Published online Nov 7, 2013. doi: 10.3748/wjg.v19.i41.6969
Revised: August 16, 2013
Accepted: September 15, 2013
Published online: November 7, 2013
Processing time: 141 Days and 18.9 Hours
The 148 Isoleucine to Methionine protein variant (I148M) of patatin-like phospholipase domain-containing 3 (PNPLA3), a protein is expressed in the liver and is involved in lipid metabolism, has recently been identified as a major determinant of liver fat content. Several studies confirmed that the I148M variant predisposes towards the full spectrum of liver damage associated with fatty liver: from simple steatosis to steatohepatitis and progressive fibrosis. Furthermore, the I148M variant represents a major determinant of progression of alcohol related steatohepatitis to cirrhosis, and to influence fibrogenesis and related clinical outcomes in chronic hepatitis C virus hepatitis, and possibly chronic hepatitis B virus hepatitis, hereditary hemochromatosis and primary sclerosing cholangitis. All in all, studies suggest that the I148M polymorphism may represent a general modifier of fibrogenesis in liver diseases. Remarkably, the effect of the I148M variant on fibrosis was independent of that on hepatic steatosis and inflammation, suggesting that it may affect both the quantity and quality of hepatic lipids and the biology of non-parenchymal liver cells besides hepatocytes, directly promoting fibrogenesis. Therefore, PNPLA3 is a key player in liver disease progression. Assessment of the I148M polymorphism will possibly inform clinical practice in the future, whereas the determination of the effect of the 148M variant will reveal mechanisms involved in hepatic fibrogenesis.
Core tip: The 148 Isoleucine to Methionine protein variant (I148M) of patatin-like phospholipase domain-containing 3 (PNPLA3) has recently been identified as a major determinant of liver fat content. Several studies conducted in different ethnicities confirmed that I148M influences the full spectrum of liver damage: from simple steatosis to nonalcoholic steatohepatitis and progressive fibrosis to hepatocellular carcinoma. Furthermore, I148M turned out to represent a major determinant of progression of alcohol related steatohepatitis, and to influence fibrosis progression and related clinical outcomes in chronic hepatitis C virus hepatitis, as well as other in liver diseases. All in all, studies suggest that the PNPLA3 I148M polymorphism may represent a general modifier of fibrogenesis in liver diseases.
- Citation: Dongiovanni P, Donati B, Fares R, Lombardi R, Mancina RM, Romeo S, Valenti L. PNPLA3 I148M polymorphism and progressive liver disease. World J Gastroenterol 2013; 19(41): 6969-6978
- URL: https://www.wjgnet.com/1007-9327/full/v19/i41/6969.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i41.6969
The clinical evolution of chronic liver diseases is highly variable, and genetic factors plays a key role in determining the inter-individual susceptibility towards end-stage liver disease and hepatocellular carcinoma. It is now established that all common diseases, including type 2 diabetes, atherosclerosis, and nonalcoholic fatty liver disease (NAFLD) among liver diseases[1], exhibit a heritable component of susceptibility accounting for 30%-50% of risk. Indeed, also liver diseases can be considered complex traits that result from environmental exposures, e.g., diet and physical inactivity for NAFLD, excessive alcohol intake for alcoholic liver disease (ALD), or infection for chronic viral hepatitis, acting on a susceptible polygenic background comprising multiple independent modifiers[2]. These are generally represented by common genetic variants with a mild effect or rare variants associated with a more marked phenotype[3].
Despite initial hypothesis-driven, case-control studies identified some genetic loci associated with the progression of liver damage, the genetic determinants of NAFLD remained obscure until recently[1]. By 2008, the first genomewide association studies in the field of hepatic steatosis allowed to identify the rs738409 variant, by an hypothesis free drive approach, as the single major genetic determinant of hepatic fat content[4,5]. This sequence variation is a C > G single nucleotide change, encoding for the 148 Isoleucine to Methionine protein variant (I148M) of Patatin-like phospholipase domain-containing 3 (PNPLA3). The main purpose of this review is to provide an overview of the current knowledge of the PNPLA3 I148M polymorphism role in the progression of liver disease.
Ectopic fat accumulation in the liver related to systemic insulin resistance represents the prototypical manifestation of NAFLD[6]. With a prevalence of 20%-34% and rising, NAFLD is now the most frequent liver disease in industrialized countries[7,8]. In a minority of susceptible individuals, steatosis, that is excessive fat accumulation in the liver (> 5%), is associated with oxidative hepatocellular damage, inflammation and activation of fibrogenesis, i.e. nonalcoholic steatohepatitis (NASH)[9,10], which can progress to cirrhosis and hepatocellular carcinoma[11,12].
NAFLD is epidemiologically associated with obesity and metabolic syndrome. The pathogenesis is related to adipose tissue insulin resistance[13], leading to an increased flux of free fatty acids to the liver[14], increased lipogenesis induced by hyperinsulinemia, abnormal intra-hepatic lipid metabolism and dietary factors. Hepatic fat accumulation, in turn, worsens insulin resistance and liver damage, determining an increased risk of both cardiovascular and liver related mortality[15-17].
Besides hepatocytes, two cell types play a key role in the pathogenesis of NASH. Kupffer cells are the hepatic macrophages that under basal conditions are involved in the maintenance of immune homeostasis, but during NASH become activated by intestinal bacterial products and oxidized lipids via Toll-like receptor-4 and secrete reactive oxygen species, chemokines and several cytokines, thereby orchestrating inflammation[18]. Hepatic stellate cells are hepatic pericytes localized between sinusoidal endothelial cells and the hepatocytes, which in quiescent conditions store lipids and retinoids, secrete extracellular matrix, and regulate blood flow. Upon activation in NASH, stellate cells release retinoids, undergo myofibroblast transition, secrete type 1 collagen and a variety of fibrogenic mediators thereby initiating the process of fibrogenesis[19,20].
The risk of NAFLD is highly variable even in individuals with obesity and type 2 diabetes. Furthermore, even if the majority of obese subjects with metabolic syndrome develop simple steatosis, only about one third has NASH, and a minority progresses to more severe forms of the disease.
Epidemiological, familial and twin studies provide evidence for a component of hereditability of liver fat content and NAFLD[21,22]. Indeed, NAFLD is more prevalent in Hispanics compared to Europeans, and less common in African-Americans, and this difference is not explained by diabetes and obesity[22,23]. Family studies demonstrated a strong heritability of NASH[21,24,25]. Accordingly, twin studies shown that, in subjects without viral hepatitis and alcohol abuse, alanine aminotransferases (ALT) levels and liver fat content are strongly heritable traits, with genetic factors explaining up to 60% of variability[26,27]. Overall, evidences indicate that about half of steatosis variability, determined by biochemical indices or noninvasive assessment of liver fat, is inherited[1]. Therefore, several hypothesis-driven studies tried to evaluate the role of candidate genetic variants in the susceptibility to NAFLD and progressive NASH, with the goal of identifying disease markers or potential drug targets, but with inconsistent results[1].
In 2008, two independent genome-wide association studies linked the common rs738409 polymorphism of PNPLA3 (I148M) with hepatic fat content and ALT levels[4,5]. In particular, a genomewide scan of the association of non-synonymous sequence variations in the Dallas Heart Study revealed a very strong association of increased fat content with a single missense variant, I148M, in PNPLA3[4].
Remarkably, the association between PNPLA3 I148M and liver fat was independent of major differences in body composition, diabetes and serum lipoprotein levels. Furthermore, the 148M at risk allele was more prevalent in Hispanics [minor allele frequency (MAF): 0.49] than in Europeans (MAF: 0.23), and less common in Afro-Americans (MAF: 0.17) explaining a consistent fraction of the inter-ethnic variability in NAFLD susceptibility[4,28].
Since then, several studies and a recent meta-analysis have replicated the association between the I148M polymorphism and NAFLD in all ethnic groups, both in adults and in the developmental age[28-41]. The association of the I148M variant with hepatic lipid content is exposed in the presence of other risk factors, such as severe obesity[32], visceral adiposity[42], increased intake of sugars[43] or omega-6 poly-unsaturated fatty acids[44], and other genetic factors[45,46]. Vice versa, weight loss results in a rapid decrease of intra-hepatic fat and of indices of liver damage in subjects homozygous for the 148M variant[47]. All in all, these data suggest that the 148M variant becomes a critical factor determining hepatocellular fat accumulation when stressing factors such as increased flux of free fatty acids related to adipose tissue insulin resistance in visceral obesity, increased lipogenesis stimulated by hyperinsulinemia and carbohydrates, or altered lipid metabolism intervene.
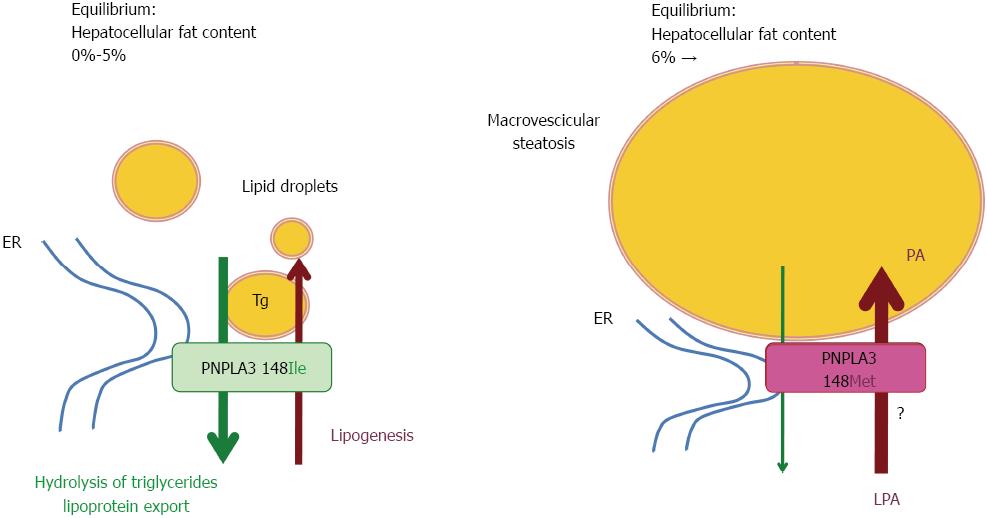
PNPLA3, also called adiponutrin, encodes a 481 amino acid protein with a molecular mass of approximately 53 kDa that in humans is mainly expressed in intracellular membrane fractions in hepatocytes[48], and is induced in the liver after feeding and during insulin resistance by the master regulator of lipogenesis Steroid Regulatory Element Binding Protein-1c[49].
Wild-type (148I) PNPLA3 has lipolytic activity towards triglycerides[48,50]. The 148M mutation determines a critical aminoacidic substitution next to the catalytic domain, likely reducing the access of substrates and reducing the PNPLA3 enzymatic activity towards glycerolipids, thereby leading to the development of macrovescicular steatosis[48,50]. However, other reported a gain of lipogenic function associated with the 148M variant, which would acquire the ability to synthesize phosphatidic acid from lysophosphatidic acid[51]. In addition, results deriving from murine models gave contradictory results[52-55]. The issue of the functional consequences of the I148M polymorphism is therefore still intensively debated, and it may be hypothesized that PNPLA3 has additional physiological substrates. Human studies have also suggested a possible direct or indirect influence of PNPLA3 genotype on adipose tissue biology[56,57], which however awaits replication.
A model depicting hypothetical mechanisms of hepatic fat accumulation associated with the I148M PNPLA3 polymorphism is shown in Figure 1.
Even if steatosis severity is a risk factor for NASH and progressive disease in NAFLD[58], the association is not invariable, and hepatocellular fat is believed to represent more a epiphenomenon of insulin resistance and altered lipid metabolism than the key driver underpinning liver damage progression. Indeed, accumulation of neutral lipids in cytoplasmic droplets is now retained to represent a protective response towards the increased burden of hepatotoxic free fatty acids and other lipids[14]. Therefore, the first question arising after the discovery of PNPLA3 genotype as the major determinant of hepatic fat content, was whether the I148M polymorphism decreased liver damage favoring accumulation of fatty acids in lipid droplets or conversely increased the susceptibility to develop progressive NASH and fibrogenesis.
The answer came soon, as the 148M allele was linked with NASH[30], and our group first reported that homozygosity for the 148M allele was associated with an 3.3-fold increased risk of both NASH and liver fibrosis in two independent cohorts of European subjects with histological NAFLD[31]. The association between PNPLA3 I148M and the severity of fibrosis in NAFLD was almost contemporarily replicated by independent groups in adults[34,35] and in the pediatric population[59], and confirmed by a recent meta-analysis[36].
ALD shares many pathophysiological features with NAFLD[18], most notably steatohepatitis being the key driver of fibrogenesis and liver damage progression. Indeed, candidate gene studies demonstrated that the I148M polymorphism is also strongly associated with the risk of developing ALD and with the susceptibility towards cirrhosis in alcohol abusers of different ethnic groups[60-62]. In one study in German subjects, the I148M polymorphism alone explained as much as 26% of cirrhosis variability in alcohol abusers[61]. Furthermore, the earlier the age of the increase at risk alcohol intake the stronger the effect of the PNPLA3 148M mutation has on the cirrhosis susceptibility[63].
The next natural question was clearly whether PNPLA3 genotype represents a modifier of progression of other liver diseases in which steatosis plays a key role in the pathogenesis. Chronic hepatitis C virus (CHC), a leading cause of end stage liver disease and hepatocellular carcinoma in many Western countries[64], is frequently characterized by steatosis, occurring in more than half of patients. The presence of steatosis has been associated with more aggressive histological features, faster progression of fibrosis, and poorer response to therapy[65-68]. Hepatic steatosis favors hepatitis C virus (HCV) life-cycle[69], and both viral and host factors are believed to contribute to its pathogenesis[67,70-73]. It became soon clear that the I148M polymorphism is a major determinant of the susceptibility to steatosis in also CHC, in particular in patients not infected by genotype 3 strains that per se strongly induces steatosis by altering very low density lipoproteins export[74-77]. This model is also consistent with the recent finding of a nonsense mutation of APOB in humans causing hypo-beta-alipoproteinemia and a massive history of severe steatosis associated with development of hepatocellular carcinoma in carriers of this mutation[78].
Studies that specifically evaluated the association between the I148M PNPLA3 variant and fibrosis progression in CHC are reported in Table 1. As in NAFLD and ALD, the effect of the 148M mutation was not limited to predisposition to steatosis, but extended towards progressive fibrosis and cirrhosis development[75-77,79]. Interestingly, the size effect of the association of the I148M polymorphism with fibrosis appeared larger in subjects with at risk alcohol intake (> 30 g/d in males/females)[76,80], suggesting the existence of an interaction between different triggering factors and PNPLA3 genotype in fibrogenesis. Furthermore, genetic factors influencing immunological response towards HCV, i.e., IL28B region polymorphisms, may influence the association between PNPLA3 and steatosis[81].
We could speculate that when steatosis inducing stressors such as obesity and insulin resistance, excess alcohol intake, and HCV infection stress the liver, in the presence of the “normal”148I PNPLA3 allele the damage will result in simple uncomplicated steatosis, whereas the 148M“at risk” allele will favor steatohepatitis and fibrogenesis, with progression towards cirrhosis and its complication in susceptible individuals[82].
Finally, some studies indicate that during treatment with peg-interferon plus ribavirin the I148M polymorphism may affect sustained virological response (i.e., cure rate)[75] and viral kinetics[83], especially in difficult to cure CHC patients with advanced fibrosis[79]. However, the clinical impact of PNPLA3 on the response to therapy will likely be modest in the new era of direct antiviral agents[79].
Having established that the I148M polymorphism is a modifier of the natural history of liver diseases associated with steatosis, i.e., NAFLD, ALD and CHC, the possible role of I148M in determining the susceptibility to steatohepatitis and progressive liver damage in other liver diseases is becoming the subject of investigation.
As it affects more than 350 million people worldwide and is a leading causes of liver-related mortality[84], chronic hepatitis B virus (CHB) infection represented the next disease in which the role of PNPLA3 genotype had to be understood. Steatosis is indeed commonly observed also in CHB, and overall evidence suggests that it contributes to fibrosis progression[85-88]. Recent data from our group obtained in a relatively large cohort of European CHB patients with histological evaluation of liver damage indicate that the 148M variant predisposes to steatosis[89]. In patients with overweight or a positive history of alcohol intake, the I148M polymorphism predisposes also to severe steatosis, which in this population was associated with more severe fibrosis[89]. Additional studies will be required to test the interaction between the I148M genetic variant and acquired risk factors in the pathogenesis of progressive fibrosis and on related clinical outcomes also in CHB infection.
Hereditary hemochromatosis represents another interesting disease model. In fact, in a homogeneous genetic background in subjects homozygous for the C282Y mutation of the HFE gene, activation of fibrogenesis is caused by progressive hepatocellular iron overload via generation of oxidative stress[90,91] in the presence of precipitating factors, among which steatosis has a major role[92]. In a large series of Italian HFE C282Y homozygous patients with hemochromatosis, we showed that the I148M polymorphism is a strong predictor of the presence of steatosis and higher liver enzymes levels, and it is also associated with the severity of fibrosis[93]. A possible interaction with other genetic forms of liver disease (i.e., Wilson disease) may also be hypothesized and studies on this topic would help understanding the whole picture of PNPLA3 gene interaction with liver stressors.
Finally, it has been reported that in primary sclerosing cholangitis, an autoimmune cholestatic liver disease characterized by inflammatory changes of major bile ducts, the 148M PNPLA3 variant is associated with increased mortality[94]. The effect of PNPLA3 genotype was evident in the subgroup of patients with severe disease, i.e., males with stenosis of the main duct, but unfortunately it could not be determined whether the association was mediated by steatosis and faster progression of fibrosis.
Although much work has clearly yet to be done in these and many other forms of liver damage, collectively these initial studies suggest that PNPLA3 I148M is a promising candidate general modifier of fibrogenesis in liver diseases.
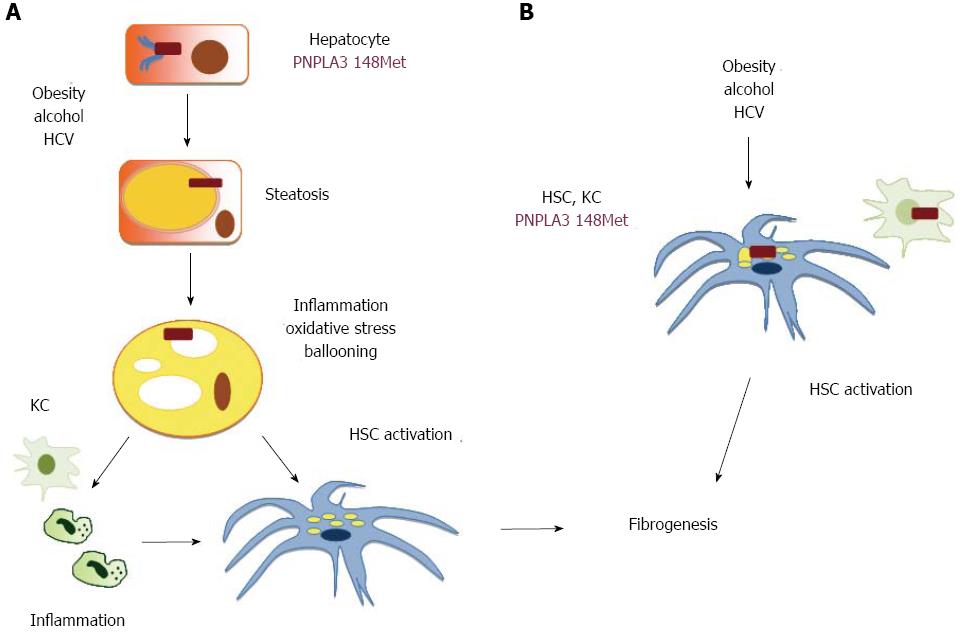
Intriguingly, the association between the I148M polymorphism and NASH appears to be independent of the severity of steatosis[31], thus suggesting that this genetic variant not only influences the overall amount of hepatocellular fat, but by impacting on the concentration or subcellular localization of specific lipid species directly modulates inflammation. It should not be forgotten that several lipids behave as inflammatory mediators acting through specific receptors[9]. Alternatively, if the 148M mutation slows down triglycerides kinetics between cell compartments, it could be speculated that renders them more susceptible to lipoperoxidation, leading to oxidative stress, and in turn to hepatocellular damage and inflammation[9]. These hypothetical mechanisms linking the PNPLA3 148M mutation with hepatic fibrogenesis are shown in Figure 2. In the aforementioned scenario, shown in panel A, hepatocellular damage and the release of inflammatory mediators would lead to secondary activation of Kupffer cells amplifying the inflammatory cascade and cell death, and of hepatic stellate cells with initiation of fibrogenesis.
Even more striking is though the observation that patients the I148M polymorphism is associated in NAFLD with advanced fibrosis independently of NASH[31], and in CHC with cirrhosis independently of steatosis, ALT levels, and hepatic necroinflammatory activity[75]. It could therefore be envisioned that the 148M mutation also directly influences the activation of non-parenchymal hepatic cells in response to hepatotoxic insults, as shown in Figure 2B. This hypothesis needs to be addressed and the potential mechanisms investigated by experimental studies.
Last but not least, evidence is also accumulating that the I148M polymorphism predisposes to hepatocellular carcinoma, a common complication of cirrhosis and the fifth cause of cancer worldwide, with a clinically significant increment in risk, thereby representing a potentially useful biomarker[75,95-97]. We have recently reviewed elsewhere the clinical studies supporting such an association and the potential mechanisms involved[3]. To summarize, data indicate that the 148M PNPLA3 mutation favors hepatic carcinogenesis in steatohepatitis as well as in other liver diseases, and the mechanism is partly independent of the predisposition towards fibrogenesis and cirrhosis.
In conclusion, PNPLA3 is a novel key player in liver disease progression. Assessment of the I148M polymorphism will possibly inform clinical practice in the future, whereas the determination of the physiological role of wild-type PNPLA3 and the 148M variant will reveal mechanisms involved in hepatic fibrogenesis and carcinogenesis and hopefully identify novel therapeutic targets.
P- Reviewers: Loguercio C, Rosenthal P S- Editor: Zhai HH L- Editor: A E- Editor: Wu HL
| 1. | Dongiovanni P, Anstee QM, Valenti L. Genetic predisposition in NAFLD and NASH: impact on severity of liver disease and response to treatment. Curr Pharm Des. 2013;19:5219-5238. [PubMed] |
| 2. | Hirschhorn JN, Gajdos ZK. Genome-wide association studies: results from the first few years and potential implications for clinical medicine. Annu Rev Med. 2011;62:11-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Valenti L, Dongiovanni P, Ginanni Corradini S, Burza MA, Romeo S. PNPLA3 I148M variant and hepatocellular carcinoma: a common genetic variant for a rare disease. Dig Liver Dis. 2013;45:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461-1465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2701] [Cited by in RCA: 2594] [Article Influence: 152.6] [Reference Citation Analysis (0)] |
| 5. | Yuan X, Waterworth D, Perry JR, Lim N, Song K, Chambers JC, Zhang W, Vollenweider P, Stirnadel H, Johnson T. Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am J Hum Genet. 2008;83:520-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 360] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 6. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844-1850. [PubMed] |
| 7. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [PubMed] |
| 8. | Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112-117. [PubMed] |
| 9. | Day CP. From fat to inflammation. Gastroenterology. 2006;130:207-210. [PubMed] |
| 10. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [PubMed] |
| 11. | Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140. [PubMed] |
| 12. | Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 919] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 13. | Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E, Rizzetto M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634-642. [PubMed] |
| 14. | Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 788] [Article Influence: 43.8] [Reference Citation Analysis (2)] |
| 15. | Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369-1375. [PubMed] |
| 16. | Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341-1350. [PubMed] |
| 17. | Dongiovanni P, Valenti L, Rametta R, Daly AK, Nobili V, Mozzi E, Leathart JB, Pietrobattista A, Burt AD, Maggioni M. Genetic variants regulating insulin receptor signalling are associated with the severity of liver damage in patients with non-alcoholic fatty liver disease. Gut. 2010;59:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Valenti L, Fracanzani AL, Fargion S. The immunopathogenesis of alcoholic and nonalcoholic steatohepatitis: two triggers for one disease? Semin Immunopathol. 2009;31:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Lee YS, Jeong WI. Retinoic acids and hepatic stellate cells in liver disease. J Gastroenterol Hepatol. 2012;27 Suppl 2:75-79. [PubMed] |
| 20. | Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195-206. [PubMed] |
| 21. | Schwimmer JB, Celedon MA, Lavine JE, Salem R, Campbell N, Schork NJ, Shiehmorteza M, Yokoo T, Chavez A, Middleton MS. Heritability of nonalcoholic fatty liver disease. Gastroenterology. 2009;136:1585-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 358] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 22. | Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49:791-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 23. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1619] [Article Influence: 115.6] [Reference Citation Analysis (1)] |
| 24. | Struben VM, Hespenheide EE, Caldwell SH. Nonalcoholic steatohepatitis and cryptogenic cirrhosis within kindreds. Am J Med. 2000;108:9-13. [PubMed] |
| 25. | Willner IR, Waters B, Patil SR, Reuben A, Morelli J, Riely CA. Ninety patients with nonalcoholic steatohepatitis: insulin resistance, familial tendency, and severity of disease. Am J Gastroenterol. 2001;96:2957-2961. [PubMed] |
| 26. | Makkonen J, Pietiläinen KH, Rissanen A, Kaprio J, Yki-Järvinen H. Genetic factors contribute to variation in serum alanine aminotransferase activity independent of obesity and alcohol: a study in monozygotic and dizygotic twins. J Hepatol. 2009;50:1035-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Bathum L, Petersen HC, Rosholm JU, Hyltoft Petersen P, Vaupel J, Christensen K. Evidence for a substantial genetic influence on biochemical liver function tests: results from a population-based Danish twin study. Clin Chem. 2001;47:81-87. [PubMed] |
| 28. | Hernaez R, McLean J, Lazo M, Brancati FL, Hirschhorn JN, Borecki IB, Harris TB, Nguyen T, Kamel IR, Bonekamp S. Association between variants in or near PNPLA3, GCKR, and PPP1R3B with ultrasound-defined steatosis based on data from the third national health and nutrition examination survey. Clin Gastroenterol Hepatol. 2013;11:1183-1190.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Kotronen A, Johansson LE, Johansson LM, Roos C, Westerbacka J, Hamsten A, Bergholm R, Arkkila P, Arola J, Kiviluoto T. A common variant in PNPLA3, which encodes adiponutrin, is associated with liver fat content in humans. Diabetologia. 2009;52:1056-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 30. | Sookoian S, Castaño GO, Burgueño AL, Gianotti TF, Rosselli MS, Pirola CJ. A nonsynonymous gene variant in the adiponutrin gene is associated with nonalcoholic fatty liver disease severity. J Lipid Res. 2009;50:2111-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 31. | Valenti L, Al-Serri A, Daly AK, Galmozzi E, Rametta R, Dongiovanni P, Nobili V, Mozzi E, Roviaro G, Vanni E. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1209-1217. [PubMed] |
| 32. | Romeo S, Sentinelli F, Dash S, Yeo GS, Savage DB, Leonetti F, Capoccia D, Incani M, Maglio C, Iacovino M. Morbid obesity exposes the association between PNPLA3 I148M (rs738409) and indices of hepatic injury in individuals of European descent. Int J Obes (Lond). 2010;34:190-194. [PubMed] |
| 33. | Romeo S, Sentinelli F, Cambuli VM, Incani M, Congiu T, Matta V, Pilia S, Huang-Doran I, Cossu E, Loche S. The 148M allele of the PNPLA3 gene is associated with indices of liver damage early in life. J Hepatol. 2010;53:335-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 34. | Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52:894-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 381] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 35. | Speliotes EK, Butler JL, Palmer CD, Voight BF, Hirschhorn JN. PNPLA3 variants specifically confer increased risk for histologic nonalcoholic fatty liver disease but not metabolic disease. Hepatology. 2010;52:904-912. [PubMed] |
| 36. | Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 739] [Article Influence: 52.8] [Reference Citation Analysis (1)] |
| 37. | Nobili V, Svegliati-Baroni G, Alisi A, Miele L, Valenti L, Vajro P. A 360-degree overview of paediatric NAFLD: recent insights. J Hepatol. 2013;58:1218-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 38. | Kitamoto T, Kitamoto A, Yoneda M, Hyogo H, Ochi H, Nakamura T, Teranishi H, Mizusawa S, Ueno T, Chayama K. Genome-wide scan revealed that polymorphisms in the PNPLA3, SAMM50, and PARVB genes are associated with development and progression of nonalcoholic fatty liver disease in Japan. Hum Genet. 2013;132:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 39. | Peng XE, Wu YL, Lin SW, Lu QQ, Hu ZJ, Lin X. Genetic variants in PNPLA3 and risk of non-alcoholic fatty liver disease in a Han Chinese population. PLoS One. 2012;7:e50256. [PubMed] |
| 40. | Hotta K, Yoneda M, Hyogo H, Ochi H, Mizusawa S, Ueno T, Chayama K, Nakajima A, Nakao K, Sekine A. Association of the rs738409 polymorphism in PNPLA3 with liver damage and the development of nonalcoholic fatty liver disease. BMC Med Genet. 2010;11:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 41. | Lin YC, Chang PF, Hu FC, Yang WS, Chang MH, Ni YH. A common variant in the PNPLA3 gene is a risk factor for non-alcoholic fatty liver disease in obese Taiwanese children. J Pediatr. 2011;158:740-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Giudice EM, Grandone A, Cirillo G, Santoro N, Amato A, Brienza C, Savarese P, Marzuillo P, Perrone L. The association of PNPLA3 variants with liver enzymes in childhood obesity is driven by the interaction with abdominal fat. PLoS One. 2011;6:e27933. [PubMed] |
| 43. | Davis JN, Lê KA, Walker RW, Vikman S, Spruijt-Metz D, Weigensberg MJ, Allayee H, Goran MI. Increased hepatic fat in overweight Hispanic youth influenced by interaction between genetic variation in PNPLA3 and high dietary carbohydrate and sugar consumption. Am J Clin Nutr. 2010;92:1522-1527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 44. | Santoro N, Savoye M, Kim G, Marotto K, Shaw MM, Pierpont B, Caprio S. Hepatic fat accumulation is modulated by the interaction between the rs738409 variant in the PNPLA3 gene and the dietary omega6/omega3 PUFA intake. PLoS One. 2012;7:e37827. [PubMed] |
| 45. | Santoro N, Zhang CK, Zhao H, Pakstis AJ, Kim G, Kursawe R, Dykas DJ, Bale AE, Giannini C, Pierpont B. Variant in the glucokinase regulatory protein (GCKR) gene is associated with fatty liver in obese children and adolescents. Hepatology. 2012;55:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 46. | Valenti L, Alisi A, Nobili V. Unraveling the genetics of fatty liver in obese children: additive effect of P446L GCKR and I148M PNPLA3 polymorphisms. Hepatology. 2012;55:661-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Sevastianova K, Santos A, Kotronen A, Hakkarainen A, Makkonen J, Silander K, Peltonen M, Romeo S, Lundbom J, Lundbom N. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am J Clin Nutr. 2012;96:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 48. | He S, McPhaul C, Li JZ, Garuti R, Kinch L, Grishin NV, Cohen JC, Hobbs HH. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285:6706-6715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 433] [Cited by in RCA: 495] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 49. | Huang Y, He S, Li JZ, Seo YK, Osborne TF, Cohen JC, Hobbs HH. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proc Natl Acad Sci USA. 2010;107:7892-7897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 307] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 50. | Pirazzi C, Adiels M, Burza MA, Mancina RM, Levin M, Ståhlman M, Taskinen MR, Orho-Melander M, Perman J, Pujia A. Patatin-like phospholipase domain-containing 3 (PNPLA3) I148M (rs738409) affects hepatic VLDL secretion in humans and in vitro. J Hepatol. 2012;57:1276-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 51. | Kumari M, Schoiswohl G, Chitraju C, Paar M, Cornaciu I, Rangrez AY, Wongsiriroj N, Nagy HM, Ivanova PT, Scott SA. Adiponutrin functions as a nutritionally regulated lysophosphatidic acid acyltransferase. Cell Metab. 2012;15:691-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 238] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 52. | Basantani MK, Sitnick MT, Cai L, Brenner DS, Gardner NP, Li JZ, Schoiswohl G, Yang K, Kumari M, Gross RW. Pnpla3/Adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J Lipid Res. 2011;52:318-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 53. | Li JZ, Huang Y, Karaman R, Ivanova PT, Brown HA, Roddy T, Castro-Perez J, Cohen JC, Hobbs HH. Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis. J Clin Invest. 2012;122:4130-4144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 225] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 54. | Kumashiro N, Yoshimura T, Cantley JL, Majumdar SK, Guebre-Egziabher F, Kursawe R, Vatner DF, Fat I, Kahn M, Erion DM. Role of patatin-like phospholipase domain-containing 3 on lipid-induced hepatic steatosis and insulin resistance in rats. Hepatology. 2013;57:1763-1772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 55. | Chen W, Chang B, Li L, Chan L. Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology. 2010;52:1134-1142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 56. | Santoro N, Kursawe R, D’Adamo E, Dykas DJ, Zhang CK, Bale AE, Calí AM, Narayan D, Shaw MM, Pierpont B. A common variant in the patatin-like phospholipase 3 gene (PNPLA3) is associated with fatty liver disease in obese children and adolescents. Hepatology. 2010;52:1281-1290. [PubMed] |
| 57. | Valenti L, Rametta R, Ruscica M, Dongiovanni P, Steffani L, Motta BM, Canavesi E, Fracanzani AL, Mozzi E, Roviaro G. The I148M PNPLA3 polymorphism influences serum adiponectin in patients with fatty liver and healthy controls. BMC Gastroenterol. 2012;12:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 58. | Chalasani N, Wilson L, Kleiner DE, Cummings OW, Brunt EM, Unalp A. Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with non-alcoholic fatty liver disease. J Hepatol. 2008;48:829-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 59. | Valenti L, Alisi A, Galmozzi E, Bartuli A, Del Menico B, Alterio A, Dongiovanni P, Fargion S, Nobili V. I148M patatin-like phospholipase domain-containing 3 gene variant and severity of pediatric nonalcoholic fatty liver disease. Hepatology. 2010;52:1274-1280. [PubMed] |
| 60. | Tian C, Stokowski RP, Kershenobich D, Ballinger DG, Hinds DA. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet. 2010;42:21-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 343] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 61. | Stickel F, Buch S, Lau K, Meyer zu Schwabedissen H, Berg T, Ridinger M, Rietschel M, Schafmayer C, Braun F, Hinrichsen H. Genetic variation in the PNPLA3 gene is associated with alcoholic liver injury in caucasians. Hepatology. 2011;53:86-95. [PubMed] |
| 62. | Stickel F, Hampe J. Genetic determinants of alcoholic liver disease. Gut. 2012;61:150-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 63. | Burza MA, Molinaro A, Attilia ML, Rotondo C, Attilia F, Ceccanti M, Ferri F, Maldarelli F, Maffongelli A, De Santis A. PNPLA3 I148M (rs738409) genetic variant and age at onset of at-risk alcohol consumption are independent risk factors for alcoholic cirrhosis. Liv Int. 2013;in press. |
| 64. | Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 908] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 65. | Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358-1364. [PubMed] |
| 66. | Akuta N, Suzuki F, Tsubota A, Suzuki Y, Someya T, Kobayashi M, Saitoh S, Arase Y, Ikeda K, Kumada H. Efficacy of interferon monotherapy to 394 consecutive naive cases infected with hepatitis C virus genotype 2a in Japan: therapy efficacy as consequence of tripartite interaction of viral, host and interferon treatment-related factors. J Hepatol. 2002;37:831-836. [PubMed] |
| 67. | Rubbia-Brandt L, Quadri R, Abid K, Giostra E, Malé PJ, Mentha G, Spahr L, Zarski JP, Borisch B, Hadengue A. Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3. J Hepatol. 2000;33:106-115. [PubMed] |
| 68. | Westin J, Nordlinder H, Lagging M, Norkrans G, Wejstål R. Steatosis accelerates fibrosis development over time in hepatitis C virus genotype 3 infected patients. J Hepatol. 2002;37:837-842. [PubMed] |
| 69. | Bassendine MF, Sheridan DA, Felmlee DJ, Bridge SH, Toms GL, Neely RD. HCV and the hepatic lipid pathway as a potential treatment target. J Hepatol. 2011;55:1428-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 70. | Czaja AJ, Carpenter HA, Santrach PJ, Moore SB. Host- and disease-specific factors affecting steatosis in chronic hepatitis C. J Hepatol. 1998;29:198-206. [PubMed] |
| 71. | Monto A, Alonzo J, Watson JJ, Grunfeld C, Wright TL. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology. 2002;36:729-736. [PubMed] |
| 72. | Kumar D, Farrell GC, Fung C, George J. Hepatitis C virus genotype 3 is cytopathic to hepatocytes: Reversal of hepatic steatosis after sustained therapeutic response. Hepatology. 2002;36:1266-1272. [PubMed] |
| 73. | Lerat H, Honda M, Beard MR, Loesch K, Sun J, Yang Y, Okuda M, Gosert R, Xiao SY, Weinman SA. Steatosis and liver cancer in transgenic mice expressing the structural and nonstructural proteins of hepatitis C virus. Gastroenterology. 2002;122:352-365. [PubMed] |
| 74. | Cai T, Dufour JF, Muellhaupt B, Gerlach T, Heim M, Moradpour D, Cerny A, Malinverni R, Kaddai V, Bochud M. Viral genotype-specific role of PNPLA3, PPARG, MTTP, and IL28B in hepatitis C virus-associated steatosis. J Hepatol. 2011;55:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 75. | Valenti L, Rumi M, Galmozzi E, Aghemo A, Del Menico B, De Nicola S, Dongiovanni P, Maggioni M, Fracanzani AL, Rametta R. Patatin-like phospholipase domain-containing 3 I148M polymorphism, steatosis, and liver damage in chronic hepatitis C. Hepatology. 2011;53:791-799. [PubMed] |
| 76. | Müller T, Buch S, Berg T, Hampe J, Stickel F. Distinct, alcohol-modulated effects of PNPLA3 genotype on progression of chronic hepatitis C. J Hepatol. 2011;55:732-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Trépo E, Pradat P, Potthoff A, Momozawa Y, Quertinmont E, Gustot T, Lemmers A, Berthillon P, Amininejad L, Chevallier M. Impact of patatin-like phospholipase-3 (rs738409 C > G) polymorphism on fibrosis progression and steatosis in chronic hepatitis C. Hepatology. 2011;54:60-69. [PubMed] |
| 78. | Cefalù AB, Pirruccello JP, Noto D, Gabriel S, Valenti V, Gupta N, Spina R, Tarugi P, Kathiresan S, Averna MR. A novel APOB mutation identified by exome sequencing cosegregates with steatosis, liver cancer, and hypocholesterolemia. Arterioscler Thromb Vasc Biol. 2013;33:2021-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 79. | Valenti L, Aghemo A, Stättermayer AF, Maggioni P, De Nicola S, Motta BM, Rumi MG, Dongiovanni P, Ferenci P, Colombo M. Implications of PNPLA3 polymorphism in chronic hepatitis C patients receiving peginterferon plus ribavirin. Aliment Pharmacol Ther. 2012;35:1434-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 80. | Valenti L, Colombo M, Fargion S. Modulation of the effect of PNPLA3 I148M mutation on steatosis and liver damage by alcohol intake in patients with chronic hepatitis C. J Hepatol. 2011;55:1470-1471; author reply 1471-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 81. | Valenti L, Aghemo A, Stättermayer AF. Interaction between IL28B and PNPLA3 genotypes in the pathogenesis of steatosis in chronic hepatitis C non genotype-3 patients. J Hepatol. 2012;56:1209-110; author reply 1209-1210. [PubMed] |
| 82. | Valenti L, Alisi A, Nobili V. 148M PNPLA3 variant and progressive liver disease: a new paradigm in hepatology. Hepatology. 2012;56:1883-1889. [PubMed] |
| 83. | Rembeck K, Maglio C, Lagging M, Christensen PB, Färkkilä M, Langeland N, Buhl MR, Pedersen C, Mørch K, Norkrans G. PNPLA 3 I148M genetic variant associates with insulin resistance and baseline viral load in HCV genotype 2 but not in genotype 3 infection. BMC Med Genet. 2012;13:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2400] [Article Influence: 184.6] [Reference Citation Analysis (0)] |
| 85. | Park SH, Kim DJ, Lee HY. Insulin resistance is not associated with histologic severity in nondiabetic, noncirrhotic patients with chronic hepatitis B virus infection. Am J Gastroenterol. 2009;104:1135-1139. [PubMed] |
| 86. | Petta S, Cammà C, Di Marco V, Macaluso FS, Maida M, Pizzolanti G, Belmonte B, Cabibi D, Di Stefano R, Ferraro D. Hepatic steatosis and insulin resistance are associated with severe fibrosis in patients with chronic hepatitis caused by HBV or HCV infection. Liver Int. 2011;31:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 87. | Wong VW, Wong GL, Yu J, Choi PC, Chan AW, Chan HY, Chu ES, Cheng AS, Chim AM, Chan FK. Interaction of adipokines and hepatitis B virus on histological liver injury in the Chinese. Am J Gastroenterol. 2010;105:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 88. | Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chan HY, Chan FK, Sung JJ, Chan HL. Metabolic syndrome increases the risk of liver cirrhosis in chronic hepatitis B. Gut. 2009;58:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 89. | Viganò M, Valenti L, Lampertico P, Facchetti F, Motta BM, D’Ambrosio R, Romagnoli S, Dongiovanni P, Donati B, Fargion S. Patatin-like phospholipase domain-containing 3 I148M affects liver steatosis in patients with chronic hepatitis B. Hepatology. 2013;58:1245-1252. [PubMed] |
| 90. | Gualdi R, Casalgrandi G, Montosi G, Ventura E, Pietrangelo A. Excess iron into hepatocytes is required for activation of collagen type I gene during experimental siderosis. Gastroenterology. 1994;107:1118-1124. [PubMed] |
| 91. | Pietrangelo A. Hereditary hemochromatosis--a new look at an old disease. N Engl J Med. 2004;350:2383-2397. [PubMed] |
| 92. | Wood MJ, Powell LW, Ramm GA. Environmental and genetic modifiers of the progression to fibrosis and cirrhosis in hemochromatosis. Blood. 2008;111:4456-4462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 93. | Valenti L, Maggioni P, Piperno A, Rametta R, Pelucchi S, Mariani R, Dongiovanni P, Fracanzani AL, Fargion S. Patatin-like phospholipase domain containing-3 gene I148M polymorphism, steatosis, and liver damage in hereditary hemochromatosis. World J Gastroenterol. 2012;18:2813-2820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 94. | Friedrich K, Rupp C, Hov JR, Steinebrunner N, Weiss KH, Stiehl A, Brune M, Schaefer PK, Schemmer P, Sauer P. A frequent PNPLA3 variant is a sex specific disease modifier in PSC patients with bile duct stenosis. PLoS One. 2013;8:e58734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 95. | Trepo E, Guyot E, Ganne-Carrie N, Degre D, Gustot T, Franchimont D, Sutton A, Nahon P, Moreno C. PNPLA3 (rs738409 C > G) is a common risk variant associated with hepatocellular carcinoma in alcoholic cirrhosis. Hepatology. 2012;55:1307-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 96. | Falleti E, Fabris C, Cmet S, Cussigh A, Bitetto D, Fontanini E, Fornasiere E, Bignulin S, Fumolo E, Bignulin E. PNPLA3 rs738409C/G polymorphism in cirrhosis: relationship with the aetiology of liver disease and hepatocellular carcinoma occurrence. Liver Int. 2011;31:1137-1143. [PubMed] |
| 97. | Burza MA, Pirazzi C, Maglio C, Sjöholm K, Mancina RM, Svensson PA, Jacobson P, Adiels M, Baroni MG, Borén J. PNPLA3 I148M (rs738409) genetic variant is associated with hepatocellular carcinoma in obese individuals. Dig Liver Dis. 2012;44:1037-1041. [PubMed] |