Published online Nov 7, 2013. doi: 10.3748/wjg.v19.i41.7160
Revised: August 19, 2013
Accepted: September 15, 2013
Published online: November 7, 2013
Processing time: 164 Days and 21.1 Hours
AIM: To evaluate the feasibility of diagnostic and therapeutic transgastric (TG) peritoneoscopic interventions with a forward-viewing endoscopic ultrasound (FV-EUS).
METHODS: This prospective endoscopic experimental study used an animal model. Combined TG peritoneoscopic interventions and EUS examination of the intra-abdominal organs were performed using an FV-EUS on 10 animal models (1 porcine and 9 canine). The procedures carried out include EUS evaluation and endoscopic biopsy of intraperitoneal organs, EUS-guided fine needle aspiration (EUS-FNA), EUS-guided radiofrequency ablation (EUS-RFA), and argon plasma coagulation (APC) for hemostatic control. The animals were kept alive for 7 d, and then necropsy was performed to evaluate results and complications.
RESULTS: In all 10 animals, TG peritoneoscopy, followed by endoscopic biopsy for the liver, spleen, abdominal wall, and omentum, was performed successfully. APC helped control minor bleeding. Visualization of intra-abdominal solid organs with real-time EUS was accomplished with ease. Intraperitoneal EUS-FNA was successfully performed on the liver, spleen, and kidney. Similarly, a successful outcome was achieved with EUS-RFA of the hepatic parenchyma. No adverse events were recorded during the study.
CONCLUSION: Peritoneoscopic natural orifice transluminal endoscopic surgery (NOTES) interventions through FV-EUS were feasible in providing evaluation and performing endoscopic procedures. It promises potential as a platform for future EUS-based NOTES.
Core tip: Recently, the forward-viewing endoscopic ultrasound (FV-EUS) was developed, however, peritoneoscopic natural orifice transluminal endoscopic surgery (NOTES) interventions with an FV-EUS has never been discussed. In this study, transgastric peritoneoscopy with FV-EUS, real-time EUS, EUS-guided fine needle aspiration, EUS-guided radiofrequency ablation, and bleeding control were successfully undertaken. FV-EUS will broaden the prospects of NOTES interventions to endoscopists, and the NOTES interventions with an FV-EUS might be performed in the various conditions.
- Citation: Jeong SU, Aizan H, Song TJ, Seo DW, Kim SH, Park DH, Lee SS, Lee SK, Kim MH. Forward-viewing endoscopic ultrasound-guided NOTES interventions: A study on peritoneoscopic potential. World J Gastroenterol 2013; 19(41): 7160-7167
- URL: https://www.wjgnet.com/1007-9327/full/v19/i41/7160.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i41.7160
Natural orifice transluminal endoscopic surgery (NOTES) reaches the target organ by inserting the endoscope through a natural orifice (e.g., mouth, anus, vagina, or urethra) and entering the peritoneal cavity by making an incision on the luminal wall. In the years after the first described NOTES by Kalloo et al[1] in 2004, a wide range of NOTES procedures with a transgastric (TG) endoscopic approach to access the peritoneal cavity have been reported. Several studies, mainly performed using animal models, have been feasible for a variety of procedures, including fallopian tube ligation[2], cholecystectomy[3], biliary anastomosis[4], gastrojejunostomy[5], splenectomy[6], and partial hysterectomy[7]. NOTES with flexible peritoneoscopy enables the examination of the peritoneal cavity with minimal invasiveness. By avoiding abdominal incisions, these successful NOTES procedures have the potential to offer less postoperative pain and reduced postoperative recovery time while avoiding hernia formation, adhesions, surface incision infection and scarring[8]. Primarily confined to the proponents of NOTES in the surgical discipline, these procedures offered a viable alternative to laparoscopic surgery, especially in patients deemed at high risk for complications.
In assessing the peritoneal cavity, the anterior wall of the stomach is usually the ideal incision site while the posterior wall may be selected to explore the retroperitoneum[9-11]. However, the TG approach has inherent risks, such as access site bleeding, adjacent organ injury during gastrotomy creation, or gastric content leakage, giving rise to infection in the peritoneal cavity. Apart from infection, bleeding is one of the most common complications. Given these considerations, the endoscopic ultrasound (EUS) has been used to avoid and mitigate the risk of injury to extraluminal structures, as well as to detect neighboring vessels by using color Doppler imaging[12,13]. In these studies, an oblique-viewing, curved, linear-array endoscopic ultrasound (OV-EUS) was used to acquire real-time images of the vessels and structures outside the gastrointestinal tract during access into the peritoneal cavity. After making an incision on the gastric wall, the OV-EUS must be exchanged for endoscopy to perform the subsequent NOTES procedures, because it provides oblique-viewing images different from the direction of the echoendoscopic movement.
To overcome several limitations of OV-EUS in EUS-interventions, a forward-viewing endoscopic ultrasound (FV-EUS) was developed. The FV-EUS simultaneously offers a straight endoscopic view and an ultrasound image. In several studies, FV-EUS was successfully tested in EUS-interventions, such as the drainage of pancreatic pseudocysts[14,15], EUS-guided fine needle aspiration (EUS-FNA)[16], and celiac plexus neurolysis[17]. In addition, it is now possible to go beyond the gut wall with the FV-EUS for intraluminal to intraperitoneal EUS evaluation. Although it showed advantages in other EUS-interventions, studies have yet to suggest the possibility of FV-EUS in NOTES procedures. Hence, this study was conducted to evaluate the technical feasibility and safety profile of the FV-EUS in a variety of procedures related to diagnostic and therapeutic TG peritoneoscopic interventions.
A mini pig (40 kg) and 9 dogs (mean weight, 18 kg; weight range, 15-20 kg) were used. Approval of the Institutional Animal Care and Use Committee was obtained before initiation of the study. All animals were fasted for 24 h but permitted water ad libitum. Anesthetic induction was achieved with a drug combination of tiletamine and Zolazepam (7.5 mg/kg) (Zoletil 50, Virbac, South Korea) and Xylazine Hydrochloride (2 mg/kg) (Rompun: Bayer, South Korea) and maintained on 1.5% isoflurane (Forane, JW pharmaceutical, South Korea) following endotracheal intubation. Cardiopulmonary parameters were monitored throughout the procedure.
Under general anesthesia, an FV-EUS (UCT 160J-AL5, Olympus, Tokyo, Japan) was advanced into the esophagus and stomach. The access site on the anterior gastric wall was first evaluated under real-time image and Doppler guidance to exclude adjacent organs and interfering vessels. A single-lumen microknife needle (Boston Scientific, Natick, MA) was used to create and puncture a small hole in the anterior gastric wall. Through the puncture site, a standard 0.035-guidewire (Jagwire; Boston Scientific, Natick, MA) was advanced through the microknife into the abdominal cavity. The microknife was then withdrawn with the guidewire left in situ.
This portal of access was then dilated with a 20-mm controlled radial expansion (CRE) balloon (Boston Scientific, Natick, MA). The balloon was held in place for 1 min. The radially expanded puncture formed a circular gastrotomy that granted passage of the FV-EUS into the peritoneum. The resultant entry allowed air insufflation through the echoendoscope to expand the peritoneal cavity for improved visualization.
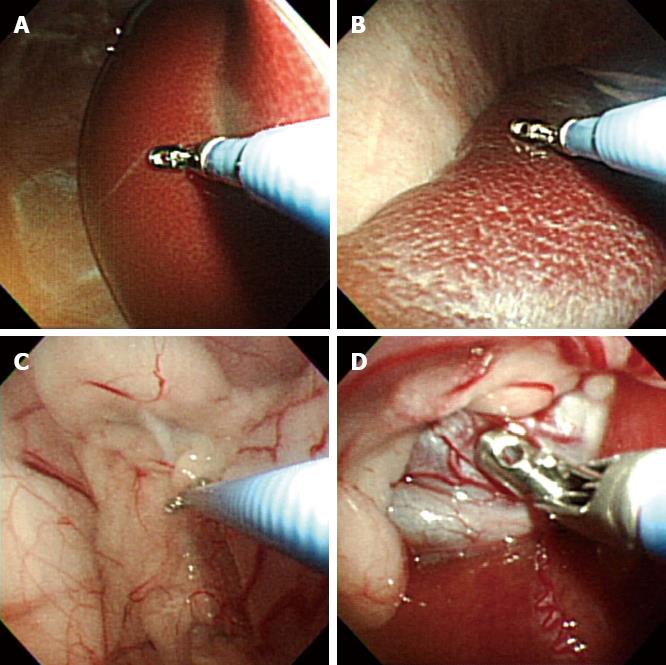
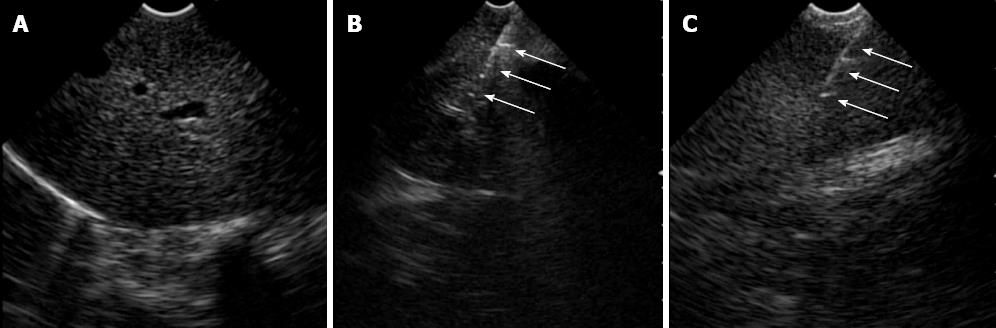
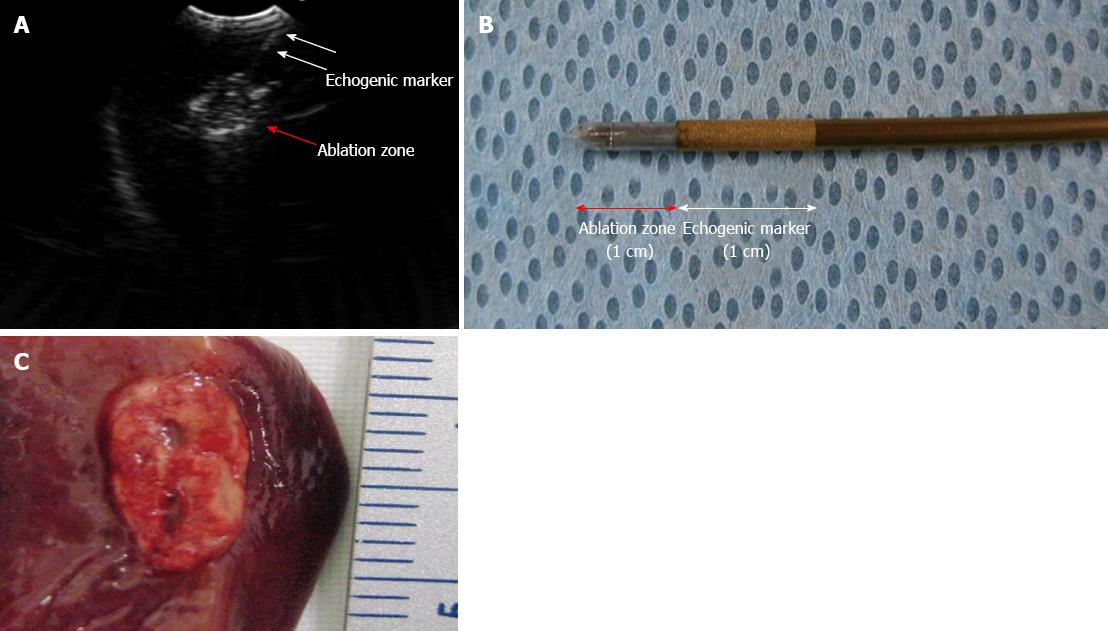
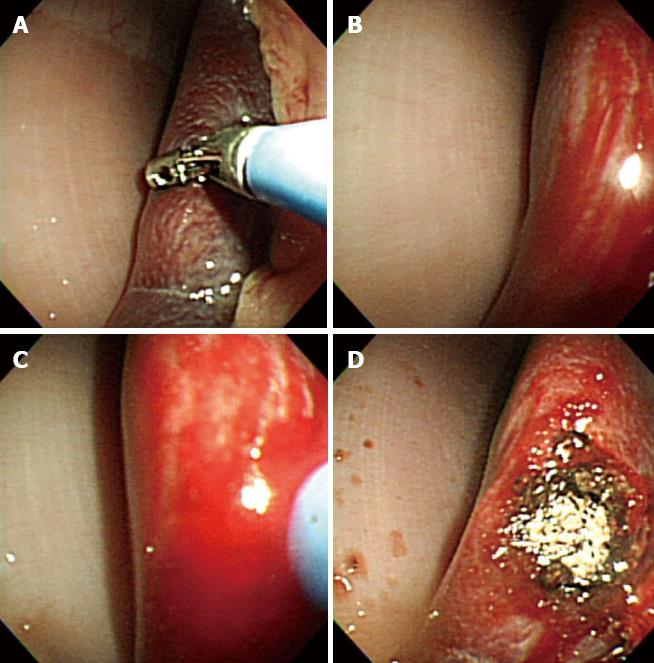
After access into the peritoneal cavity with the FV-EUS, the following procedures were performed: (1) Peritoneoscopy and endoscopic biopsy of the liver, spleen, abdominal wall, and omentum using a rat-tooth biopsy forceps (Olympus, Tokyo, Japan) (Figure 1); (2) Real-time FV-EUS examination of the intraperitoneal solid organs (Figure 2); (3) EUS-FNA with a 19G (Cook Medical Inc., Winston-Salem, NC) aspiration needle on the liver, spleen, and kidney (Figure 2); (4) FV-EUS-guided radiofrequency ablation (EUS-RFA) with the newly developed 18G RFA needle (Starmed, Seoul, South Korea) on the hepatic parenchyma (Figure 3); and (5)Argon plasma coagulation (APC) for hemostatic control of artificially induced bleeding at the liver and spleen (Figure 4).
The gastrotomy site was closed with endoscopic hemoclips. After the procedure, antibiotics and analgesics were administered and the regular diet was introduced 24 h later. The animals were kept alive for 7 d and then sacrificed. Necropsy was performed to evaluate macroscopically the EUS-FNA and EUS-RFA lesions, as well as any gross anatomical injuries to the intraperitoneal organs and infective complications.
This study was performed on 1 pig and 9 dogs. After gastrostomy, the FV-EUS, using forward optic view to enter the peritoneal cavity, and diagnostic TG peritoneoscopy for various intraperitoneal organs was undertaken safely and easily in all animals. Endoscopic biopsies of the liver, spleen, abdominal wall, and omentum were also completed successfully without complications in all 10 animals. APC was successfully used to control minor artificial bleeding caused by deliberate multiple-forceps biopsy and poking on the liver and spleen in 4 animals. Real-time EUS images were acquired with ample clarity and ease while observing the deeper portions of the intra-abdominal organs. When the scope contacted the target organ, the endoscopic view was switched to the sonographic view, and EUS-FNA from the peritoneal cavity was successfully performed on the liver, spleen, and kidney in the 9 dogs.
The EUS-RFA was undertaken when the equipment was made available. In the EUS-RFA, the power was set to 50 watts, and the duration was 1 min. The EUS-RFA of the hepatic parenchyma was equally successful in 6 animals by using the RFA needle (Table 1).
| Procedure | 1 (pig) | 2 (dog) | 3 (dog) | 4 (dog) | 5 (dog) | 6 (dog) | 7 (dog) | 8 (dog) | 9 (dog) | 10 (dog) |
| Peritoneoscopy | + | + | + | + | + | + | + | + | + | + |
| Multiple biopsies | + | + | + | + | + | + | + | + | + | + |
| EUS-FNA | Liver | Liver, kidney | Liver, spleen | Liver, spleen | Liver, spleen | Liver, spleen, kidney, | Liver, spleen, kidney | Liver, spleen, kidney | Liver, spleen, kidney | |
| EUS-RFA | Liver | Liver | Liver | Liver | Liver | Liver | ||||
| Argon plasma coagulation | + | + | + | + |
All the animals survived for 7 d without any obvious pattern of behavioral distress. Necropsy revealed no apparent or gross anatomical damage to the intraperitoneal organs related to these diagnostic and therapeutic procedures. The closure of the gastrotomy orifice was accomplished using 6 to 7 endoscopic hemoclips. No significant peritoneal adhesions or peritonitis were seen in the necropsies. In addition, neither intraperitoneal infectious complications nor abscesses were detected in the animals.
EUS-guided therapeutic interventions are performed with the OV-EUS. The major disadvantage of the OV-EUS is that the echoendoscope occasionally accesses the targeted area at an acute angle. Because of the acute angle, the force of accessory advancement may cause the scope to push away from the target organ. Another limitation of the OV-EUS is the lack of forward-viewing endoscopy. It requires reorientation in switching from a sonographic to endoscopic view. As a result of technological advances and a surge in new therapeutic modalities for EUS-guided procedures, FV-EUS was developed to overcome the disadvantages of OV-EUS.
The FV-EUS facilitated needle or device insertion and deployment[18]. Unlike the OV-EUS an important advantage offered with the FV-EUS is that the axis and optics of the echoendoscope is in line with the accessory channel. This straight alignment not only provides the operator easier deployment and manipulation of needle and devices through the working channel but also renders better transmission of force to the tip of the accessory device or needle[17]. Furthermore, the FV-EUS could be manipulated to secure a perpendicular puncture trajectory instead of the angulated puncture direction in OV-EUS, thereby, preventing the “pushback” phenomenon or moving away from the gut wall[19]. This ensures that the echoendoscope could be kept more easily in its intended position during therapeutic interventions.
Of course, the FV-EUS has disadvantages: narrow ultrasound scanning range, absence of an elevator, and incapability of using a balloon at the tip of the echoendoscope. However, these disadvantages of FV-EUS did not affect its maneuverability or outcomes[15,19,20]. Overall, the FV-EUS facilitates EUS-guided therapeutic procedures.
With the introduction of the FV-EUS, its use in TG NOTES peritoneoscopy could mark the evolution from mainly a diagnostic modality to the prospect of carrying out a wide range of peritoneoscopic interventional procedures. This is possible because of certain advantages afforded by the FV-EUS, namely, improved maneuverability guided by forward optics, wider distal-end range of angulation, a shorter and smaller distal tip in front of the view, the facility to deploy needles and other accessory devices along the axis of the scope, and the ability to switch readily between sonographic and endoscopic views without the need for frequent echoendoscope reorientation[14]. By using FV-EUS to identify and avoid extraluminal organs and vessels, the gastrotomy site created on the anterior abdominal wall was accomplished without intra- or post-procedural complications. Neither bleeding from the gastric wall during its incision nor injury to the contiguous organs on entry into the peritoneal cavity was observed. FV-EUS enhanced safety by providing real-time images of the anticipated path of the microknife puncture. Hence, with the advantages mentioned earlier, the transmural microknife puncture through the gastric layers, advancement of the guidewire via the fistula into the peritoneal cavity, and fistula dilatation with the CRE balloon were successful in all 10 animal cases. In particular, the FV-EUS circumvents the need of a second endoscopic procedure for peritoneal cavity entry and, thereby, reducing overall procedure time[12].
In the current animal study, biopsies were taken from the liver, spleen, anterior abdominal wall, and omentum successfully with the FV-EUS. This was exemplified in the minimal resistance and enhanced facility the operator encountered during the procedure. In the event of bleeding, APC was used to attain control. Hemostasis was achieved effectively for minor bleeding, which was deliberately induced by the forceps biopsy at the liver and spleen in 4 dogs. Electrocautery could also be used to prevent further bleeding from the biopsy sites[21]. These findings suggest that liver and splenic biopsies via the TG peritoneoscopic approach could be accomplished uneventfully and without major bleeding complications.
The EUS-FNA and EUS-RFA needles were clearly visualized extruding from the working channel of the FV-EUS and then they were inserted directly into the various organs under real-time EUS imaging. The FV-EUS, with its ability to switch readily between endoscopic and sonographic views, diminished manipulative reorientation of the echoendoscope during EUS-FNA or EUS-RFA, greatly improving technical performance. This translated into successful attempts at EUS-FNA with a 19G needle on the liver, spleen, and kidney in all 9 dogs. Likewise, EUS-RFA with an 18G RFA needle at 50W for 1 min to the hepatic parenchyma was successfully duplicated in 5 dogs and 1 pig.
Necropsy findings revealed a well-demarcated RFA ablation zone in the hepatic parenchyma while FNA needle puncture marks were seen on the intra-abdominal organs. Therefore, the design of the FV-EUS enabled the operator to target lesions within and external to the organs with relative ease, which greatly improved the ability to perform diagnostic and therapeutic procedures. This suggests that the new EUS-RFA method is able to treat the mass of intraperitoneal solid organs by using the newly developed RFA-needle.
The improvement in intraperitoneal maneuverability of the FV-EUS results in adequate visualization of all four abdominal quadrants and intestinal loops[22]. As modern imaging techniques tend to understage around 10%-40% of GI malignancies[23,24], peritoneoscopy with intraperitoneal FV-EUS could provide adequate minimally invasive staging of GI malignancies, especially for pancreatic and stomach cancers, prior to surgical resection. Therefore, by providing better diagnostic accuracy, the FV-EUS, with its extra ability to see through solid organs, might be a preferred substitute to staging laparoscopy in detecting peritoneal carcinomatosis and small metastatic tumors. In addition, endoscopic visualization of the anterior abdominal wall could be easily achieved by looking up to the abdominal wall rather than looking back with angled laparoscopes.
Necropsy findings in this study did not reveal any organ injury or infective complications related to the TG peritoneoscopy. Nevertheless, bacterial contamination and infection in the abdomen is a genuine concern for a gastrotomy site. Donatsky et al[25] reported that in TG NOTES with over-the-scope-clip closure, intra-abdominal chronic abscesses were discovered in 3 of 10 pigs at necropsy, although all the animals survived during the study period. The study concluded that peritoneal contamination did occur, which warranted implementation other than the use of single-dose prophylactic antibiotics to prevent infective complications. In contrast, a study by Narula et al[26] revealed that despite the presence of contamination measured by an increase in the bacterial colony-forming units, no clinically significant spillage into the peritoneum that resulted in abscess formation was seen. These conflicting results would require further evaluation to prevent post-gastrostomy septic complications, including peritonitis.
The effectiveness of current suture techniques and the perforation risk following closure of the gastrotomy site remain unsettled issues. Unsatisfactory closure of the transluminal access site has resulted in several animal cases of microabscesses, peritonitis, and death[27]. Although the endoclips used in this study did not give rise to any adverse complications, mucosal closure with endoclips has been shown unreliable and it could result in substantial air and gastric fluid leakage[28]. The presence of tissue edema and widely opposing incisional edges considerably impede satisfactory tissue approximation. Without achieving full thickness closure, the potential for gastric fluid leakage and spontaneous perforation risk definitely exist. Until now, the unavailability of a simple and safe closure technique continued to impede the progress of NOTES procedure.
This study was limited in its small number of animal cases, as well the substantial difference in porcine and canine abdominal anatomy that may limit the relevance of the study findings in relation to clinical human applicability. Despite the limitation, this animal study is the first report that suggested the possibility of FV-EUS in NOTES procedures and show that the FV-EUS was very efficient as a modality of NOTE interventions. It is possible to go beyond the gut wall with the FV-EUS from intraluminal to intraperitoneal EUS evaluation, enabling freedom to assess many areas within the abdominal cavity, including the pelvic region. Armed with a sonographic window, extraluminal peritoneoscopic evaluation with the FV-EUS would enable assessment beyond visual inspection by providing views and accessibility to lesions within solid intraperitoneal organs and structures, thereby, broadening the appeal of NOTES peritoneoscopic interventional procedures to the endoscopist.
In conclusion, TG NOTES combined with EUS-guided peritoneoscopic interventions and intraperitoneal, as well as intraluminal, EUS could be achieved with the FV-EUS. This study ably demonstrated the utility and success of FV-EUS in both diagnostic and therapeutic peritoneoscopic interventions in animal models, which adds to the growing armamentarium available for NOTES procedures. Even though concerns remain, embracing this strategy is essential for further development of EUS-guided NOTES interventions.
We wish to thank Olympus Medical Systems, Tokyo, Japan for generous providing the prototype echoendoscope for evaluation.
Natural orifice transluminal endoscopic surgery (NOTES) is a new surgical technique and it has the potential to offer less operation related complications. However, the therapeutic transgastric (TG) approach to access the peritoneal cavity has inherent risks, such as bleeding and adjacent organ injury. The endoscopic ultrasound (EUS) has been used to avoid the risk of injury to extraluminal structures and oblique-viewing, curved, linear-array echoendoscope (OV-EUS) have been used in NOTES interventions. Recently, the forward-viewing endoscopic ultrasound (FV-EUS) was developed and successfully tested in EUS-guided interventions. The FV-EUS is regarded as an ergonomic and viable endoscopic modality to perform TG peritoneoscopic interventions via NOTES. However, peritoneoscopic NOTES interventions through FV-EUS have never been discussed.
The FV-EUS was developed to overcome the limitations of OV-EUS which provides oblique-viewing images different from the direction of the echoendoscopic movement. Several experimental studies through FV-EUS have been feasible for a variety of EUS-interventions, such as the drainage of pancreatic pseudocysts, EUS-FNA, and celiac plexus neurolysis.
FV-EUS has not been used previously for NOTES access. In their animal experiments, the authors aim to investigate the use of the FV-EUS for the performance of standard NOTES interventions. This study ably demonstrates the utility and success of FV-EUS in both diagnostic and therapeutic peritoneoscopic interventions, and it suggests that FV-EUS can improve safety of the NOTES access to the peritoneal cavity. In conclusion, this animal study is the first report that suggested the possibility of FV-EUS in NOTES procedures and show that the FV-EUS was very efficient as a modality of NOTES interventions.
Armed with a sonographic window, extraluminal peritoneoscopic evaluation with the FV-EUS would enable assessment beyond visual inspection by providing views and accessibility to lesions within various intraperitoneal structures. Therefore, FV-EUS will broaden the prospects of NOTES interventions to endoscopists and gastroenterologists, and the NOTES interventions through a FV-EUS might be performed in the various conditions.
NOTES: NOTES is an experimental surgical technique. NOTES reaches the target organ by inserting the endoscope through a natural orifice (e.g., mouth, anus, vagina, or urethra) and entering the peritoneal cavity by making an incision on the luminal wall, thus avoiding any external incisions. FV-EUS: FV-EUS has both an forward endoscopic view and a sonographic view, plus a working channel in alignment with the endoscope shaft. It is able to deploy needles and other accessory devices along the axis of the scope, and has a wider angulation range of the tip.
The authors performed the first animal study of NOTES procedures using forward view EUS guidiance. The study confirm the feasibility of FV-EUS guidedNOTES. It is an interesting study that provide important information on future studies of NOTES under FV-EUS guidance.
P- Reviewers: Ahmed F, Lok KH S- Editor: Zhai HH L- Editor: A E- Editor: Ma S
| 1. | Kalloo AN, Singh VK, Jagannath SB, Niiyama H, Hill SL, Vaughn CA, Magee CA, Kantsevoy SV. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117. [PubMed] |
| 2. | Jagannath SB, Kantsevoy SV, Vaughn CA, Chung SS, Cotton PB, Gostout CJ, Hawes RH, Pasricha PJ, Scorpio DG, Magee CA. Peroral transgastric endoscopic ligation of fallopian tubes with long-term survival in a porcine model. Gastrointest Endosc. 2005;61:449-453. [PubMed] |
| 3. | Park PO, Bergström M, Ikeda K, Fritscher-Ravens A, Swain P. Experimental studies of transgastric gallbladder surgery: cholecystectomy and cholecystogastric anastomosis (videos). Gastrointest Endosc. 2005;61:601-606. [PubMed] |
| 4. | Bergström M, Ikeda K, Swain P, Park PO. Transgastric anastomosis by using flexible endoscopy in a porcine model (with video). Gastrointest Endosc. 2006;63:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Kantsevoy SV, Jagannath SB, Niiyama H, Chung SS, Cotton PB, Gostout CJ, Hawes RH, Pasricha PJ, Magee CA, Vaughn CA. Endoscopic gastrojejunostomy with survival in a porcine model. Gastrointest Endosc. 2005;62:287-292. [PubMed] |
| 6. | Kantsevoy SV, Hu B, Jagannath SB, Vaughn CA, Beitler DM, Chung SS, Cotton PB, Gostout CJ, Hawes RH, Pasricha PJ. Transgastric endoscopic splenectomy: is it possible? Surg Endosc. 2006;20:522-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 232] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | Merrifield BF, Wagh MS, Thompson CC. Peroral transgastric organ resection: a feasibility study in pigs. Gastrointest Endosc. 2006;63:693-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | McGee MF, Rosen MJ, Marks J, Onders RP, Chak A, Faulx A, Chen VK, Ponsky J. A primer on natural orifice transluminal endoscopic surgery: building a new paradigm. Surg Innov. 2006;13:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Feretis C, Kalantzopoulos D, Koulouris P, Kolettas C, Archontovasilis F, Chandakas S, Patsea H, Pantazopoulou A, Sideris M, Papalois A. Endoscopic transgastric procedures in anesthetized pigs: technical challenges, complications, and survival. Endoscopy. 2007;39:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Hazey JW, Narula VK, Renton DB, Reavis KM, Paul CM, Hinshaw KE, Muscarella P, Ellison EC, Melvin WS. Natural-orifice transgastric endoscopic peritoneoscopy in humans: Initial clinical trial. Surg Endosc. 2008;22:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | von Delius S, Feussner H, Wilhelm D, Karagianni A, Henke J, Schmid RM, Meining A. Transgastric in vivo histology in the peritoneal cavity using miniprobe-based confocal fluorescence microscopy in an acute porcine model. Endoscopy. 2007;39:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Elmunzer BJ, Schomisch SJ, Trunzo JA, Poulose BK, Delaney CP, McGee MF, Faulx AL, Marks JM, Ponsky JL, Chak A. EUS in localizing safe alternate access sites for natural orifice transluminal endoscopic surgery: initial experience in a porcine model. Gastrointest Endosc. 2009;69:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Fritscher-Ravens A, Ghanbari A, Cuming T, Kahle E, Niemann H, Koehler P, Patel K. Comparative study of NOTES alone vs. EUS-guided NOTES procedures. Endoscopy. 2008;40:925-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Voermans RP, Eisendrath P, Bruno MJ, Le Moine O, Devière J, Fockens P. Initial evaluation of a novel prototype forward-viewing US endoscope in transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc. 2007;66:1013-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Trevino JM, Varadarajulu S. Initial experience with the prototype forward-viewing echoendoscope for therapeutic interventions other than pancreatic pseudocyst drainage (with videos). Gastrointest Endosc. 2009;69:361-365. [PubMed] |
| 16. | Kida M, Araki M, Miyazawa S, Ikeda H, Kikuchi H, Watanabe M, Imaizumi H, Koizumi W. Fine needle aspiration using forward-viewing endoscopic ultrasonography. Endoscopy. 2011;43:796-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Eloubeidi MA. Initial evaluation of the forward-viewing echoendoscope prototype for performing fine-needle aspiration, Tru-cut biopsy, and celiac plexus neurolysis. J Gastroenterol Hepatol. 2011;26:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Irisawa A, Imaizumi H, Hikichi T, Takagi T, Ohira H. Feasibility of interventional endoscopic ultrasound using forward-viewing and curved linear-array echoendoscope: a literature review. Dig Endosc. 2010;22 Suppl 1:S128-S131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Iwashita T, Nakai Y, Lee JG, Park do H, Muthusamy VR, Chang KJ. Newly-developed, forward-viewing echoendoscope: a comparative pilot study to the standard echoendoscope in the imaging of abdominal organs and feasibility of endoscopic ultrasound-guided interventions. J Gastroenterol Hepatol. 2012;27:362-367. [PubMed] |
| 20. | De Lusong MA, Shah JN, Soetikno R, Binmoeller KF. Treatment of a completely obstructed colonic anastomotic stricture by using a prototype forward-array echoendoscope and facilitated by SpyGlass (with videos). Gastrointest Endosc. 2008;68:988-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Mintz Y, Horgan S, Cullen J, Ramamoorthy S, Chock A, Savu MK, Easter DW, Talamini MA. NOTES: the hybrid technique. J Laparoendosc Adv Surg Tech A. 2007;17:402-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Voermans RP, van Berge Henegouwen MI, Bemelman WA, Fockens P. Feasibility of transgastric and transcolonic natural orifice transluminal endoscopic surgery peritoneoscopy combined with intraperitoneal EUS. Gastrointest Endosc. 2009;69:e61-e67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Mertz HR, Sechopoulos P, Delbeke D, Leach SD. EUS, PET, and CT scanning for evaluation of pancreatic adenocarcinoma. Gastrointest Endosc. 2000;52:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 198] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Phoa SS, Reeders JW, Rauws EA, De Wit L, Gouma DJ, Laméris JS. Spiral computed tomography for preoperative staging of potentially resectable carcinoma of the pancreatic head. Br J Surg. 1999;86:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Donatsky AM, Andersen L, Nielsen OL, Holzknecht BJ, Vilmann P, Meisner S, Jørgensen LN, Rosenberg J. Pure natural orifice transluminal endoscopic surgery (NOTES) with ultrasonography-guided transgastric access and over-the-scope-clip closure: a porcine feasibility and survival study. Surg Endosc. 2012;26:1952-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Narula VK, Hazey JW, Renton DB, Reavis KM, Paul CM, Hinshaw KE, Needleman BJ, Mikami DJ, Ellison EC, Melvin WS. Transgastric instrumentation and bacterial contamination of the peritoneal cavity. Surg Endosc. 2008;22:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Ryou M, Fong DG, Pai RD, Sauer J, Thompson CC. Evaluation of a novel access and closure device for NOTES applications: a transcolonic survival study in the porcine model (with video). Gastrointest Endosc. 2008;67:964-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Shabbir A, Liang S, Lomanto D, Ho KY, So JB. Closure of gastrotomy in natural orifice transluminal endoscopic surgery: a feasibility study using an ex vivo model comparing endoloop with endoclip. Dig Endosc. 2011;23:130-134. [PubMed] |