INTRODUCTION
The liver is the largest abdominal organ. It occupies a substantial portion of the upper abdominal cavity. Abnormalities in the position or number of liver parts are considered rare developmental anomalies. They are typically asymptomatic, and incidental detection, though extremely rare, may occur during an operation or autopsy. The incidence of ectopic liver is 0.24%-0.56%, according to data described in laparoscopic or autopsy studies[1-3], but this estimate seems high. Most authors distinguish two types of ectopic liver. The first is an accessory liver lobe connected to the liver, and the second is a truly ectopic liver. Collan classified four types. The first is the ectopic liver, which is not connected to the mother liver, but is typically attached to the gallbladder or intra-abdominal ligaments. The second is a microscopic ectopic liver, which is occasionally found in the gallbladder wall. The third is a large accessory liver lobe, attached to the mother liver by a stalk (pedunculated liver). The fourth is a small, accessory liver lobe attached to the mother liver[4]. Here, we presented two manifestations of ectopic livers.
CASE REPORT
Case 1
A 31-year-old male patient was admitted with an 8-h history of pain in the right lower abdominal quadrant with a gradual onset. The patient reported nausea, but no vomiting, normal bowel function, and normal miction. He was subfebril, but no infection was observed. His medical history included pollinosis. He took no regular medication and had no previous surgeries.
The clinical examination showed right lower quadrant abdominal pain with tenderness. The bowel sounds were diminished. The patient was hemodynamically stable without any signs of sepsis (temperature 37.5 °C, noninvasive blood pressure 120/80 mmHg, heart rate 76 beats/min, respiratory rate 14 breaths/min). The white blood count was 14800 cells/mL and C-reactive protein was 12.3 mg/L. Other biochemical results were normal and the urinalysis revealed no pathological findings. An abdominal ultrasound showed a small amount of pericaecal fluid. No other abnormal findings were identified by ultrasound in other parts of the abdomen or pelvis.
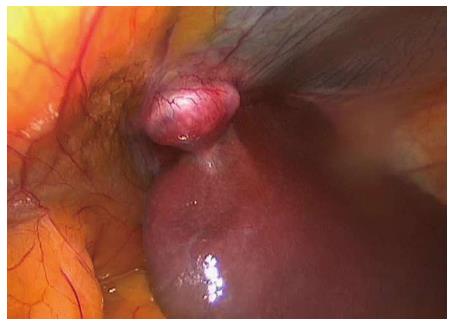
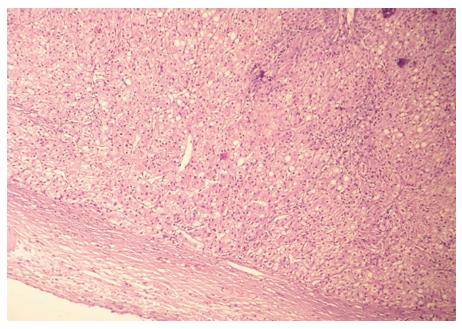
The signs and symptoms suggested appendicitis; therefore, we performed an acute laparoscopic appendectomy. First, antibiotic therapy was introduced. Initially, the routine diagnostic laparoscopy revealed perforated appendicitis with circumscript peritonitis. Incidentally, a small oval tumor (3 cm × 2 cm × 2 cm) was found in the ligamentum hepato umbilicalis next to the liver (Figure 1). No other pathological signs were observed. The appendectomy was performed, followed by an intraoperative lavage of the peritoneal cavity. The tumor was excised. The operation and the postoperative recovery were uncomplicated. The patient was discharged on the third postoperative day. The histology of the appendix revealed an ulcerophlegmonous appendix. The histopathological examination of the tumor from the ligamentum hepato umbilicalis revealed liver tissue with moderate steatosis and a thick fibrotic capsule (Figure 2). The specimen examination showed that the tumor was completely separate, with no connection to the liver. It was classified as the first type of ectopic liver according to the Collan classification. No further therapy was required.
Figure 1 Small oval tumor (3 cm × 2 cm × 2 cm) was found in the ligamentum hepatoumbilicalis next to the liver.
Figure 2 Histopathology of the tumor from the ligamentum hepatoumbilicalis (liver tissue with moderate steatosis and a thick fibrotic capsule).
Case 2
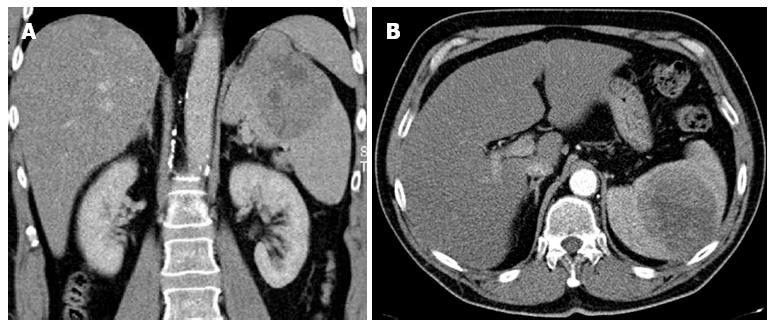
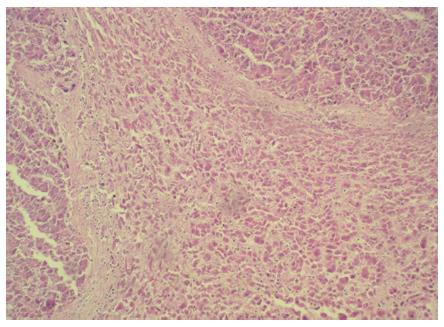
During an ultrasound examination, a 59-year-old male was incidentally diagnosed with a tumor (10 cm × 8 cm × 6 cm) on the upper pole of the spleen. The finding was confirmed with a computed tomography (CT) scan (Figure 3), and a biopsy was performed. A histological examination of the biopsy specimen, including immunohistochemistry, raised the suspicion of a metastatic hepatocellular carcinoma, but bile production was not caught and renal carcinoma could not be reliably ruled out. To detect the primary tumor location, we performed a positron emission tomography-computed tomography examination. This showed a localized accumulation of F-18 fluorodeoxyglucose only in the suspicious tumor. There was no other pathological finding in the abdominal or thoracic cavities. The medical history included toxonutritive hepatopathy (alcoholic liver disease) and chronic gastritis. The biochemical results were within normal limits, and oncomarkers (carcinoembryonic antigen, alfa-fetaprotein, CA 19-9) were negative. A perioperative examination confirmed that the tumor was located on the upper pole of the spleen and was connected to diaphragm, but did not invade other surrounding tissues. It was classified as the first type of ectopic liver according to the Collan classification. No other pathology was found in the abdomen. A splenectomy was performed, with partial diaphragm resection and reconstruction. The postoperative recovery was uneventful. A definitive histological examination, including immunohistochemistry, confirmed a hepatocellular carcinoma (HCC) in the spleen tissue (Figure 4). Two small additional tumor sites (satellite tumors) were found in addition to the main lesion. Several investigations showed that the orthotopic liver tissue was negative for HCC, but a histological verification (a biopsy of the mother liver tissue) was not performed. Due to the high risk of tumor recurrence (additional tumor sites were found), we initialized a targeted adjuvant therapy with sorafenib. The American Association for the Study of Liver Diseases (AASLD) practice guidelines on the management of hepatocellular carcinoma do not recommend the routine use of adjuvant therapy with sorafenib (recurrence rates reduction was not reliably proven)[5], nevertheless, there are data available that indicate that sorafenib was effective for treating patients with advanced HCC[6,7].
Figure 3 Tumor (10 cm × 8 cm × 6 cm) on the upper pole of the spleen.
A: Computed tomography scan, arterial phase with a coronal reconstruction; B: Computed tomography scan, arterial phase - axial orientation.
Figure 4 Histopathology of the hepatocellular carcinoma found in an ectopic liver in the spleen.
DISCUSSION
In development, the hepatic diverticulum comprises the liver and biliary tree, and it appears late in the third week or early in the fourth week of gestation. The foregut endoderm of the hepatic diverticulum develops into the liver parenchyma (hepatocytes) and the epithelial lining of the biliary tract. The hepatic diverticulum divides to form a small ventral portion, the future gall bladder, and a larger cranial portion, the liver primordium. Developmental errors are relatively rare in the liver. Other errors in foregut development are more frequently observed, like errors in pancreas or duodenum formation[8]. Liver tissue can migrate to various organs during embryogenesis. Sites of ectopic liver include the gallbladder, spleen, retroperitoneum, pancreas, adrenal gland, portal vein, diaphragm, thorax, gastric serosa, testes, and umbilical vein[9]. Most authors distinguish ectopic and accessory liver formations, based on whether there is a connection to the mother liver. The Collan Classification mentioned above is not widely used. In many cases, it is difficult to make a clear distinction between ectopic liver and accessory liver. The precise incidence of ectopic liver or accessory liver is unknown. Examination of several studies indicated that the incidence is approximately 0.24%-0.56%. Watanabe’s series of 1060 patients revealed an incidence of 0.47% for ectopic liver and 0.09% for accessory liver. These numbers could be over-estimated, because histological verification was not performed in all cases. Ectopic and accessory liver are typically asymptomatic, but occasionally they cause unexpected problems, like intra-abdominal bleeding or hepatocarcinogenesis. The first clinical sign of ectopic or accessory liver could be an acute complication that leads to acute surgery and diagnosis of ectopic liver. Various clinical symptoms, like recurrent abdominal pain and impaired liver function could be caused by ectopic or accessory liver, but in the majority of cases, an ectopic/accessory liver remains undetected.
In some cases, the ectopic or accessory liver may undergo torsion, infarction, rupture, or other disorders. Torsion and subsequent infarction of an accessory liver lobe has been described in children and adults[10-14]. Ladurner presented a very interesting case of a patient with hepatic ischemia caused by complete vascular occlusion due to a twisted accessory liver lobe. In that case, the accessory liver lobe produced serious, life-threatening problems, and an orthotopic liver transplantation was performed[15]. Ito reported a small omphalocele that involved an accessory liver lobe embedded in the cranial portion of the amniotic sac. In that case, the pedicle of liver tissue was markedly elongated[16].
An ectopic or accessory liver can lead to benign or malignant diseases. Benign cases in the literature report hemangiomas, adenomas, or focal nodular hyperplasia associated with an ectopic or accessory liver[17-19]. Benign lesions seem to be less frequent; however, the higher frequency of malignancies could be based on the fact that many benign lesions remain undiagnosed because they are asymptomatic. Moreover, due to their abnormal locations, asymptomatic lesions may be misdiagnosed in the absence of histology.
The ectopic liver has been associated with malignancies more often than with benign lesions. Many authors have pointed out that ectopic liver tissue is more predisposed to malignancy than normal liver tissue. Ectopic livers have completely functional architecture, but may be metabolically handicapped; this may facilitate carcinogenesis. Ectopic liver tissue also has increased neoplastic potential compared to orthotopic liver tissue. This may have given rise to the hypothesis that ectopic livers are particularly predisposed to the development of hepatocellular cancer. A high incidence of hepatocellular cancer in ectopic livers was described in Japan[20]. In most cases, a malignant tumor was found in the ectopic liver, but not in the mother liver. Ectopic or accessory livers with cancer may be amenable to surgical resection. Many case reports have described surgical treatments. Some authors suggested that the outcome after resection to remove hepatocellular cancer was superior when it involved an ectopic or accessory liver, compared to when it involved the mother liver. However, long-term follow-up data are poor.
Many anatomical locations have been described for ectopic livers with cancer[20-23]. The favorable outcome after resection of ectopic livers could depend on the specific anatomical location[24,25]. Shigemori described a case of ectopic hepatocellular carcinoma in the jejunum[26]. Cardona et al[27] reported a case of a primary, well-differentiated hepatocellular carcinoma arising from ectopic liver tissue in the pancreas. Leone presented interesting data regarding three cases of hepatocellular carcinomas that arose in ectopic livers. The clinical presentations were very interesting; one patient reported dull epigastric pain; the second reported abrupt onset with signs and symptoms of acute abdomen caused by intra-abdominal bleeding; and the third presented with an unexplained, progressive increase in alpha-fetoprotein serum levels[28]. Seo et al[29] reported a case of hepatocellular carcinoma that arose from hepatic parenchyma located in the left subphrenic space in the upper portion of the gastrorenal ligament. The preoperative diagnosis was a nonspecific stomach mass, with suspicion of gastrointestinal stromal tumor. An operation was performed laparoscopically. Takavasu reported another case with high serum alpha-fetoprotein combined with a suspicion of a submucosal stomach tumor[30]. The two latter cases diagnosed the ectopic liver postoperatively, after histological examination.
It is important to consider an ectopic/accessory liver when evaluating perihepatic lesions. It is common to misdiagnose an ectopic liver as a malignant tumor. Stattaus suggested that the diagnosis should be based on a biopsy of ectopic liver or a NMR with liver specific contrast[31]. However, all investigative imaging methods are limited for diagnosing an ectopic/accessory liver, due to its limited volume. Despite the low incidence and rare complications of ectopic/accessory live, it is necessary to maintain an awareness of this possibility. Because this entity presents with a broad spectrum of clinical symptomatology, it is rarely diagnosed; thus, most discoveries of ectopic and accessory liver are incidental. An elevation in serum alpha-fetoprotein and lack of focus in liver CT image may be the first signs of malignant transformation in an ectopic liver. The suspicion of hepatocellular carcinoma in an ectopic liver is substantial reason for radical surgical removal of an ectopic liver found incidentally. When hepatocellular carcinoma is definitely, histologically confirmed in an ectopic liver, it should be treated with the same approaches used for treating carcinoma in the mother liver (National Comprehensive Cancer Network Guidelines).