Published online Nov 14, 2013. doi: 10.3748/wjg.v19.i42.7302
Revised: October 2, 2013
Accepted: October 19, 2013
Published online: November 14, 2013
Processing time: 151 Days and 17.5 Hours
Drug absorption in patients with chronic pancreatitis might be affected by the pathophysiology of the disease. The exocrine pancreatic insufficiency is associated with changes in gastrointestinal intraluminal pH, motility disorder, bacterial overgrowth and changed pancreatic gland secretion. Together these factors can result in malabsorption and may also affect the efficacy of pharmacological intervention. The lifestyle of chronic pancreatitis patients may also contribute to gastrointestinal changes. Many patients limit their food intake because of the pain caused by eating and in some cases food intake is more or less substituted with alcohol, tobacco and coffee. Alcohol and drug interaction are known to influence the pharmacokinetics by altering either drug absorption or by affecting liver metabolism. Since patients suffering from chronic pancreatitis experience severe pain, opioids are often prescribed as pain treatment. Opioids have intrinsic effects on gastrointestinal motility and hence can modify the absorption of other drugs taken at the same time. Furthermore, the increased fluid absorption caused by opioids will decrease water available for drug dissolution and may hereby affect absorption of the drug. As stated above many factors can influence drug absorption and metabolism in patients with chronic pancreatitis. The factors may not have clinical relevance, but may explain inter-individual variations in responses to a given drug, in patients with chronic pancreatitis.
Core tip: In patients with chronic pancreatitis several pathophysiological factors can account for malabsorption and may also affect the efficacy of pharmacological intervention by reduced drug absorption. For example it can be speculated that changes in gastrointestinal intraluminal pH, motility disorder, bacterial overgrowth and changed pancreatic gland secretion may contribute. The lifestyle of chronic pancreatitis patients may also be a factor to gastrointestinal changes. The factors may not have clinical relevance, but may explain inter-individual variations in responses to a given drug, in patients with chronic pancreatitis.
- Citation: Olesen AE, Brokjaer A, Fisher IW, Larsen IM. Pharmacological challenges in chronic pancreatitis. World J Gastroenterol 2013; 19(42): 7302-7307
- URL: https://www.wjgnet.com/1007-9327/full/v19/i42/7302.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i42.7302
Chronic pancreatitis is a persistent inflammation of the pancreas that results in irreversible morphological changes and impairment of both exocrine and endocrine functions[1]. The etiology of chronic pancreatitis is multi-factorial and the risk factors include alcohol and nicotine consumption, hereditary factors, efferent duct obstructions, immunological factors or rare metabolic disorders[2]. It is well known that pancreatitis patients suffer from malabsorption[3-5] and it could be hypothesized that this would also affect drug absorption.
Drug absorption in patients with gastrointestinal disorders can be influenced by alterations in several factors. For example; gastric and intestinal motility, changes in the mucosal surface area available for drug absorption, and altered physical and chemical properties of the intestinal luminal content. These properties are usually changed in combination and the degree of each factor impact is dependent on the duration and severity of the disease[6]. Despite this, most of the data about bioavailability of orally administered drugs are obtained from healthy individuals.
The knowledge about drug absorption in patients with chronic pancreatitis is limited. It has been demonstrated that the pharmacokinetic profile of pregabalin was not extensively affected by chronic pancreatitis[7]. However, inter-individual variations were found and several factors affecting drug pharmacokinetic profiles in patients with chronic pancreatitis may be relevant to consider.
This aim of this review was therefore to evaluate different factors which could possibly affect drug absorption in chronic pancreatitis patients leading to pharmacological challenges in this patient group. Moreover, suggestions on how to diminish the impact of these factors will be provided.
Two primary factors influencing bioavailability of orally administered medications is the amount of drug absorbed, and metabolism by the liver. Hence, any factor influencing the gastrointestinal tract can alter drug absorption, such as gastric pH, regional blood flow, mucosal surface area and gut motility[8].
Chronic pancreatitis is a clinical condition in which exocrine pancreatic insufficiency occur leading to secondary maldigestion. Several causes of exocrine pancreatic insufficiency may be associated with changes in gastrointestinal physiology such as: (1) changes in gastrointestinal intraluminal pH; (2) motility disorders; (3) bacterial overgrowth; and (4) pancreatic secretion.
Together these factors can result in malabsorption and may also affect the efficacy of pharmacological treatment in exocrine pancreatic insufficiency[8]. The main clinical manifestation of exocrine pancreatic insufficiency is fat malabsorption, identified as steatorrhea[4]. Steatorrhea leads to deficit of fat-soluble vitamins (A, D, E and K) with consequent clinical manifestations[4]. Moreover, fat malabsorption may affect absorption of lipophilic drug formulations and as such deserves particular attention[6].
Changes in intraluminal pH: Low intraluminal pH in the upper small intestine might be a factor in the pathogenesis of fecal loss of bile acids in pancreatic insufficiency[4]. The drug’s ability to cross membranes is determined by the environmental pH and the acid dissociation constant (pKa). Therefore, decreased intraluminal pH may affect drug absorption. However, this may be of variable importance, as changes in intraluminal pH might be negligible in some cases and more pronounced in others.
Motility disorders: The rate of gastric emptying and intestinal transit is abnormal in patients with chronic pancreatitis and this may affect the efficacy of treatments[8]. Gastric emptying can be accelerated due to diarrhea[4] or decreased by, e.g., opioids. Because most drugs are absorbed through the small intestines, delayed gastric emptying will prolong the time to peak concentration and delay the onset of the action of a drug[9]. Clinicians should be aware that delayed gastric emptying can delay the onset of action of a medication if administered orally[9]. On the other hand accelerated gastric emptying will have the opposite effect. Taken together gastric emptying is likely to be a rate limiting step in drug absorption unless normal absorption (prolonged release) is slow[10].
Bacterial overgrowth: One mechanism which has been hypothesized between maldigestion and intestinal alterations relates to bacterial overgrowth in the small intestine. It has been speculated that lack of coordination between motor activity and peak of secretory activity in the gastrointestinal tract may reduce the effectiveness of the “housekeeper” function and thereby contribute to the intestinal bacterial overgrowth often observed in patients with chronic pancreatitis[11]. Bacterial overgrowth might either contribute to diarrhea or account for the persistence of diarrhea[4]. Moreover, bacterial overgrowth might give rise to bile acid malabsorption and changes in intestinal permeability[4,12]. Bacterial overgrowth may interfere with the normal intestinal environment and lead to atrophic mucosa with structural abnormalities which can decrease drug absorption[13].
Pancreatic gland secretion: The pancreatic gland normally secretes more than 2 L of juice per day (high protein content) with enzymes able to digest lipids, proteins and carbohydrates. Patients with exocrine pancreatic insufficiency exhibit decreased pancreatic bicarbonate secretion resulting in reduction in duodenal pH postprandial, leading to inactivation of orally administered exogenous enzymes[6]. In the same line acidic inactivation of pancreatic enzymes is considered a significant reason for failure of drug therapy[8].
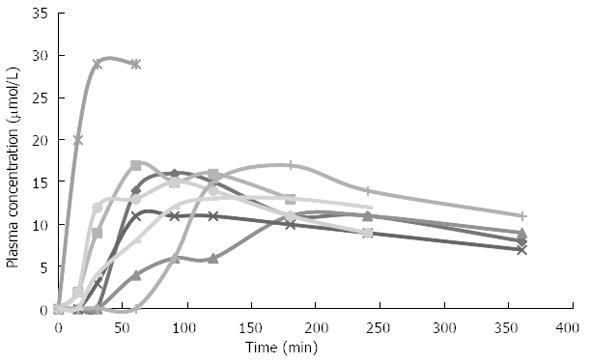
A combination of the above mentioned factors may affect drug absorption in patients with chronic pancreatitis. Figure 1 illustrates the variance in absorption of pregabalin in a group of women with chronic pancreatitis.
To our knowledge only one study investigated hepatic drug metabolism in patients with chronic pancreatitis and found reduced drug elimination capacity in this group of patients[14]. The results were most probably explained by the patients’ general state, with a fairly overt malnutrition, although theoretically, a subclinical, probably alcohol-induced, liver affection could not be ruled out[14]. Interestingly, the study results yielded the need for caution upon administration of drugs that are biotransformed in the liver.
Thus, to optimize the efficacy of pharmaceutical treatment, the management of exocrine pancreatic insufficiency should ideally be individually tailored to account for both the underlying cause and any associated disturbance in gastrointestinal physiology.
The gastrointestinal physiological changes in chronic pancreatitis can be further affected by patients’ lifestyle. Many patients limit their food intake because of the pain caused by eating and in some cases food intake is more or less substituted with tobacco and coffee[15]. Due to malabsorption and lifestyle factors, chronic pancreatitis patients are likely to have a lower body mass index than those not suffering from this disease[15]. Thus, body mass index is an easy accessible but important factor to consider in dose decision in these patients, as drug disposition might be affected. This is especially important when drugs with a narrow therapeutic index, such as, e.g., warfarin and digoxin are prescribed[14].
Furthermore, decreased food intake, could be an important factor in several cases of chronic pancreatitis as food intake will affect some of the physiological processes, e.g., changes in pH, reduced gastric emptying time, increased gall secretion, increased motility, increased gastrointestinal and liver blood flow. Most of these processes are already affected by the pancreatitis and hence these changes could possibly affect drug bioavailability. Consequently, reduced food intake in itself will worsen gastrointestinal physiological processes.
Insufficient food intake due to nausea, anorexia or alcoholism may also be of some significance[16]. Alcohol abuse is a well known etiological factor (alcoholic chronic pancreatitis) and the lifestyle of the alcoholic chronic pancreatitis patient group is in general characterized by excessive alcohol consumption and smoking. Together, these factors can be further accompanied by insufficient food intake and will eventually also lead to malnutrition.
Alcohol can be used as a central nervous system depressant to relieve pain and concerns about the disease. Therefore many patients with chronic pancreatitis continue their alcohol consumption throughout disease progression. In relation to alcohol induced chronic pancreatitis it is relevant to consider how the lifestyle with regard to alcohol- and alcohol related dietary habits may provide pharmacologically challenges. The most frequent pharmacological interaction is the combination of alcohol with other depressors of the central nervous system[17]. Moreover, alcohol drug interactions can influence the pharmacokinetics of a drug by altering the drug absorption or by affecting the liver metabolism. The effect will vary with, e.g., the amount of alcohol consumed, the nature of the drug, the dosage and how the drug is administered. There are various interactions between alcohol and drugs. Antihistamines, analgesics and antidepressants are examples of drugs which may interact with alcohol.
The liver is the primary site of drug metabolism and the cytochrome P450 mixed-function oxidase enzyme system, is primarily responsible for this process. Alcohol inhibits the oxidation of drugs by cytochrome P450 isoenzyme (CYP2E1). In contrast, chronic alcohol consumption will cause induction of the CYP2E1 leading to increased drug metabolism. Previously, concerns have been raised regarding interactions of paracetamol and alcohol. However, paracetamol interaction is only a problem when ingestion of alcohol is suddenly stopped. Consequently, the hepatic glutathione is unable to detoxify which leads to irreversible hepatic damage. Therefore, in chronic alcoholic patients the consumption of alcohol should not be suspended on prescribing paracetamol[17]. Additionally, extensive alcohol consumption may result in gastric mucosal injury and hereby further affect drug absorption[8].
Smoking may increase hepatic drug metabolism to a significant extent[14] and may in addition result in induction of the cytochrome oxidases[18]. It has also been demonstrated that body mass index is associated with cigarette smoking in chronic alcoholic-associated pancreatitis patients; the more cigarettes smoked per day, the lower the mean body mass index[19]. This is in line with the hypothesis that alcohol consumption and smoking can cause insufficient food intake and hereby affecting drug absorptions.
Abdominal pain is usually the symptom that causes patients with chronic pancreatitis to seek medical attention. In parallel, they can have a history of alcoholic abuse making opioids, with their associated abuse potential together with other side effects as, for example, bowel dysfunction, less suitable for these patients and other treatment regimens should be considered[7]. Recently it was demonstrated that pregabalin was superior to placebo for attenuation of experimental visceral pain in chronic pancreatitis patients[20]. Therefore, pregabalin may be used to treat pain in patients with chronic pancreatitis when there is a conflict or concern with abuse.
A summary of pathophysiological effects on drug absorption is given in Table 1.
| Pathophysiology | Effect on drug absorption |
| Low intraluminal pH | The drugs ability to cross the luminal wall |
| Motility disorder | Delayed drug effect |
| Bacterial overgrowth | Decreased absorption due to diarrhea or structural abnormalities |
| Pancreatic secretion | Lack of enzymes leads to inactivation of pro-drugs |
| Steatorrhea | Problems with absorption of lipophilic drugs |
| Low body mass index | Increased plasma concentration |
The physiological processes affected by chronic pancreatitis are widespread. This section will focus on how to treat the pathophysiology of chronic pancreatitis in order to reduce the impact of factors possibly affecting drug absorption.
Enzyme therapy: The main goal with enzyme treatment is to achieve optimal enzyme activity in the duodenum[21] and hereby improve the nutritional status, preventing weight loss, vitamin deficiencies and exocrine pancreatic insufficiency related symptoms, resulting in steatorrhea. The most widely used enzyme preparation is porcine pancreatin. The preparation contains a mixture of protease, lipase and amylase[22]. Löhr et al[23] analyzed the effectiveness of different preparations and concluded that overall pancreatin preparation replacements must contain high lipase activity. Lipase of porcine pancreatin is destroyed by protease and acids, thus it is necessary to protect the pancreatin against the influence of gastric acids. Another factor that is of great importance is the particle size and the rate of which the porcine pancreatin is released into the duodenum. The best particle size is assumed to be a diameter of ≤ 2 mm, since these particles leaves the stomach at the same time as solid food. The enzymes should be released within 30 min[24]. Pancreatic enzyme supplements improve fat absorption[25], and hence reduces steatorrhea[26] and this may have beneficial effects on drug absorption. In contrast high-dose enzyme replacement therapy with or without gastric acid suppression may cause additional challenges related to drug absorption and interactions if additional drug therapy is required[6]. Thus, enzyme treatment can either enhance or complicate drug absorption in different aspects and this should be considered in the pharmacological management of clinical symptoms.
More invasive treatment is recommended for patients with pancreatic duct stones and pancreatic obstruction in whom standard medical therapy is not sufficient. The goals of endoscopy and surgery are to decompress ducts, dilate stricture with stent placement and preserve pancreatic tissue and adjacent organs[27,28]. Endoscopic therapy should be the first-line option because it is less invasive than surgery[29]. Surgery should be the first-line option in patients in whom endoscopic therapy failed or those with pancreatic mass with suspicion of malignancy[29]. It has been assumed that invasive procedures designed to improve drainage of the main pancreatic duct, will result in decreased pain. It could therefore be hypothesized that pain attenuation can improve the lifestyle and hereby indirectly affecting drug absorption. There is consensus that endoscopical and surgical management have both benefits and harms[26] and it has been suggested that management may include medical, endoscopic and surgical approaches with the interaction between various specialties, calling for a concerted multidisciplinary approach[28]. Moreover, as surgery has a low rate of success with attendant morbidity, mortality and slow recovery rates, it is not considered an option for optimizing absorption of drug in the pharmacological management.
Patients with chronic pancreatitis are often advised to eat small meals with low-fat content (< 20 g of fat) in an attempt to decrease the need of pancreatic secretion[30]. Low-fat diets decreases the amount of overall fat presented to the intestine for digestion and absorption, and may be helpful alleviating steatorrhea[26] and hereby could lead to better conditions for some drug absorptions. However, a systematic review of benefits and harms of low-fat diet in chronic pancreatitis found no studies of sufficient quality to confirm the effect of low-fat diet[31]. Thus, if people are given pancreatic enzyme supplements, they are usually advised to maintain a normal diet, as there is no need to lower fat intake alongside enzyme supplementation[31].
Overeating is dissuaded, instead smaller meals on a more frequent basis is preferred. Frequent meals will also benefit gastric motility and result in more normal gastrointestinal conditions, and hereby improve drug absorption. The restriction in fat intake should be monitored by a dietitian who follows the total caloric intake and the diet should compensate the loss in caloric intake by carbohydrate-enriched diet. On a carbohydrate-enriched diet 65%-70% of the total daily energy intake should derive from carbohydrate. Hereby, it is possible for the patient to gain weight, which again will be beneficial in several ways, e.g., in relation to drug absorption, where body weight and body composition is directly related to drug distribution volumes.
In general alcohol consumption and smoking should be diminished or avoided to reduce the impact on the pharmacokinetic profiles. Total alcohol abstinence is only recommended for patients whose chronic pancreatitis is derived from alcohol abuse.
Patients with painful chronic pancreatitis are often treated with opioids which lead to diverse issues and alterations in the gastrointestinal tract. Opioids have intrinsic effects on gastrointestinal motility and can modify the absorption of other drugs taken at the same time[10]. It has been demonstrated that, e.g., tramadol is an effective oral opioid analgesic for reducing pain in people with chronic pancreatitis, but it is also associated with gastrointestinal adverse effect[26]. Opioids will affect opioid receptors in the enteric nervous system and will cause changes in motility, sphincter function and secretion which affect absorption leading to opioid induced bowel dysfunction[32].
Opioid induced decreased motility occurs throughout the entire gastrointestinal tract. In the circular muscle in the small and large intestine, opioids induce increased resting contractile tone and decreases tonic inhibition of the muscle tone, which leads to increased tone in the circular muscle layer. This is accompanied by occasional occurrence of high-amplitude, non-propulsive phasic contractions enhanced by rhythmic contractions and associated changes in smooth muscle electrical activity. These motility abnormalities result in decreased propulsive forward peristalsis, and increased segmental contraction which in clinical settings manifests as constipation, abdominal cramps and gut spasm[33-35]. An additional consequence of this peristaltic disruption is stasis of luminal contents, which leads to increased passive fluid absorption[36]. Furthermore, intestinal fluid secretion is inhibited by opioids directly via the enteric nervous system. In the sympathetic nervous system opioids also increase activity and thereby decrease the secretion. Serotonin and noradrenaline as terminal transmitters seems to dominate the local effect[37,38]. An overall decreased gut secretion of intestinal fluids takes place and together leads to harder and dryer stools[36-38]. Therefore, patients treated with opioids might have even more pronounced decreased motility which will complicate drug absorption further. Furthermore, as solid drug forms must dissolve before absorption can occur; dissolution rate determines availability of the drug for absorption. The increased fluid absorption caused by opioid effects will decrease water available for drug dissolution and may hereby affect absorption. If dissolution is slower than absorption it becomes the rate-limiting step.
Several factors might affect drug absorption and metabolism in patients with chronic pancreatitis: gastric pH, regional blood flow, mucosal surface area and gut motility. The impact of these factors on drug absorption may be reduced by treating the pathophysiology of chronic pancreatitis. Treatment can be pharmacological management, enzyme therapy, endoscopic therapy or surgical management. Moreover, as gastrointestinal physiological changes in chronic pancreatitis can be affected by patients’ lifestyle, lifestyle changes may lead to more optimal drug absorption. However, issues raised in this review may not have clinical relevance, but could explain part of the variation observed in drug effects in this patient group.
P- Reviewers: Hardt PD, Hackert T, Lee SW S- Editor: Wen LL L- Editor: A E- Editor: Zhang DN
| 1. | Chen JM, Férec C. Genetics and pathogenesis of chronic pancreatitis: the 2012 update. Clin Res Hepatol Gastroenterol. 2012;36:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1341] [Article Influence: 111.8] [Reference Citation Analysis (0)] |
| 3. | Hammer HF. Pancreatic exocrine insufficiency: diagnostic evaluation and replacement therapy with pancreatic enzymes. Dig Dis. 2010;28:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Pezzilli R. Chronic pancreatitis: maldigestion, intestinal ecology and intestinal inflammation. World J Gastroenterol. 2009;15:1673-1676. [PubMed] |
| 5. | Forsmark CE. Chronic pancreatitis and malabsorption. Am J Gastroenterol. 2004;99:1355-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Dressman JB, Lennernäs H. Oral Drug Absorption, Prediction and Assessment. New York: Marcel Dekker Inc 2005; . |
| 7. | Olesen AE, Olofsen E, Olesen SS, Staahl C, Andresen T, Dahan A, Drewes AM. The absorption profile of pregabalin in chronic pancreatitis. Basic Clin Pharmacol Toxicol. 2012;111:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Bruno MJ, Haverkort EB, Tytgat GN, van Leeuwen DJ. Maldigestion associated with exocrine pancreatic insufficiency: implications of gastrointestinal physiology and properties of enzyme preparations for a cause-related and patient-tailored treatment. Am J Gastroenterol. 1995;90:1383-1393. [PubMed] |
| 9. | Smith BS, Yogaratnam D, Levasseur-Franklin KE, Forni A, Fong J. Introduction to drug pharmacokinetics in the critically ill patient. Chest. 2012;141:1327-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Prescott LF. Gastric emptying and drug absorption. Br J Clin Pharmacol. 1974;1:189-190. [PubMed] |
| 11. | Pieramico O, Dominguez-Muñoz JE, Nelson DK, Böck W, Büchler M, Malfertheiner P. Interdigestive cycling in chronic pancreatitis: altered coordination among pancreatic secretion, motility, and hormones. Gastroenterology. 1995;109:224-230. [PubMed] |
| 12. | Mathias JR, Clench MH. Review: pathophysiology of diarrhea caused by bacterial overgrowth of the small intestine. Am J Med Sci. 1985;289:243-248. [PubMed] |
| 13. | Domínguez-Muñoz JE. Pancreatic exocrine insufficiency: diagnosis and treatment. J Gastroenterol Hepatol. 2011;26 Suppl 2:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Andersen V, Sonne J, Larsen S. Antipyrine, oxazepam, and indocyanine green clearance in patients with chronic pancreatitis and healthy subjects. Scand J Gastroenterol. 1999;34:813-817. [PubMed] |
| 15. | Turner RC, Brazionis LB, McDermott R. Intake patterns of food nutrients and other substances associated with chronic pancreatitis. Pancreatology. 2013;13:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Armbrecht U. [Chronic pancreatitis: weight loss and poor physical performance - experience from a specialized rehabilitation centre]. Rehabilitation (Stuttg). 2001;40:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 17. | Gómez-Moreno G, Guardia J, Cutando A. Interaction of paracetamol in chronic alcoholic patients. Importance for odontologists. Med Oral Patol Oral Cir Bucal. 2008;13:E235-E238. [PubMed] |
| 18. | Acheson DW, Hunt LP, Rose P, Houston JB, Braganza JM. Factors contributing to the accelerated clearance of theophylline and antipyrine in adults with exocrine pancreatic disease. Clin Sci (Lond). 1989;76:377-385. [PubMed] |
| 19. | Talamini G, Vaona B, Bassi C, Bovo P, Damoc T, Mastromauro M, Falconi M, Vantini I, Cavallini G, Pederzoli P. Alcohol intake, cigarette smoking, and body mass index in patients with alcohol-associated pancreatitis. J Clin Gastroenterol. 2000;31:314-317. [PubMed] |
| 20. | Olesen SS, Graversen C, Olesen AE, Frøkjaer JB, Wilder-Smith O, van Goor H, Valeriani M, Drewes AM. Randomised clinical trial: pregabalin attenuates experimental visceral pain through sub-cortical mechanisms in patients with painful chronic pancreatitis. Aliment Pharmacol Ther. 2011;34:878-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Ferrone M, Raimondo M, Scolapio JS. Pancreatic enzyme pharmacotherapy. Pharmacotherapy. 2007;27:910-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Sikkens EC, Cahen DL, Kuipers EJ, Bruno MJ. Pancreatic enzyme replacement therapy in chronic pancreatitis. Best Pract Res Clin Gastroenterol. 2010;24:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Löhr JM, Hummel FM, Pirilis KT, Steinkamp G, Körner A, Henniges F. Properties of different pancreatin preparations used in pancreatic exocrine insufficiency. Eur J Gastroenterol Hepatol. 2009;21:1024-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Mössner J, Keim V. Pancreatic enzyme therapy. Dtsch Arztebl Int. 2010;108:578-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Waljee AK, Dimagno MJ, Wu BU, Schoenfeld PS, Conwell DL. Systematic review: pancreatic enzyme treatment of malabsorption associated with chronic pancreatitis. Aliment Pharmacol Ther. 2009;29:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Kocher HM, Kadaba R. Chronic pancreatitis. Clin Evid (Online). 2011;2011. [PubMed] |
| 27. | Banks PA, Conwell DL, Toskes PP. The management of acute and chronic pancreatitis. Gastroenterol Hepatol (N Y). 2010;6:1-16. [PubMed] |
| 28. | Oza VM, Kahaleh M. Endoscopic management of chronic pancreatitis. World J Gastrointest Endosc. 2013;5:19-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Jabłońska B. Is endoscopic therapy the treatment of choice in all patients with chronic pancreatitis? World J Gastroenterol. 2013;19:12-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Shea JC, Hopper IK, Blanco PG, Freedman SD. Advances in nutritional management of chronic pancreatitis. Curr Gastroenterol Rep. 2000;2:323-326. [PubMed] |
| 31. | Kocher HM, Froeling FE. Chronic pancreatitis. Clin Evid (Online). 2008;2008. [PubMed] |
| 32. | Brock C, Olesen SS, Olesen AE, Frøkjaer JB, Andresen T, Drewes AM. Opioid-induced bowel dysfunction: pathophysiology and management. Drugs. 2012;72:1847-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 33. | Telford GL, Condon RE, Szurszewski JH. Opioid receptors and the initiation of migrating myoelectric complexes in dogs. Am J Physiol. 1989;256:G72-G77. [PubMed] |
| 34. | Frantzides CT, Cowles V, Salaymeh B, Tekin E, Condon RE. Morphine effects on human colonic myoelectric activity in the postoperative period. Am J Surg. 1992;163:144-148; discussion 144-148. [PubMed] |
| 35. | Sarna SK, Otterson MF. Small intestinal amyogenesia and dysmyogenesia induced by morphine and loperamide. Am J Physiol. 1990;258:G282-G289. [PubMed] |
| 36. | Thomas J. Opioid-induced bowel dysfunction. J Pain Symptom Manage. 2008;35:103-113. [PubMed] |
| 37. | Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 2004;16 Suppl 2:17-28. [PubMed] |
| 38. | De Luca A, Coupar IM. Insights into opioid action in the intestinal tract. Pharmacol Ther. 1996;69:103-115. [PubMed] |