Published online Oct 7, 2013. doi: 10.3748/wjg.v19.i37.6296
Revised: July 5, 2013
Accepted: July 30, 2013
Published online: October 7, 2013
Processing time: 171 Days and 4.5 Hours
A 49 years old Vietnamese male with a history of thalassemia, presented with gastrointestinal symptoms and signs of hemolysis. He was diagnosed with yersinia enterocolitis. Yersinia is a gram-negative rod that most frequently occurs in children especially during the winter months. In the current case, the bone marrow biopsy showed hemophagocytosis along with positive cultures for Yersinia. The microorganism likely triggered hemophagocytosis. This syndrome, also known as, hemophagocytic lymphohistiocytosis, is defined by fever for more than 7 d, cytopenia of two or more cell lines, hemophagocytosis, hepatitis, serum ferritin greater than 500, jaundice, lymphadenopathy, and hepatosplenomegaly. This disorder can be either familial or secondary to a strong immunologic activation. Both have an overwhelming activation of T-cells and macrophages.
Core tip: In the current case, the bone marrow biopsy showed hemophagocytosis along with positive cultures for Yersinia. The microorganism likely triggered hemophagocytosis. This syndrome, also known as, hemophagocytic lymphohistiocytosis, is defined by fever for more than 7 d, cytopenia of two or more cell lines, hemophagocytosis, hepatitis, serum ferritin greater than 500, jaundice, lymphadenopathy, and hepatosplenomegaly. This disorder can be either familial or secondary to a strong immunologic activation. Both have an overwhelming activation of T-cells and macrophages.
- Citation: Selsky N, Forouhar F, Wu GY. An ironic case of liver infections: Yersinia enterocolitis in the setting of thalassemia. World J Gastroenterol 2013; 19(37): 6296-6298
- URL: https://www.wjgnet.com/1007-9327/full/v19/i37/6296.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i37.6296
Yersinia is a gram-negative rod that most frequently occurs in children especially during the winter months. Transmission is largely food and waterborne. Pigs are frequently colonized with strains that cause human illness. Incubation typically lasts 2-6 d followed by a diarrheal period that can last up to three weeks. Symptoms include nausea, vomiting, and abdominal pain. Most strains of Yersinia grow poorly in typical agar solutions because the bacteria lack a mechanism for the efficient uptake of iron. Individuals who have iron overload due to either primary or secondary hemochromatosis are at increased risk of infection, and are also at higher risk to develop severe infections. Complications of severe infection can include diffuse ulcerating ileitis and colitis, intussusception, perforation, toxic megacolon, cholangitis, mesenteric vein thrombosis, and hemophagocytic lymphohistiocytosis. Post-infectious complications include erythema nodosum and reactive arthritis. Treatment, reserved only for severe systemic infections, should consist of a 3rd generation cephalosporin and gentamicin for 3 wk. Genetic studies on this patient showed a loss of three alpha globin genes indicating the presence of Hb H disease. This lack of alpha globin causes a relative increase in the number of beta globin chains which can aggregate to form unstable tetramers. The tetramers have abnormal oxygen dissociation curves reflected in poor delivery of oxygen to the periphery, as well as precipitation of the hemoglobin tetramers as Heinz bodies. These precipitants can induce phagocytosis of red blood cells and a chronic hemolytic anemia which in turn leads to an increase in serum hepcidin levels with resultant elevated iron transport across the gut mucosa. Over time, this leads to a systemic iron overload which can also be exacerbated iatrogenically by blood transfusions.
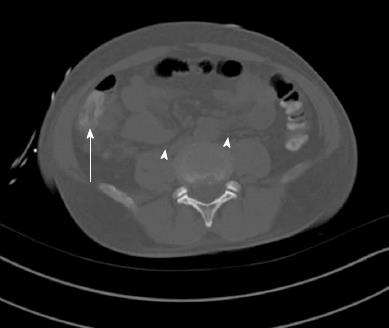
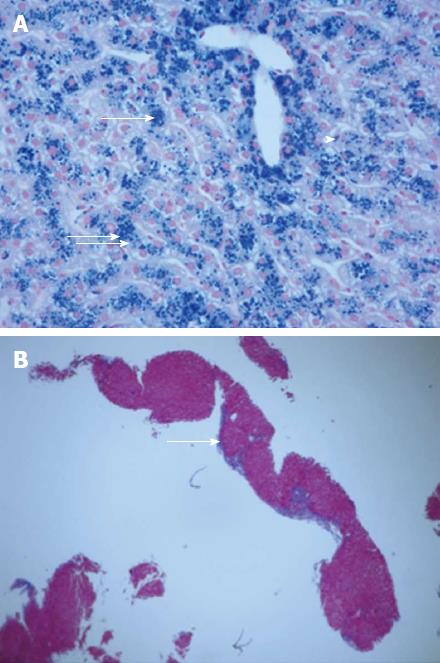
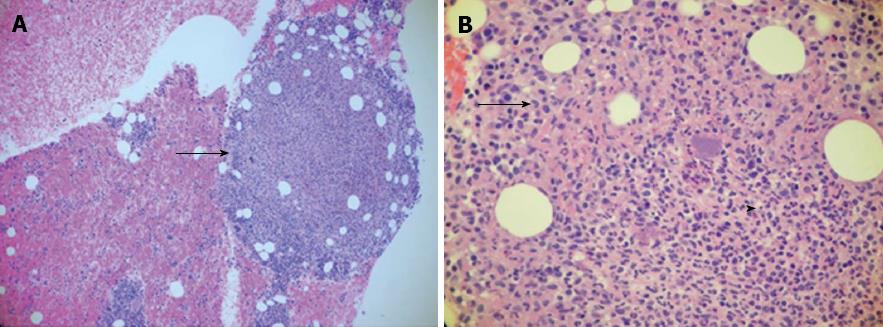
A 49-year-old Vietnamese male, with a history of malaria 27 years ago was well until 5 d prior to admission when he developed dark urine associated with fevers, chills, and night sweats. This was followed by non-bloody diarrhea, and right upper quadrant abdominal pain as well as nausea and non-bloody vomiting. He denied any IV drug abuse, sick contacts, or travel history. He drank alcohol socially, but not to excess. On physical exam, he had a temperature of 104.3 °C, Blood pressure of 102/59 mmHg, heart rate of 100 beats/min, and saturation of 92% on room air. Generally, he was pale, diaphoretic, and sclerae were icteric. Abdominal examination revealed some right upper quadrant tenderness, but no rebound or guarding, and no hepatosplenomegaly. He had no rashes or stigmata of chronic liver disease. His laboratory studies showed a hemoglobin of 6.1 (13.8-18.0) g/dL with an MCV of 58 (80-100) fL, a white cell count of 10.6 (4.8-10.5) 103/μL, and a platelet count of 75 (150-400) 103/μL. Aspartate aminotransferase and alanine aminotransferase were 168 and 160 (5-40 and 7-56) U/L, respectively with a total bilirubin of 3.3 (0.3-1.9) mg/dL, and a direct bilirubin of 1.1 (0-0.3) mg/dL. Haptoglobin was < 15 (41-165). A peripheral smear demonstrated marked anisopoikilocytosis with schistocytes and target cells. Iron saturation was initially normal, 29%, with a ferritin of 6148 (12-300) mg/dL. Subsequent testing revealed persistently high iron saturation, 80%, and ferritin levels > 1000 mg/dL. Glucose and electrolytes were normal. Computerized tomography (CT) of the abdomen showed a normal biliary tree, proximal ascending colon mural thickening with surrounding adenopathy and pericolonic stranding as well as bilateral pleural effusions (Figure 1). Stool, blood, and bone marrow cultures were all positive for Yersinia enterocolitica. The patient was positive for HBsAg with a viral load of 270000 IU. Genetic testing revealed mutations of three alpha globin genes making a diagnosis of alpha thalassemia (Hb H). A liver biopsy showed 3+ iron in hepatocytes with a portal to central gradient (Figure 2A), and chronic inflammation with early septum formation (Figure 2B). Bone marrow biopsy revealed iron overload, and a granuloma (Figure 3A), and hemophagocytic lymphohistiocytosis (Figure 3B)[1]. The patient was started on piperacillin/tazobactam/gentamicin and transfused to a hemoglobin level of 10 g/dL with rapid clinical improvement. He was discharged on a 3-wk course of oral ciprofloxacin, and a follow up CT of the abdomen showed resolution of the bowel thickening and disappearance of the fat stranding. In addition, he was treated with oral deferasirox (Exjade) and entecavir. His ferritin level decreased to 842 by 12 wk. His liver enzyme levels returned to normal, and his HBV viral load became undetectable.
In the current case, the bone marrow biopsy showed hemophagocytosis along with positive cultures for Yersinia. The microorganism likely triggered hemophagocytosis[2]. This syndrome, also known as, hemophagocytic lymphohistiocytosis, is defined by: fever for more than 7 d, cytopenia of two or more cell lines, hemophagocytosis, hepatitis, serum ferritin greater than 500, jaundice, lymphadenopathy, and hepatosplenomegaly. This disorder can be either familial or secondary to a strong immunologic activation. Both have an overwhelming activation of T-cells and macrophages.
In this patient, the chronic anemia due to thalassemia, or anemia in combination with the hepatitis B caused a secondary hemochromatosis[3]. This increased the risk of Yersinia infection, and likely was responsible for the severity of the systemic infection[4].
This patient will need iron chelation therapy and close monitoring for development of hepatocellular carcinoma because of the heightened risk with his coexisting HBV infection.
P- Reviewer Geboes K S- Editor Zhai HH L- Editor A E- Editor Zhang DN
| 1. | Au WY, Lam WW, Chu WW, Tam S, Wong WK, Lau J, Yeung YM, Liu HS, Liang R. Organ-specific hemosiderosis and functional correlation in Chinese patients with thalassemia intermedia and hemoglobin H disease. Ann Hematol. 2009;88:947-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Pallister C, Rotstein OD. Yersinia enterocolitica as a cause of intra-abdominal abscess: the role of iron. Can J Surg. 2001;44:135-136. [PubMed] |
| 3. | Sebastiani G, Tempesta D, Alberti A. Hepatic iron overload is common in chronic hepatitis B and is more severe in patients coinfected with hepatitis D virus. J Viral Hepat. 2012;19:e170-e176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Xu L, Chen EQ, Lei J, Liu L, Zhou TY, Gao Z, Tang H. Pre-core/basal-core promoter and reverse transcriptase mutations in chronic HBV infected-patients. Hepatogastroenterology. 2012;59:212-215. [PubMed] |