm for ablation of HCC tumors 3.1 to 5.0 cm.
LTP was defined by the presence of a nodular lesion that was enhanced during the hepatic arterial phase and washed out by the delayed phase and was found along the peripheral margin of the low-attenuated ablative zone. IDR was defined by a lesion with similar characteristics that did not contact the original ablation zone in the liver. Overall survival was defined as the interval between date of initial therapy and date of death, or the last follow-up examination for living patients.
This is a good prospective cohort study in which the authors analyzed the survival benefit of radiofrequency AMs > 1.0 cm for HCC tumors 3.1 to 5.0 cm. The results are interesting and suggest that for HCC tumors 3.1 to 5.0 cm, AMs > 1.0 cm could reduce chance of recurrence compared with narrower AMs of 0.5-1.0 cm, emphasizing the need for a more defensive strategy, using wider AMs > 1.0 cm for ablation of HCC tumors 3.1 to 5.0 cm.
P- Reviewers: Cucchetti A, Radu A, Xu HX S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg. 2009;249:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 241] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 2. | Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 407] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 3. | Nakazawa T, Kokubu S, Shibuya A, Ono K, Watanabe M, Hidaka H, Tsuchihashi T, Saigenji K. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007;188:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 271] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 4. | Livraghi T. Radiofrequency ablation of hepatocellular carcinoma. Surg Oncol Clin N Am. 2011;20:281-99, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Kim H, Rhim H, Choi D, Lim HK, Kim YS, Lee WJ, Joh JW. Recurrence and treatment pattern in long-term survivors with hepatocellular carcinoma: a comparison between radiofrequency ablation and surgery as a first-line treatment. World J Surg. 2010;34:1881-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, Watanabe Y, Kojiro M, Sata M. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 331] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 7. | Zytoon AA, Ishii H, Murakami K, El-Kholy MR, Furuse J, El-Dorry A, El-Malah A. Recurrence-free survival after radiofrequency ablation of hepatocellular carcinoma. A registry report of the impact of risk factors on outcome. Jpn J Clin Oncol. 2007;37:658-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Shindoh J, Hasegawa K, Inoue Y, Ishizawa T, Nagata R, Aoki T, Sakamoto Y, Sugawara Y, Makuuchi M, Kokudo N. Risk factors of post-operative recurrence and adequate surgical approach to improve long-term outcomes of hepatocellular carcinoma. HPB (Oxford). 2013;15:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Shi M, Zhang CQ, Zhang YQ, Liang XM, Li JQ. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg. 2004;28:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 144] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (& gt; 2 and & lt; 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010;195:758-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 11. | Kim YS, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol. 2006;59:432-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Nishikawa H, Inuzuka T, Takeda H, Nakajima J, Sakamoto A, Henmi S, Matsuda F, Eso Y, Ishikawa T, Saito S. Percutaneous radiofrequency ablation therapy for hepatocellular carcinoma: a proposed new grading system for the ablative margin and prediction of local tumor progression and its validation. J Gastroenterol. 2011;46:1418-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Kim JH, Won HJ, Shin YM, Kim SH, Yoon HK, Sung KB, Kim PN. Medium-sized (3.1-5.0 cm) hepatocellular carcinoma: transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol. 2011;18:1624-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 431] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 15. | Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 634] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 16. | Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 594] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 17. | Choi D, Lim HK, Rhim H, Kim YS, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS, Yoo BC. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 245] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 18. | Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116:5452-5460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 249] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 19. | Okusaka T, Okada S, Ueno H, Ikeda M, Shimada K, Yamamoto J, Kosuge T, Yamasaki S, Fukushima N, Sakamoto M. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer. 2002;95:1931-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 181] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | McHugh PP, Gilbert J, Vera S, Koch A, Ranjan D, Gedaly R. Alpha-fetoprotein and tumour size are associated with microvascular invasion in explanted livers of patients undergoing transplantation with hepatocellular carcinoma. HPB (Oxford). 2010;12:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Ikeda K, Seki T, Umehara H, Inokuchi R, Tamai T, Sakaida N, Uemura Y, Kamiyama Y, Okazaki K. Clinicopathologic study of small hepatocellular carcinoma with microscopic satellite nodules to determine the extent of tumor ablation by local therapy. Int J Oncol. 2007;31:485-491. [PubMed] |
| 22. | Matsui O, Kadoya M, Yoshikawa J, Gabata T, Takashima T, Demachi H. Subsegmental transcatheter arterial embolization for small hepatocellular carcinomas: local therapeutic effect and 5-year survival rate. Cancer Chemother Pharmacol. 1994;33 Suppl:S84-S88. [PubMed] |
| 23. | Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, Gervais DA, Gillams AR, Kane RA, Lee FT. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20:S377-S390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 325] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 24. | Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 25. | Chen MS, Peng ZW, Xu L, Zhang YJ, Liang HH, Li JQ. Role of radiofrequency ablation in the treatment of hepatocellular carcinoma: experience of a cancer center in China. Oncology. 2011;81 Suppl 1:100-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Kong J, Kong J, Pan B, Ke S, Dong S, Li X, Zhou A, Zheng L, Sun WB. Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF-1α/VEGFA. PLoS One. 2012;7:e37266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 27. | Ke S, Ding XM, Kong J, Gao J, Wang SH, Cheng Y, Sun WB. Low temperature of radiofrequency ablation at the target sites can facilitate rapid progression of residual hepatic VX2 carcinoma. J Transl Med. 2010;8:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Kong J, Kong L, Kong J, Ke S, Gao J, Ding X, Zheng L, Sun H, Sun W. After insufficient radiofrequency ablation, tumor-associated endothelial cells exhibit enhanced angiogenesis and promote invasiveness of residual hepatocellular carcinoma. J Transl Med. 2012;10:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Ruzzenente A, Manzoni GD, Molfetta M, Pachera S, Genco B, Donataccio M, Guglielmi A. Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World J Gastroenterol. 2004;10:1137-1140. [PubMed] |
| 30. | Dodd GD, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol. 2001;177:777-782. [PubMed] |
| 31. | Fernandes ML, Lin CC, Lin CJ, Chen WT, Lin SM. Risk of tumour progression in early-stage hepatocellular carcinoma after radiofrequency ablation. Br J Surg. 2009;96:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |