Published online Jan 28, 2013. doi: 10.3748/wjg.v19.i4.597
Revised: December 2, 2012
Accepted: December 15, 2012
Published online: January 28, 2013
Processing time: 95 Days and 16.7 Hours
Here, we report a case of fulminant gastrointestinal graft-versus-host disease (GI-GVHD) with cytomegalovirus (CMV) infection in 44-year-old woman. Despite the difficulties associated with the treatment of GI-GVHD and GI-CMV disease, the mucosal findings and the clinical course showed marked improvements during long-term clinical observation. The endoscopic findings were remarkable, with diffuse sloughing mucosa in the stomach and highly active inflammation and deep discrete ulcers throughout the colon. Changes in the CMV quantitative polymerase chain reaction results were correlated with the endoscopic mucosal findings and were useful for assessing the efficacy of the treatment. Although a definite diagnosis of GI-GVHD is generally made by endoscopy with biopsy, the gross appearance of this disease can vary depending on the endoscopy. In this paper, we also conduct a literature review of patients with GI-GVHD.
- Citation: Okubo H, Nagata N, Uemura N. Fulminant gastrointestinal graft-versus-host disease concomitant with cytomegalovirus infection: Case report and literature review. World J Gastroenterol 2013; 19(4): 597-603
- URL: https://www.wjgnet.com/1007-9327/full/v19/i4/597.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i4.597
Graft-versus-host disease (GVHD) is a serious complication of allogeneic hematopoietic stem cell transplantation (HSCT) and mainly attacks the skin, gastrointestinal (GI) tract and liver[1-5]. GI-GVHD, in particular, can cause life-threatening complications, such as massive diarrhea, hemorrhage, paralytic ileus, and perforation[6,7]; therefore, the accurate diagnosis of GI-GVHD is essential. Although endoscopic observation is an indispensable diagnostic tool, various mucosal patterns are observed on endoscopy[8]. In addition, testing for cytomegalovirus (CMV) infection is necessary because the complication of GI-GVHD with CMV infection will reduce the quality of life (QoL) of the patients[9]. This reduction in the QoL occurs because CMV-related GI disease is exacerbated by the administration of steroids for the treatment of GI-GVHD when antiviral drugs are not administered[10]. Here, we report a rare case of fulminant GI-GVHD detected by endoscopy and its clinical course, and we review related literature on the endoscopic findings of GI-GVHD.
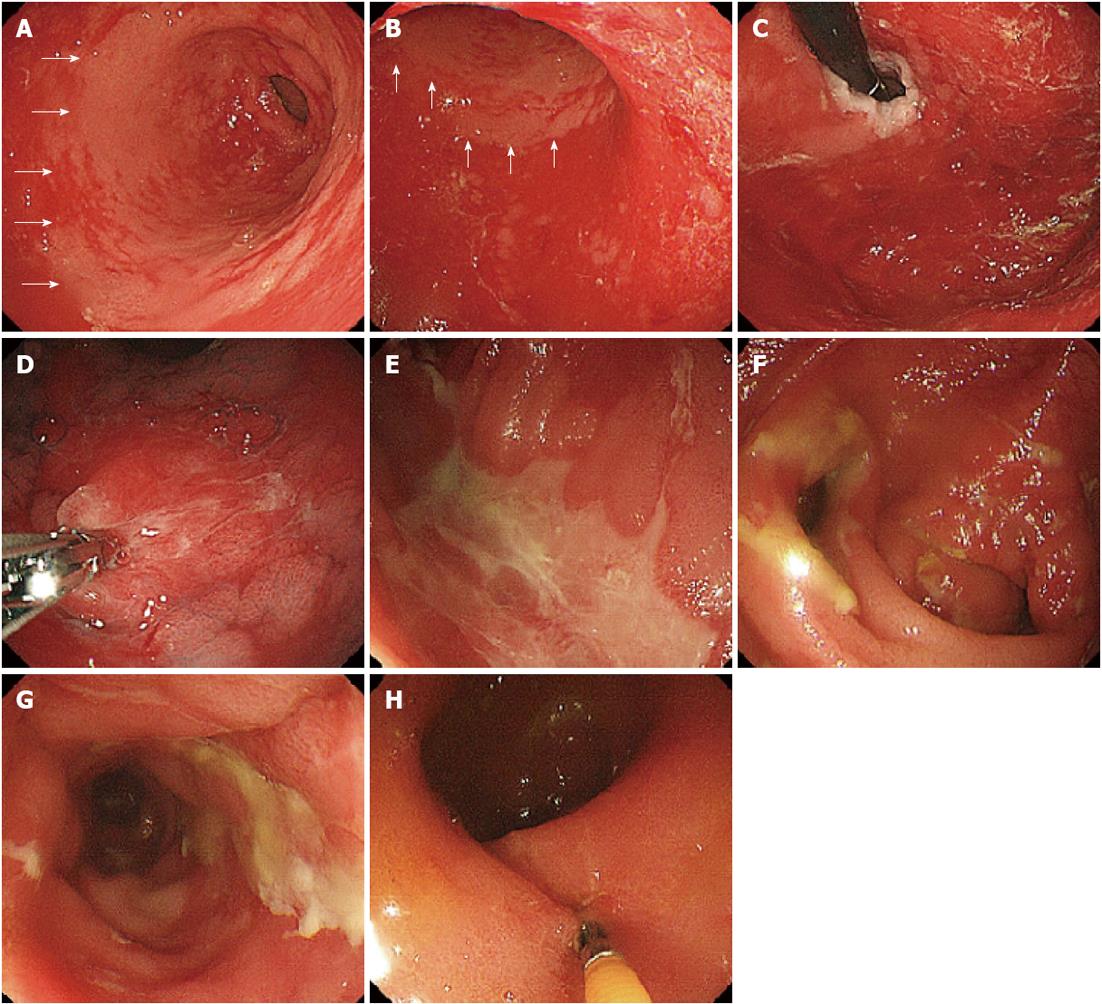
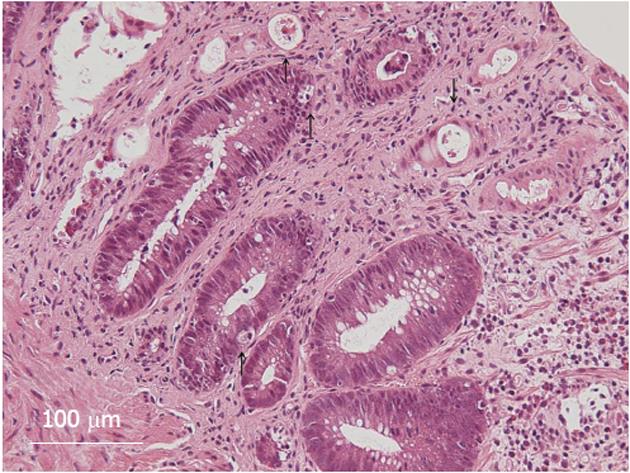
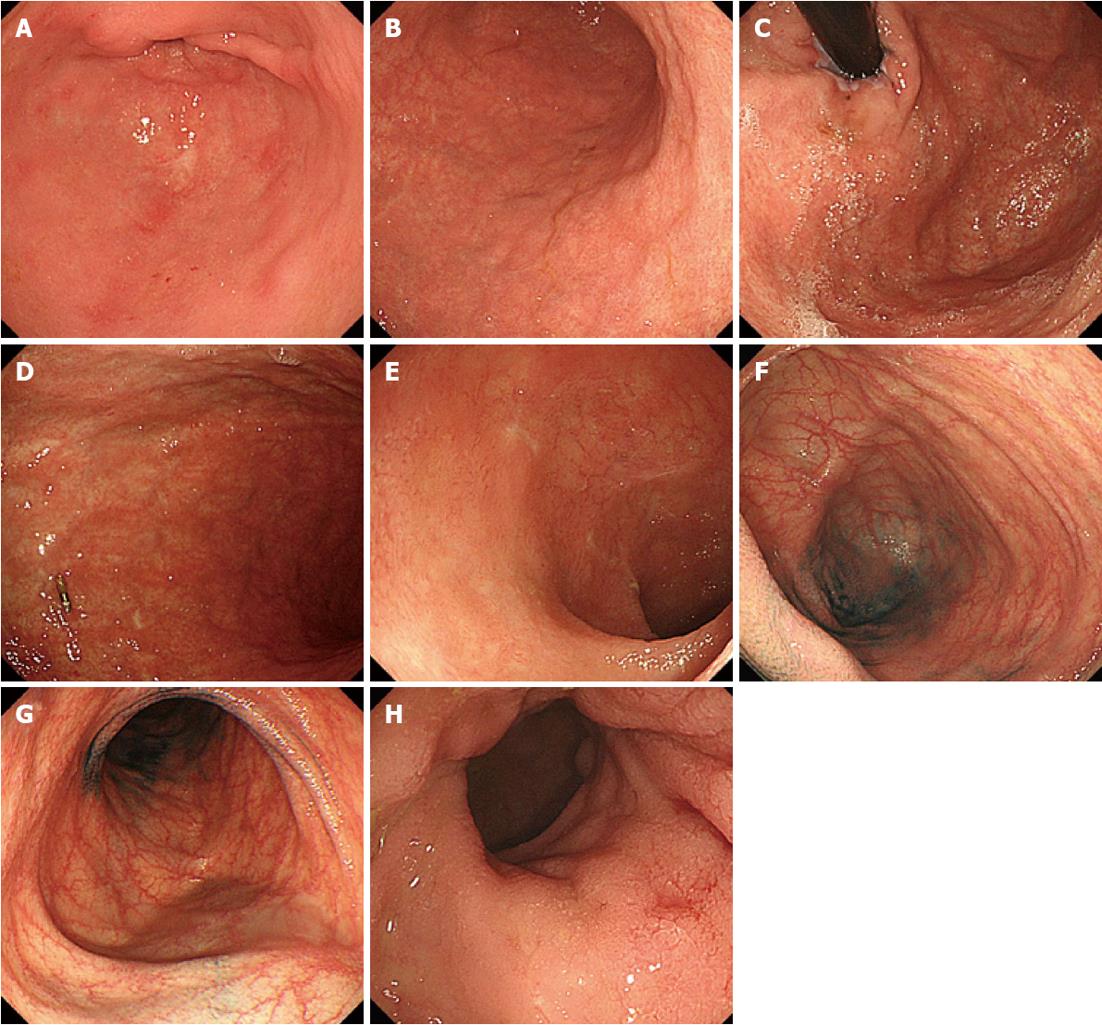
The patient, a 44-year-old woman with acute myeloid leukemia who was diagnosed 18 mo earlier, underwent allogeneic bone marrow transplantation 3 mo prior to the examination. A full-body skin rash appeared on day 26 after the transplantation. On day 85 after the transplantation, she experienced watery diarrhea more than 10 times per day and developed abdominal pain. Upper GI endoscopy showed diffuse sloughing of the mucosa with diffuse erythema and hemorrhage in the antrum and corpus of the stomach (Figure 1A-D). Lower GI endoscopy revealed multiple deep discrete ulcers with exudative and mucosal oozing in the terminal ileum end and throughout the entire colon (Figure 1E and F). Biopsy specimens from the upper and lower GI tract revealed diffuse erythema, erosions, and sloughing mucosa with active bleeding in the stomach, as well as multiple erosions and a small discrete ulcer with active bleeding in the lower GI tract. Gland apoptosis showed histological evidence of acute GVHD (Figure 2). Moreover, the biopsy specimens showed CMV infection detected by polymerase chain reaction (PCR) at remarkably elevated concentrations of 4.0 × 104 copies/μg DNA, but immunochemical staining for the CMV antibody was negative. Finally, the patient was diagnosed with severe GI-GVHD concomitant with a CMV infection. Other organ involvement included a whole-body skin rash and slight liver dysfunction.
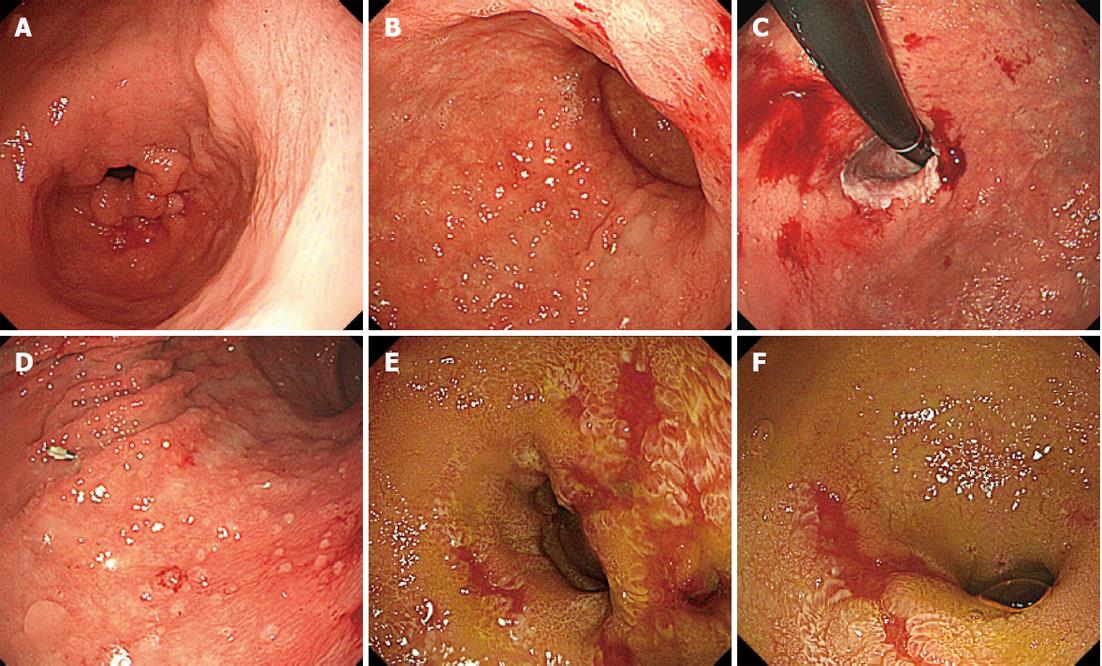
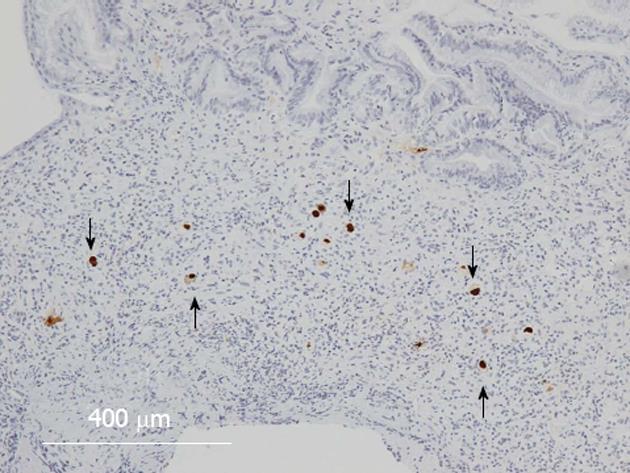
The treatment for acute GVHD was started with an initial dose of prednisolone 1 mg/kg per day (50 mg/d) and for CMV infection with an initial dose of ganciclovir (GCV) 10 mg/kg per day. After 40 d of treatment, the patient improved clinically. Then, GCV was discontinued because of thrombocytopenia that appeared 60 d after the treatment. After 64 d, however, CMV was detected in the blood by a CMV-antigenemia assay, and the maintenance dose of GCV (5 mg/kg per day) was resumed. After 70 d, upper and lower endoscopy showed that the endoscopic findings of mucosal sloughing had improved (Figure 3A-D) but that the colonic mucosa remained ulcerative and inflamed (Figure 3E and F). Both upper and lower GI tract biopsies revealed CMV-infected cells in immunohistochemically stained tissue specimens (Figure 4), and the CMV-PCR from the GI tract biopsy was 2.0 × 105 copies/μg DNA. Intravenous GCV at 5 mg/kg every 12 h as the induction therapy was re-started and then changed to the maintenance therapy, and the prednisolone dose was tapered. Although the treatment continued for an additional 102 d, the CMV-PCR from the GI tract biopsy was 1.0 × 103 copies/μg DNA. Oral valganciclovir at 900 mg/d was started, with oral prednisolone 10 mg administered every other day. After 162 d, the upper and lower endoscopy showed notable improvements in the mucosal findings (Figure 5), and the CMV-PCR count from a GI tract biopsy was within the normal range of < 4.0 × 10 copies/μg DNA.
Nausea and vomiting, appetite loss, abdominal pain, and watery diarrhea are common symptoms of GI-GVHD. Watery diarrhea appears in almost all cases of GI-GVHD and occasionally becomes chronic or causes bleeding. Therefore, this symptom is a major factor that reduces patient QoL[11]. Moreover, after HSCT, CMV infection of the GI mucosa and GI-GVHD can both cause diarrhea[12,13].
Diseases characterized by the development of GI symptoms after HSCT include not only CMV and GI-GVHD infection but also virus infections caused by enterovirus, adenovirus, rotavirus, and Epstein-Barr virus, complications with bacterial and fungus infections, and colitis associated with thrombotic microangiopathy and regimen-related toxicity. In routine clinical examination, differentiating among these diseases is difficult[14], particularly because GI-GVHD is frequently associated with CMV infection[15,16]. In this case, because of the severe GI-GVHD accompanied by CMV infection, the patient developed frequent and long-term bloody diarrhea and abdominal pain, which appeared to have led to serious symptoms such as anemia, malnutrition, and dehydration.
The endoscopic findings reflected the severity of the clinical symptoms. The lower GI tract exhibited severe signs of multiple deep ulcers, edema, and erythema, with no normal mucosa observed. The upper GI endoscopy revealed diffuse sloughing of the mucosa in the stomach, except for the antrum. Although the endoscopic findings of chronic GI-GVHD may include the characteristic esophageal web and strictures[2], various endoscopic findings are associated with acute GI-GVHD depending on the severity of the inflammation. We reviewed the English literature in the MEDLINE database by searching with “gastrointestinal”, “GVHD”, and “endoscopy” as key words (Table 1). Although there are some reports of normal mucosal findings, a large number of studies reported findings of erythema, erosions, and edema. Because the symptoms were generally mild in the upper GI tract, the lower GI tract often had relatively severe inflammatory symptoms, with occasional actively bleeding ulcers. In addition, although tortoise shell-like mucosa and pseudomembrane formation are occasionally observed, no particular findings are reportedly associated with the colorectal mucosa. Moreover, even though some papers report mucosal sloughing and ulceration[8], a wide area of sloughing mucosa along with ulcers and inflamed mucosa, as observed in the present case, has never been reported. We believe that a combination of severe GVHD and CMV infection is the pathogenesis responsible for these symptoms[13]. According to He et al[17], GI-GVHD accompanied by CMV infection causes deep, discrete ulcers. The ischemic consequences of occluded blood vessels caused by enlarged vascular endothelial cells due to CMV infection are thought to be the mechanism underlying the GI mucosal damage[18]. Similarly, ischemic alteration, in addition to the cytotoxicity caused by GVHD[19], is a likely cause of the severe symptoms in our patient.
| No. | Ref. | Patients | Involved GI tract | Endoscopic findings |
| 1 | Sultan et al[23] | Acute GVHD patients less than 18 yr old (n = 40) | Stomach, duodenum, sigmoid and rectum | Normal, erosions, ulcer |
| 2 | Kreisel et al[24] | GI-GVHD patients (n = 175) | Stomach, duodenum, colon and rectum | Normal, edema, erosions, aptha, ulcer, nonspecific |
| 3 | Aslanian et al[25] | Acute GVHD patients (n = 18) | Stomach, duodenum, total colon and rectum | Normal, erythema, edema, erosions, ulcer |
| 4 | Martínez et al[26] | GI-GVHD patients (n = 20) | Stomach, duodenum, colon and rectum | Normal, erythema, edema, erosions, ulcer, bleeding |
| 5 | Krishna et al[27] | A 49-yr-old man | Stomach, duodenum, colon and rectum | Normal, erosions |
| 6 | Al Ashgar et al[28] | A 23-yr-old male who had undergone allogeneic HSCT | Sigmoid colon and rectum | Erythema, edema |
| 7 | Varadarajan et al[29] | Acute GVHD patients (n = 11) | Stomach, duodenum, small bowel, colon and rectum | Normal, erythema, edema, erosions, ulcer, bleeding |
| 8 | He et al[17] | GI-GVHD patients (n = 32) | Colon and rectum | Erythema, edema, erosions, ulcer, bleeding, tortoise shell-like mucosa, pseudomembrane mucosa |
| 9 | Xu et al[8] | GI-GVHD patients (n = 8) | Stomach, duodenum, colon and rectum | Normal, erythema, edema, erosions, ulcer, bleeding |
| 10 | Cheung et al[30] | GI-GVHD patients (n = 44) | Stomach, duodenum, colon and rectum | Normal, erythema, edema, erosions, ulcer, nonspecific, bleeding |
| 11 | Ross et al[31] | GI-GVHD patients (n = 92) | Stomach, duodenum and rectum | Normal, erythema, edema, erosion, ulcer, nonspecific |
| 12 | Harada et al[32] | GI-GVHD patients (n = 15) | Colon and rectum | Erythema, edema, erosion, nonspecific |
| 13 | Khan et al[33] | Acute GVHD patients (n = 38) | Stomach, duodenum, colon and rectum | Edema, erosion, ulcer, bleeding, nonspecific, pseudomembrane mucosa |
| 14 | Silbermintz et al[34] | An 8-yr-old child | Small bowel | Erosion, bleeding |
| 15 | Holmberg et al[35] | GI-GVHD patients (n = 90) | Stomach, duodenum, colon and rectum | Normal, erythema, edema, ulcer |
| 16 | Onozawa et al[36] | An 18-yr-old male | Stomach, duodenum, colon and rectum | Normal, nonspecific |
| 17 | Yeh et al[37] | GI-GVHD patients (n = 4) | Stomach, duodenum, small bowel, colon and rectum | Erythema, edema, erosions, ulcer, bleeding, nonspecific |
| 18 | Schulenburg et al[38] | GI-GVHD patients (n = 29) | Stomach, duodenum, colon and rectum | Ulcer, erythema, erosion, normal |
| 19 | Cruz-Correa et al[39] | GI-GVHD patients (n = 44) | Stomach, duodenum, colon and rectum | Normal, erythema, edema, erosions, ulcer, bleeding, nonspecific |
| 20 | Wakui et al[40] | GI-GVHD patients (n = 44) | Stomach, duodenum, colon and rectum | Erythema, erosion, ulcer |
| 21 | Terdiman et al[41] | Acute GI-GVHD patients (n = 12) | Stomach, duodenum, colon and rectum | Normal, nonspecific |
The early and accurate diagnosis of CMV infection in the GI tract is the key to preventing severe symptoms, such as perforation and bleeding[20]. In this case, even though immunostaining with an anti-CMV antibody was negative, the quantitative PCR results of the biopsy specimens were positive, with a high value of 1.0 × 104 copies/μg DNA, which enabled the diagnosis of CMV-GID. The diagnostic accuracy of the quantitative PCR method using biopsy samples is reportedly superior to that of immunostaining to determine the involvement of CMV in the GI symptoms[21]. This case showed that the results of the CMV quantitative PCR were closely correlated with the post-treatment improvement of the mucosa, suggesting the usefulness of the technique for evaluating the effects of CMV treatment.
In conclusion, when endoscopic observation is performed on HSCT patients with postoperative GI symptoms, it is necessary to look for signs of mucosal sloughing and ulcers. In addition to a detailed endoscopic observation, biopsy samples should be examined for the characteristic pathological features of GVHD[22] and for signs of CMV infection. The course of this case suggests that the quantitative PCR of biopsy samples is useful for revealing CMV infection in the GI tract.
We thank Hisae Kawashiro, the clinical research coordinator, for helping with the data collection.
P- Reviewers Kiyan M, Yilmaz M S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2886] [Article Influence: 151.9] [Reference Citation Analysis (0)] |
| 2. | Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E, Moresi JM, Greenson J, Janin A, Martin PJ. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant. 2006;12:31-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 315] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | Pavletic SZ, Martin P, Lee SJ, Mitchell S, Jacobsohn D, Cowen EW, Turner ML, Akpek G, Gilman A, McDonald G. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant. 2006;12:252-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 329] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 4. | Schultz KR, Miklos DB, Fowler D, Cooke K, Shizuru J, Zorn E, Holler E, Ferrara J, Shulman H, Lee SJ. Toward biomarkers for chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: III. Biomarker Working Group Report. Biol Blood Marrow Transplant. 2006;12:126-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Martin PJ, Weisdorf D, Przepiorka D, Hirschfeld S, Farrell A, Rizzo JD, Foley R, Socie G, Carter S, Couriel D. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. Design of Clinical Trials Working Group report. Biol Blood Marrow Transplant. 2006;12:491-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Couriel D, Carpenter PA, Cutler C, Bolaños-Meade J, Treister NS, Gea-Banacloche J, Shaughnessy P, Hymes S, Kim S, Wayne AS. Ancillary therapy and supportive care of chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2006;12:375-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 223] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 7. | Irani JL, Cutler CS, Whang EE, Clancy TE, Russell S, Swanson RS, Ashley SW, Zinner MJ, Raut CP. Severe acute gastrointestinal graft-vs-host disease: an emerging surgical dilemma in contemporary cancer care. Arch Surg. 2008;143:1041-1045; discussion 1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Xu CF, Zhu LX, Xu XM, Chen WC, Wu DP. Endoscopic diagnosis of gastrointestinal graft-versus-host disease. World J Gastroenterol. 2008;14:2262-2267. [PubMed] |
| 9. | Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 927] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 10. | Beader N, Kalenić S, Labar B. [Diagnostic approach and therapy for cytomegalovirus (CMV) infection following allogeneic stem cell transplantation]. Lijec Vjesn. 2011;133:389-396. [PubMed] |
| 11. | Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295-304. [PubMed] |
| 12. | Rosen P, Armstrong D, Rice N. Gastrontestinal cytomegalovirus infection. Arch Intern Med. 1973;132:274-276. [PubMed] |
| 13. | Hoshino K, Shibata D, Miyagi T, Yamamoto Y, Arakaki S, Maeshiro T, Hokama A, Kinjo F, Takahashi K, Fujita J. Cytomegalovirus-associated gastric ulcers in a patient with dermatomyositis treated with steroid and cyclophosphamide pulse therapy. Endoscopy. 2011;43 Suppl 2 UCTN:E277-E278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Jacobsohn DA, Vogelsang GB. Acute graft versus host disease. Orphanet J Rare Dis. 2007;2:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Nachbaur D, Larcher C, Kircher B, Eibl G, Nussbaumer W, Gunsilius E, Haun M, Grünewald K, Gastl G. Risk for cytomegalovirus infection following reduced intensity allogeneic stem cell transplantation. Ann Hematol. 2003;82:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Einsele H, Ehninger G, Hebart H, Weber P, Dette S, Link H, Horny HP, Meuter V, Wagner S, Waller HD. Incidence of local CMV infection and acute intestinal GVHD in marrow transplant recipients with severe diarrhoea. Bone Marrow Transplant. 1994;14:955-963. [PubMed] |
| 17. | He JD, Liu YL, Wang ZF, Liu DH, Chen H, Chen YH. Colonoscopy in the diagnosis of intestinal graft versus host disease and cytomegalovirus enteritis following allogeneic haematopoietic stem cell transplantation. Chin Med J (Engl). 2008;121:1285-1289. [PubMed] |
| 18. | Chong KT, Gresser I, Mims CA. Interferon as a defence mechanism in mouse cytomegalovirus infection. J Gen Virol. 1983;64:461-464. [PubMed] |
| 19. | Goker H, Haznedaroglu IC, Chao NJ. Acute graft-vs-host disease: pathobiology and management. Exp Hematol. 2001;29:259-277. [PubMed] |
| 20. | Söderberg-Nauclér C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119-126. [PubMed] |
| 21. | Yoshino T, Nakase H, Ueno S, Uza N, Inoue S, Mikami S, Matsuura M, Ohmori K, Sakurai T, Nagayama S. Usefulness of quantitative real-time PCR assay for early detection of cytomegalovirus infection in patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm Bowel Dis. 2007;13:1516-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Shidham VB, Chang CC, Shidham G, Ghazala F, Lindholm PF, Kampalath B, George V, Komorowski R. Colon biopsies for evaluation of acute graft-versus-host disease (A-GVHD) in allogeneic bone marrow transplant patients. BMC Gastroenterol. 2003;3:5. [PubMed] |
| 23. | Sultan M, Ramprasad J, Jensen MK, Margolis D, Werlin S. Endoscopic diagnosis of pediatric acute gastrointestinal graft-versus-host disease. J Pediatr Gastroenterol Nutr. 2012;55:417-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Kreisel W, Dahlberg M, Bertz H, Harder J, Potthoff K, Deibert P, Schmitt-Graeff A, Finke J. Endoscopic diagnosis of acute intestinal GVHD following allogeneic hematopoietic SCT: a retrospective analysis in 175 patients. Bone Marrow Transplant. 2012;47:430-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Aslanian H, Chander B, Robert M, Cooper D, Proctor D, Seropian S, Jain D. Prospective evaluation of acute graft-versus-host disease. Dig Dis Sci. 2012;57:720-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Martínez C, Rosales M, Calvo X, Cuatrecasas M, Rodriguez-Carunchio L, Llach J, Fernández-Avilés F, Rosiñol L, Rovira M, Carreras E. Serial intestinal endoscopic examinations of patients with persistent diarrhea after allo-SCT. Bone Marrow Transplant. 2012;47:694-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Krishna SG, Barlogie B, Lamps LW, Krishna K, Aduli F, Anaissie E. Recurrent spontaneous gastrointestinal graft-versus-host disease in autologous hematopoietic stem cell transplantation. Clin Lymphoma Myeloma Leuk. 2010;10:E17-E21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Al Ashgar H, Peedikayil M, Chaudhri N, Al-Ghamdi A. Defecation of a “colon cast” as a rare presentation of acute graft-versus-host disease. Ann Saudi Med. 2009;29:231-233. [PubMed] |
| 29. | Varadarajan P, Dunford LM, Thomas JA, Brown K, Paplham P, Syta M, Schiff M, Padmanabhan S, Battiwalla M, Smiley S. Seeing what’s out of sight: wireless capsule endoscopy’s unique ability to visualize and accurately assess the severity of gastrointestinal graft-versus-host-disease. Biol Blood Marrow Transplant. 2009;15:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Cheung DY, Kim JI, Kim SS, Sung HY, Cho SH, Park SH, Han JY, Kim JK, Lee JW, Min WS. Endoscopic evaluation in gastrointestinal graft-versus-host disease: comparisons with histological findings. Dig Dis Sci. 2008;53:2947-2954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Ross WA, Ghosh S, Dekovich AA, Liu S, Ayers GD, Cleary KR, Lee JH, Couriel D. Endoscopic biopsy diagnosis of acute gastrointestinal graft-versus-host disease: rectosigmoid biopsies are more sensitive than upper gastrointestinal biopsies. Am J Gastroenterol. 2008;103:982-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Harada K, Higaki S, Hashimoto K, Hashimoto S, Oga A, Gondo T, Sakaida I. Study on the colonoscopic features of GVHD enteritis that developed after hematopoietic stem cell transplantation. Hepatogastroenterology. 2007;54:2221-2227. [PubMed] |
| 33. | Khan K, Schwarzenberg SJ, Sharp H, Jessurun J, Gulbahce HE, Defor T, Nagarajan R. Diagnostic endoscopy in children after hematopoietic stem cell transplantation. Gastrointest Endosc. 2006;64:379-385; quiz 389-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Silbermintz A, Sahdev I, Moy L, Vlachos A, Lipton J, Levine J. Capsule endoscopy as a diagnostic tool in the evaluation of graft-vs.-host disease. Pediatr Transplant. 2006;10:252-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Holmberg L, Kikuchi K, Gooley TA, Adams KM, Hockenbery DM, Flowers ME, Schoch HG, Bensinger W, McDonald GB. Gastrointestinal graft-versus-host disease in recipients of autologous hematopoietic stem cells: incidence, risk factors, and outcome. Biol Blood Marrow Transplant. 2006;12:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Onozawa M, Yonezumi M, Kawarazaki M, Izumiyama K, Chiba K, Kondo T, Ota S, Kobayashi S, Kato M, Hashino S. Usefulness of magnifying endoscopic evaluation of the terminal ileum for a patient with graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2005;84:106-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Yeh SP, Liao YM, Hsu CH, Chen CL, Shen YC, Hsueh CT, Huang HH, Chiu CF. Gastric bleeding due to graft-vs-host disease: discrepancy between endoscopic and histologic assessment. Am J Clin Pathol. 2004;122:919-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Schulenburg A, Turetschek K, Wrba F, Vogelsang H, Greinix HT, Keil F, Mitterbauer M, Kalhs P. Early and late gastrointestinal complications after myeloablative and nonmyeloablative allogeneic stem cell transplantation. Ann Hematol. 2004;83:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Cruz-Correa M, Poonawala A, Abraham SC, Wu TT, Zahurak M, Vogelsang G, Kalloo AN, Lee LA. Endoscopic findings predict the histologic diagnosis in gastrointestinal graft-versus-host disease. Endoscopy. 2002;34:808-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Wakui M, Okamoto S, Ishida A, Kobayashi H, Watanabe R, Yajima T, Iwao Y, Hisamatsu T, Hibi T, Ikeda Y. Prospective evaluation for upper gastrointestinal tract acute graft-versus-host disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 1999;23:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Terdiman JP, Linker CA, Ries CA, Damon LE, Rugo HS, Ostroff JW. The role of endoscopic evaluation in patients with suspected intestinal graft-versus-host disease after allogeneic bone-marrow transplantation. Endoscopy. 1996;28:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |