Published online Jan 28, 2013. doi: 10.3748/wjg.v19.i4.523
Revised: October 4, 2012
Accepted: December 25, 2012
Published online: January 28, 2013
Processing time: 189 Days and 0.9 Hours
AIM: To investigate primarily the prognostic value of Ki-67, as well as other parameters, in gastrointestinal stromal tumors (GISTs).
METHODS: Ki-67, c-KIT, platelet-derived growth factor receptor-alpha (PDGFRα), smooth muscle actin (SMA), CD34, S100 were stained for immunohistochemistry which was performed on formalin-fixed, paraffin-embeded sections on representative block from each case. Proliferation index counted by Ki-67 antibody was calculated as a number of positive nuclear reaction over 100 cells. Immunoreactivity for c-KIT and PDGFRα was evaluated semiquantitatively (weak, intermediate, strong) and for c-KIT type of reactivity was analyzed (cytoplasmic, membrane and "dot-like" staining). Immunoreactivity for SMA, CD34 and S100 were was evaluated as positive or negative antigen expression. Pathologic parameters investigated in this study included tumor size, cell type (pure spindle, pured epitheloid mixed spindle and epitheloid), mitotic count, hemorrhage, necrosis, mucosal ulceration. Clinical data included age, gender, primary tumor location and spread of disease. χ2 test and Student's t-test were used for comparisons of baseline characteristics. The Cox’s proportional hazard model was used for univariable and multivariable analyses. Survival rates were calculated by Kaplan-Meier method and statistical significance was determined by the log-rank test.
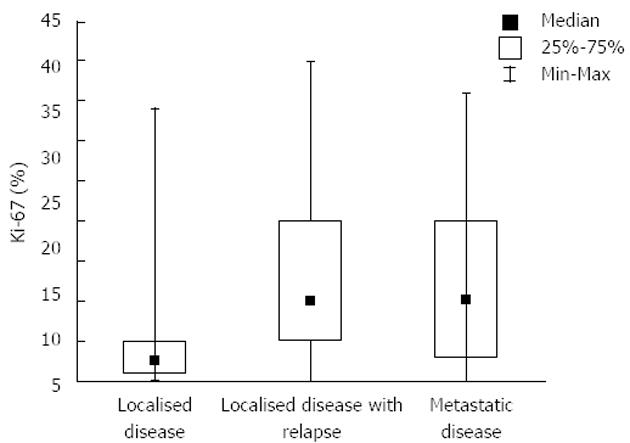
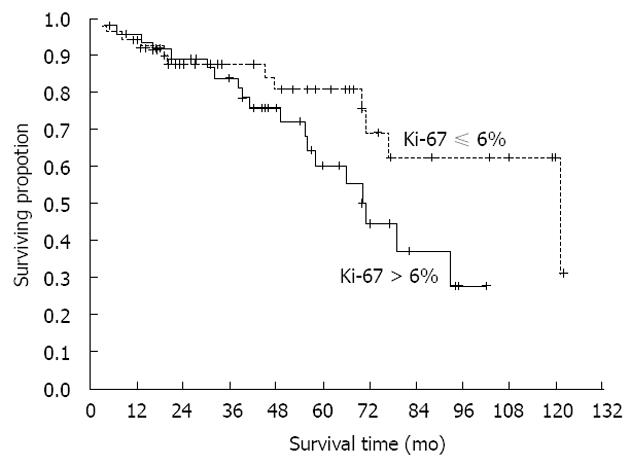
RESULTS: According to the stage of disease, there were 36 patients with localized disease, 29 patients with initially localized disease but with its recurrence in the period of follow up, and finally, 35 patients had metastatic disease from the very beginning of disease. Tumor originated most commonly in the stomach (41%), small intestine was the second most common location (36%). The mean size of primary tumors was 6.5 cm. The mean duration of follow-up was 60 mo. Multiple parameters were analyzed for their effect on overall survival, but no one reached statistical significance (P = 0.06). Analysis of time to progression/relapse in initially localized disease (univariate analysis), tumor size, mitotic count, Ki-67 and type of d-KIT distribution (cytoplasmic vs membrane/”dot-like”) showed statistically significant correlation. In multivariate analysis in the group of patients with localized disease, there were only 2 parameters that have impact on relapse, Ki-67 and SMA (P < 0.0001 and P < 0.034, respectively). Furthermore, Ki-67 was analyzed in localized disease vs localized with recurrence and metastatic disease. It was shown that there is a strict difference between these 2 groups of patients (median value was 2.5 for localized disease vs 10.0 for recurrent/metastatic disease, P < 0.0001). It was also shown that the cut-off value which is still statistically significant in terms of relapse on the level of 6%. The curves for survival on that cut-off level are significantly different (P < 0.04, Cox F).
CONCLUSION: Ki-67 presents a significant prognostic factor for GIST recurrence which could be of great importance in evaluating malignant potential of disease.
- Citation: Belev B, Brčić I, Prejac J, Golubić ZA, Vrbanec D, Božikov J, Alerić I, Boban M, Razumović JJ. Role of Ki-67 as a prognostic factor in gastrointestinal stromal tumors. World J Gastroenterol 2013; 19(4): 523-527
- URL: https://www.wjgnet.com/1007-9327/full/v19/i4/523.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i4.523
Gastrointestinal stromal tumors (GIST) present a wide spectrum of tumors with variable malignant potential[1-3]. Although this entity has been defined rather recently, retrospectively it has been shown to be the most frequent mesenchymal neoplasm arising in the gastrointestinal tract[4]. Namely, in the last few decades, GISTs were sometimes wrongly diagnosed as leiomyoma, leiomyosarcoma and schwannoma. However, immunohistochemial staining and electron microscopic studies suggested difference comparing to other smooth muscle or Schwann cells. Finally, Hirota et al[5] demonstrated in 1998. mutation of c-KIT proto-oncogen as a paradigm of single-mutation tumorogenesis. It has also been shown that GIST originates from interstitial cells of Cajal[6].
Today we also know that both c-KIT and platelet-derived growth factor receptor-alpha (PDGFRα) oncoproteins are growth factor receptors that are normally activated by specific ligand stem cell factor. Their mutations lead to an independent activation of the receptor and constitutive activation and proliferation of cells[5]. Along with KIT-expression, very often is expressed CD34 and sometimes smooth muscle actin (SMA; focal of diffuse)[7].
The prediction of GIST behavior still remains controversial after all these years and, in many cases, nonconclusive. Many prognostic factors have been suggested and investigated in previous studies[8]. It is well known and established that the size of tumor (> 5 cm), mitotic rate > 5/50 HPF and site of the tumor play an important role. Nevertheless, it is not quite possible to predict the behavior of all GISTs. According to guidelines for the assessment of likely behavior, GISTs have been traditionally divided into probably benign, malignant and uncertain/low malignant potential (Fletcher)[9].
Imatinib mesylate, a selective tyrosine kinase inhibitor, has been known to have activity against such tumors. However, no patient had a complete response to the treatment so delineating prognostic factors for patients with GIST may be important even in post imatinib era[10]. It is not clear as well whether there is a role of adjuvant treatment in some GIST patients, although some current trials are still on going[11].
Ki-67 is a nuclear proliferation associated antigen. It is expressed in the growth and synthesis phases of the cell cycle but not in G0-phase (resting phase)[12]. It is a rather reliable marker of cell proliferation although there are differences between studies of Ki-67 labeling index, ranging from 4% to 22%[13,14].
The aim of this study was to investigate the role of Ki-67 among other parameters, as a prognostic factor of clinical behavior and prognosis, along with morphological and immunohistochemical profile in 100 GIST patients.
All mesenchymal tumors of the gastrointestinal tract were retrieved from the files of the University Hospital Center Zagreb, in the period from 1997-2007. Since the immunohistochemistry has been developed on routine basis from the 2001, all specimens obtained before that time were retrospectively analyzed for c-KIT positivity. Inclusion criteria for the study were appropriate morphology and c-KIT positivity. Clinical data were retrieved from the files of the Clinic of Oncology and Surgical Oncology. Follow-up information was obtained from patients records or interviews. Clinical data included age, gender, tumor location (esophagus, stomach, small intestine, large bowel, retroperitoneum, mesentery) and spread of the disease. Spread of disease was classified into three groups: localized disease, localized disease with recurrence and metastatic disease. Pathological parameters that were assesed included tumor size, mitotic count, cell type (pure spindle, mixed spindle and epithelioid, pure epitheloid), necrosis, hemorrhagic areas and mucosal ulceration (invasion of lamina propria). Tumor size was measured in three dimensions and the largest dimension was taken into account. The mitoses were counted on 50 HPFx (400 ×). Immunohistochemistry was performed on formalin-fixed, paraffin-embedded sections on representative block from each case. Following antibodies were used: CD-117 (Dako, Glostrup, Denmark), CD34 (DAKO), PDGFRα (Novocastra, England), SMA (SMA-1, DAKO), S-100 (DAKO), Ki-67 (MIB-5, DAKO). Positive controls were included. All samples were evaluated by two experienced pathologists. Immunoreactivity for c-KIT and PDGFRα was evaluated semiquantitatively (weak, intermediate and strong), and c-KIT type of reactivity was analyzed (cytoplasmic, membrane or “dot-like” staining). Proliferating index counted by Ki-67 antibody was calculated as a number of positive nuclear reaction over 100 cells. Immunoreactivity for SMA, S100 and CD34 was evaluated as positive or negative antigen expression.
χ2 test or Student’s t-test was used for statistical comparisons of baseline characteristics. In statistical analysis of our results we also used appropriate descriptive methods (median, range, minimum and maximum). The Cox’s proportional hazard model was used in univariable and multivariable analyses. Survival rates were calculated by the Kaplan-Meier method, and statistical significance was determined by the log-rank test. The observed differences were assumed to be statistically significant if the probability of chance occurence was P < 0.05. All statistical analysis were performed by the statistical package statistica.
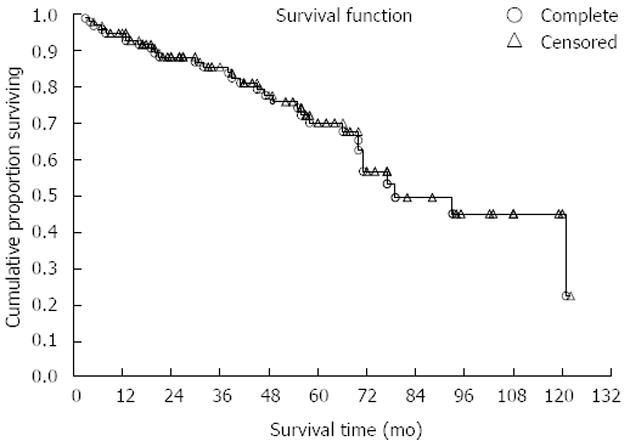
Our study comprised 100 GIST patients. Mean patients’ age was 60.5 (range 20-78) years; 56% of patients were men. Patient distribution in three groups according to sex and age is shown in Table 1. There were 36 patients presenting initially with localized disease, 29 had localized disease further with recurrence and 35 had metastatic disease from the very beginning. Tumors originated most commonly in the stomach (41%), the small intestine was the second most common location (36%), in 8% colon and rectum and in 5% retroperitoneum was involved. In 10% of cases primary site of GIST was not clearly determined, because of the wide spread of the disease. The mean size of primary tumors (in patients without metastases) was 6.5 cm and 35 patients had distant metastases in the time of diagnosis. Metastases were most often localized in the liver, all other sites were rarely involved. The mean duration of follow-up was 60 (range 28-110) mo. Survival curve for all patients included in our study is shown in Figure 1. Further on, multiple parameters were analyzed for their effect on overall survival in all patients (Table 2). Most of them showed no effect, more precisely, only 2 parameters are close to statistically significant prediction of outcome and biological behavior, on the level of P = 0.06. These are Ki-67 and type of distribution of c-KIT. On the contrary, when we analyzed time to progression/relapse in localized disease, in univariate analysis tumor size, mitotic rate, Ki-67 and type of c-KIT distribution (cytoplasmic vs membrane/“dot-like“) showed statistically significant correlation. In multivariate analysis in the group of patients with localized disease, there were only 2 parameters that have impact on relapse, Ki-67 and SMA expression (Table 3). Furthermore, when we compared Ki-67 in three different patients group it was obvious that there is a strict difference between mean value of Ki-67 in localized disease vs recurrent and metastatic disease together (median in localized disease was 2.5 vs 10.0 in recurrent and metastatic disease, P < 0.0001). It was shown that the cut-off value which is still statistically significant in terms of relapse on the level of 6% (Figure 2). Also, it has been shown that the curves for survival on that cut-off level are significantly different (P = 0.04, Cox F-test; Figure 3).
| Groups | Sex | Total | ||
| Male | Female | |||
| Localized disease | Number | 19 | 17 | 36 |
| Average age (yr) | 59.5 | 62.9 | 61.1 | |
| Recurrent disease | Number | 17 | 12 | 29 |
| Average age (yr) | 55.5 | 58.4 | 56.7 | |
| Metastatic disease | Number | 20 | 15 | 35 |
| Average | 60.7 | 48.7 | 55.5 | |
| Total | Number | 56 | 44 | 100 |
| Average age (yr) | 58.7 | 56.8 | 57.9 | |
| Parameter | Beta | Standard error | t value | P value |
| Tumor size | 0.04 | 0.03 | 1.12 | 0.26 |
| Mitosis | -0.01 | 0.01 | -0.91 | 0.35 |
| Necrosis | 0.76 | 0.73 | 1.04 | 0.29 |
| Haemorrhage | -0.63 | 0.68 | -0.93 | 0.35 |
| Ki-67 | 0.05 | 0.02 | 1.83 | 0.06 |
| c-KIT | 1.44 | 0.79 | 1.83 | 0.06 |
| SMA | 0.19 | 0.51 | 0.38 | 0.70 |
| S100 | -0.33 | 0.67 | -0.49 | 0.62 |
| CD34 | -0.34 | 0.52 | -0.66 | 0.50 |
| Cell morphology | -0.19 | 0.52 | -0.37 | 0.70 |
| Parameter | Beta | Standard error | t value | Exponent beta | P value |
| Tumor size | 0.052 | 0.031 | 1.643 | 1.053 | 0.100 |
| Number of mitosis | 0.011 | 0.014 | 0.797 | 1.011 | 0.424 |
| Necrosis | 0.525 | 0.633 | 0.830 | 1.691 | 0.406 |
| Haemorrhage | 0.865 | 0.673 | 1.285 | 2.377 | 0.198 |
| Ki-67 | 0.113 | 0.030 | 3.778 | 1.120 | 0.0001 |
| c-KIT | 0.592 | 0.536 | 1.102 | 1.807 | 0.270 |
| SMA | -1.213 | 0.575 | -2.110 | 0.297 | 0.034 |
| S100 | -0.237 | 0.771 | -0.308 | 0.788 | 0.757 |
| CD34 | -1.088 | 0.562 | -1.935 | 0.336 | 0.052 |
According to data obtained from our study, like age, sex distribution and primary localization, they are comparable to previous data in literature[8,15,16]. GISTs are rather rare tumors with very interesting biological behavior and sometimes unpredictable clinical course[17-19]. Although many reports indicated that the size and mitotic count are the most important and reliable prognostic factors[7], it has become obvious that the primary tumor localization was also very important issue[17]. Some 10 years ago, DeMatteo et al[20] showed that only tumor size is reliable predictor of survival in multivariate analysis. Some other authors found tumor size > 5 cm a poor prognostic factors[21]. However, there are only few reports of Ki-67 as a good indicator of the risk of metastases and as a prognostic factor[22]. Among other parameters, it was used for distinguishing leiomyoma and leiomyosarcoma[23]. Carrillo et al[13] stated that Ki-67 is one of the most accurate predictor of clinical behavior in GIST although it was not confirmed always and some other authors reported about Ki-67 with mitotic index as a prognostic factors in stomach GISTs[24]. In addition, we showed in our study, that there is no statistically significant difference in mean Ki-67 value between stomach- and small intestine-localization of GIST (52 patients together, 29 vs 23) (data not shown). That might support an idea of Ki-67 as a non-location-specific prognostic factor, what could be its advantage comparing to mitotic indeks which shows strong correlation to anatomic site of tumor.
One of the most important fact is observation that the level of Ki-67 is a prognostic factor for the relapse of initially localized disease (P < 0.0001), and the cut-off value is on 6%. Even in the survival setting this distinction remains statistically significant, no matter how these patients were treated (P = 0.04, Cox F-test). The expression of Ki-67 changes widely from one to another study, probably by the variety of cut-offs of various authors. Toquet et al[25] and Nakamura et al[26] described cut-off of 10%, some others on the level of 5%. In that sense, our finding is important as additional indicator of importance of Ki-67 no matter the origin of GIST, and as well this might suggest, among other factors, which patient might have a greater risk of disease relaps. Maybe it might indicate the patients suitable for adjuvant treatment after surgical resection of localized GIST, in addition to contemporary concept of high-risk disease.
Considering other analyzed parameters, no one showed statistical power and impact on survival, as we expected. Just to mention that the meaning of statistical significance of SMA in DFI (P < 0.034) should be cautiously interpreted. It might be based on the fact that we assumed SMA-positive tumors generally better differentiated ones, thus it is to expect less aggressive course of disease which means fewer recurrences.
In conclusion, although there are some differences in numerical values of Ki-67 in various studies , that may be caused by different methodology in the assessment. But Ki-67 stays very important parameter of GIST-prognosis and should not be neglected when assessing malignant potential of the GIST.
Gastrointestinal stromal tumors (GIST) have a specific tumor biology with a wide spectrum of clinical behaviors. Thus, it is of great importance to define prognostic factors which could indicate risk of recurrence or spread of disease. Ki-67 is potentially good prognostic factor.
So far, some of prognostic factors were rather well documented as the prognostic factors of further tumor behavior, for example mitotic count and tumor size, along with tumor primary localization. Ki-67 was unequivocally addressed as a prognostic factor.
This is the first study showing so clearly the significance of Ki-67 as a prognostic factor. Of importance is, that the patients included in the study were in different stage of disease, and also that comparison to other parameters have been done. It was shown that the cut-off value of Ki-67 of 6%, present the borderline value for recurrence of initially localized disease.
By understanding the meaning of Ki-67, it could be used as a parameter predicting tumor recurrence and suggest adjuvant treatment after surgery of localized disease.
Ki-67 or MKI-67 is a protein that is in humans encoded by MKI-67 gene. Ki-67 antigen is associated, and probably necessary, for cellular proliferation, it is associated with ribosomal RNA transcription. It is to assume that higher Ki-67 reveals tumor cell activity and thus predicts further behavior.
This study is a contribution to the knowledge of GIST. It has a great value in better understanding of the biology of the disease, since some other parameters have already been recognized important as prognostic factors.
P- Reviewers Chen P, Linhares E S- Editor Wen LL L- Editor A E- Editor Xiong L
| 1. | Goss GA, Merriam P, Manola J Singer S, Fletcher C, Demetri G. Clinical and pathological characteristics of gastrointestinal stromal tumors (GIST). Prog Proc Am Soc Clin Oncol. 2000;19:2000. |
| 2. | Franquemont DW. Differentiation and risk assessment of gastrointestinal stromal tumors. Am J Clin Pathol. 1995;103:41-47. [PubMed] |
| 3. | Clary BM, DeMatteo RP, Lewis JJ, Leung D, Brennan MF. Gastrointestinal stromal tumors and leiomyosarcoma of the abdomen and retroperitoneum: a clinical comparison. Ann Surg Oncol. 2001;8:290-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293-1301. [PubMed] |
| 5. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3114] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 6. | Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259-1269. [PubMed] |
| 7. | Saul SH, Rast ML, Brooks JJ. The immunohistochemistry of gastrointestinal stromal tumors. Evidence supporting an origin from smooth muscle. Am J Surg Pathol. 1987;11:464-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Nilsson B, Bümming P, Meis-Kindblom JM, Odén A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103:821-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 875] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 9. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 10. | Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 1324] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 11. | Nilsson B, Sjölund K, Kindblom LG, Meis-Kindblom JM, Bümming P, Nilsson O, Andersson J, Ahlman H. Adjuvant imatinib treatment improves recurrence-free survival in patients with high-risk gastrointestinal stromal tumours (GIST). Br J Cancer. 2007;96:1656-1658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Seidal T, Edvardsson H. Expression of c-kit (CD117) and Ki67 provides information about the possible cell of origin and clinical course of gastrointestinal stromal tumours. Histopathology. 1999;34:416-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Carrillo R, Candia A, Rodriguez-Peralto JL, Caz V. Prognostic significance of DNA ploidy and proliferative index (MIB-1 index) in gastrointestinal stromal tumors. Hum Pathol. 1997;28:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Rudolph P, Gloeckner K, Parwaresch R, Harms D, Schmidt D. Immunophenotype, proliferation, DNA ploidy, and biological behavior of gastrointestinal stromal tumors: a multivariate clinicopathologic study. Hum Pathol. 1998;29:791-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Saund MS, Demetri GD, Ashley SW. Gastrointestinal stromal tumors (GISTs). Curr Opin Gastroenterol. 2004;20:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Kindblom LG, Meis-Kindblom J, Bumming P, Dimitrijevic S, Miret M, Dortok A. Incidence, prevalence, phenotype and biologic spectrum of gastrointestinal stromal tumors (GIST) - A population-based study of 600 cases. Ann Oncol. 2003;13 (Suppl 5): 157. |
| 17. | Strickland L, Letson GD, Muro-Cacho CA. Gastrointestinal stromal tumors. Cancer Control. 2008;8:252-261. [PubMed] |
| 18. | Gupta P, Tewari M, Shukla HS. Gastrointestinal stromal tumor. Surg Oncol. 2008;17:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1177] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 20. | Dematteo RP, Gold JS, Saran L, Gönen M, Liau KH, Maki RG, Singer S, Besmer P, Brennan MF, Antonescu CR. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer. 2008;112:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 346] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 21. | Kwon SJ. Surgery and prognostic factors for gastric stromal tumor. World J Surg. 2001;25:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Ohdaira H, Ohyama S, Yamaguchi T, Yanagisawa A, Kato Y, Urashima M. Ki67 and tumor size as prognostic factors of gastrointestinal stromal tumors. JMAJ. 2005;48:586-592. |
| 23. | Matsuda K, Watanabe H, Nishikura K, Endoh Y, Ajioka Y, Hitomi J. Gastric myogenic tumors Its Ki-67 mitotic count, myogenic markers, histogenesis of sarcoma and differentiation from gastric stromal tumor. Stomach and Intestine (Tokyo). 1995;30:1109-1124. |
| 24. | Nagasako Y, Misawa K, Kohashi S, Hasegawa K, Okawa Y, Sano H, Takada A, Sato H. Evaluation of malignancy using Ki-67 labeling index for gastric stromal tumor. Gastric Cancer. 2003;6:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Toquet C, Le Néel JC, Guillou L, Renaudin K, Hamy A, Heymann MF, Simon-Valla S, Le Borgne J, Maugard C, Fiche M. Elevated (& gt; or = 10%) MIB-1 proliferative index correlates with poor outcome in gastric stromal tumor patients: a study of 35 cases. Dig Dis Sci. 2002;47:2247-2253. [PubMed] |
| 26. | Nakamura N, Yamamoto H, Yao T, Oda Y, Nishiyama K, Imamura M, Yamada T, Nawata H, Tsuneyoshi M. Prognostic significance of expressions of cell-cycle regulatory proteins in gastrointestinal stromal tumor and the relevance of the risk grade. Hum Pathol. 2005;36:828-837. [PubMed] |