Published online Oct 7, 2013. doi: 10.3748/wjg.v19.i37.6221
Revised: July 1, 2013
Accepted: August 12, 2013
Published online: October 7, 2013
Processing time: 163 Days and 20.8 Hours
AIM: To evaluate the efficacy and safety of grasper type scissors (GTS) for endoscopic submucosal dissection (ESD) of gastric epithelial neoplasia.
METHODS: The study was performed by 4 endoscopists in 4 institutions affiliated to The Catholic University of Korea. ESD was performed in 76 consecutive patients with gastric epithelial neoplasia by using the GTS (37 patients) or the hook knife plus coagrasper (HKC) (39 patients). The complete resection rate, complication rate, total time elapsed and elapsed time per square centimeter of the dissected specimen were analyzed between the GTS and HKC group.
RESULTS: The mean age of the GTS group was 62.3 ± 11.4 years and mean age of the HKC group was 65.6 ± 10.1 years. Differentiated adenocarcinoma was found in 32.4% in the GTS group and 33.3% in the HKC group. The procedures were performed without interruption in every case in both groups. The en bloc resection rates of both groups were 100%. The total time elapsed during the procedure was 44.54 ± 21.72 min in the GTS group and 43.77 ± 21.84 min in the HKC group (P = 0.88) and the time elapsed per square centimeter of the resected lesion was 7.53 ± 6.35 min/cm2 in the GTS group and 6.92 ± 5.93 min/cm2 in the HKC group (P = 0.66). The overall complication rate was not significantly different between the two groups.
CONCLUSION: GTS is a safe and effective device for ESD compared with HKC. ESD can be performed with GTS alone, which can reduce the costs for ESD.
Core tip: Many types of knives have been developed and used for endoscopic submucosal dissection (ESD). We modified the grasping type scissors forceps and developed a novel grasper type scissors (GTS). The aim of this study was to evaluate the efficacy and safety of GTS compared to hook knife plus coagrasper (HKC) for ESD of gastric epithelial neoplasia. The procedures were performed without interruption in every case in both groups. GTS is a safe and effective device for ESD of gastric epithelial neoplasia compared with HKC. ESD can be performed with GTS alone, which can reduce the costs for ESD.
- Citation: Chung WC, Kim BW, Lim CH, Kim TH, Park JM, Kim JS. Grasper type scissors for endoscopic submucosal dissection of gastric epithelial neoplasia. World J Gastroenterol 2013; 19(37): 6221-6227
- URL: https://www.wjgnet.com/1007-9327/full/v19/i37/6221.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i37.6221
Endoscopic mucosal resection (EMR) is an endoscopic technique developed for removal of sessile or flat neoplasms confined to the superficial layers (mucosa and submucosa) of the gastrointestinal tract. EMR is typically used for removal of lesions smaller than 20 mm. En bloc resection of lesions larger than 20 mm is difficult by EMR thus increasing the risk of local recurrence[1-4]. Newly developed endoscopic techniques and devices have helped to overcome the limitations of EMR in terms of lesion size, location, and presence of fibrotic scarring.
Endoscopic submucosal dissection (ESD) allows en bloc resection of larger (usually more than 20 mm) lesions as well as subepithelial gastrointestinal lesions[5]. Various cutting devices - insulated tipped (IT) knife, hook knife, flex knife, triangular knife, and fork knife and so on-have been developed. Grasping-type scissors forceps (GSF) have a 0.8-mm-wide and 6-mm-long serrated cutting edge to facilitate the grasping of tissues. The outer side of the forceps is insulated and the forceps are able to rotate to the desired location. GSF can be used for excision by accurately gripping the submucosal tissue of the target lesion[6-8]. This device has been used safely and effectively for ESD in other organs such as the colorectum and duodenum[9-12]. However, it was not designed for cutting tissues and sometimes it was not optimal when used for dissecting lesions. Furthermore, rotating the GSF to the desired location is frequently difficult.
The newly developed grasper type scissors (GTS) can grasp and cut a piece of tissue using an electrosurgical current. Unlike GSF, the tip of the knife is not insulated and has a thin cutting blade to facilitate submucosal dissection. Theoretically, it possesses both advantages of the GSF and the flex knife.
The aim of this study was to evaluate the efficacy and safety of the novel GTS knife, which can be used for both dissection and hemostasis, for ESD of gastric epithelial neoplasia and compare it to hook knife plus coagrasper (HKC), which is one of the most commonly used knives in South Korea.
The advantages and disadvantages of ESD with GTS, as well as alternative endoscopic options (e.g., ESD with a conventional device, EMR) were discussed with each patients. All patients gave their written informed consent to the designated intervention. This study protocol was approved by the Institutional Review Board of The Catholic University of Korea.
Patients with gastric epithelial neoplasia were consecutively enrolled between May 2010 and April 2012. The study was a prospective, randomized, multi-center, comparative trial. It was performed by 4 endoscopists in 4 institutions affiliated with The Catholic University of Korea (Incheon St. Mary’s Hospital, St. Vincent’s Hospital, Seoul St. Mary’s Hospital, and Bucheon St. Mary’s Hospital). All of the endoscopists were experienced with ESD and had performed ESD in over 200 cases. Before this study, two endoscopists (Kim BW, Lim CH) had used hook knife as the main device and the other two endoscopists (Chung WC, Kim TH) had used flex knife as the main device.
Adults (> 18 years) with histopathologic diagnosis of gastric epithelial neoplasia and without evidence of lymph-node involvement documented by abdominal computed tomography (CT) and/or endoscopic ultrasound (EUS) were included in this study. The lesions met the expanded criteria for local resection proposed by Gotoda[5,13,14]. Differentiated mucosal cancers of any size without ulceration or scarring, differentiated mucosal cancers < 30 mm in diameter with ulceration or scarring, or differentiated cancers with minimal submucosal invasion (< 500 μm deep in the submucosa starting from the muscularis mucosae) were enrolled. The diameter of the lesions without ulcers was limited to a maximum of 60 mm. Patients with conditions that might have substantial effects on our study results (e.g., serum creatinine > 2.5 mg/dL, total bilirubin > 3.0 mg/dL, platelet < 100000/mm3), patients who were consuming anti-platelet agents, patients with a history of previous gastric surgery and patients who did not consent to the study were excluded.
An estimated sample size of 37 subjects per group would give an 80% power to detect a difference in resection rate of the GTS compared to the HKC (assumed to have a complete resection rate of 90%), with a two-sided α = 0.05. With a 10% drop out rate, 40 patients had to be recruited for each group.
All patients were randomly assigned to receive one of the knives-hook knife (Olympus; Tokyo, Japan) plus coagrasper (Olympus; Tokyo, Japan) or grasper type scissors (Alton Medical Instruments; Shanghai, China) (Figure 1) after evaluation with abdominal CT and/or EUS. Randomization codes (A-1 to A-10, B-1 to B-10) were packed into sealed opaque envelopes by an individual, who was not involved in screening and enrolment of the subjects to ensure concealment of allocation. In each of the study institute, twenty patients were enrolled.
The diameter of the scissors is 2.4 mm and the serrated cutting edges are 4-mm-long. The outer side of the forceps except the tip is insulated so that electrosurgical current energy is concentrated at the blade to avoid burning the surrounding tissue. Unlike GSF, the tip of this knife is not insulated and has a thin cutting blade, so that it can facilitate the dissection of submucosal layer such as a flex knife. Furthermore, the forceps can be rotated to the desired location.
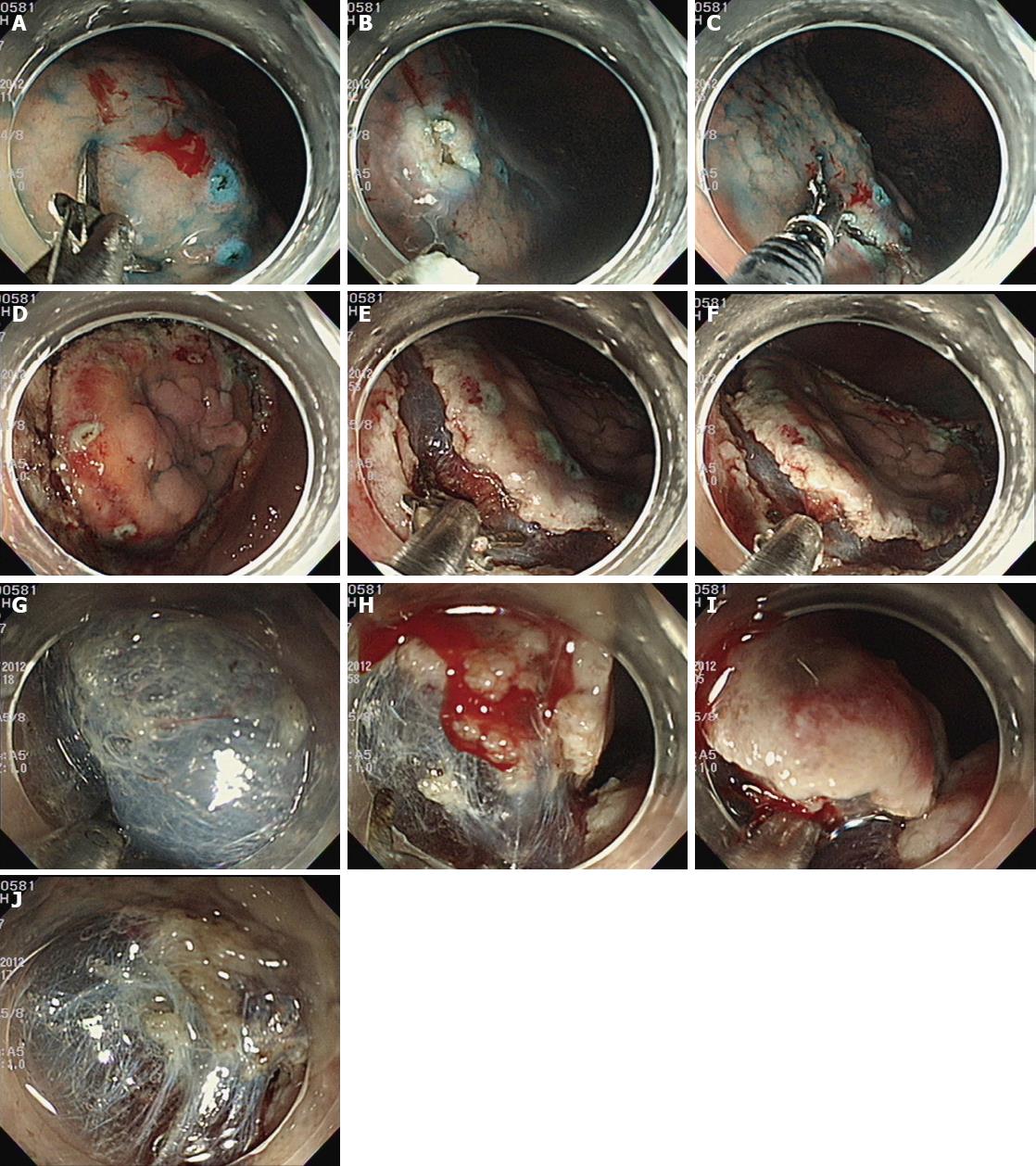
A conventional gastroscope (GIF-Q240J or GIF-H260Z; Olympus, Tokyo, Japan) fitted with a transparent distal attachment (D-201-11304, Olympus) was used for the ESD procedures regardless of the ESD devices. Patients were sedated with intravenous midazolam (0.1 mg/kg) while in the endoscopic suite, and conscious sedation was maintained with additional injections during the procedure. After spraying indigo carmine dye, circumferential markings using argon plasma coagulation were made at 5 mm distances around the outside margin of the lesion, with 2 mm intervals between each marking dot. Hypertonic saline/epinephrine solution (1:10000) and indigo carmine mixture was injected into the submucosal layer until the mucosa was raised and additional injections were repeated as necessary during the procedure. After the lesion was lifted, mucosal incision was performed by using each type of knife and an electrosurgical generator (Erbe; Tübingen, Germany). Electrical current was set as endocut-I for hook knife and as endocut-Q for GTS. After incision around the lesion, dissection was conducted with either the hook knife or GTS (Figure 2).
Electrical current was set as forced coagulation for hook knife and as swift coagulation for GTS. When bleeding occurred during dissection, saline irrigation was performed. If the endoscopist performed the procedure with a hook knife, coagrasper was used for hemostasis according to the endoscopists’ instructions, with an electrical current of 80 W for soft coagulation. With GTS, hemostasis was performed with an electrical current of 80 W for soft coagulation mode.
We compared the endoscopic appearance of tumors, location of tumors, en bloc resection rate, complete resection rate, size of resected specimens, histopathologic findings, operation time, and complication rates between the two groups. Complete resection was defined as lateral and vertical margins of the specimen being free from tumor involvement. Marking of the first dot and withdrawal of the endoscope were measured as the procedure time.
Bleeding was identified when melena, hematochezia, or the presence of fresh bloody vomitus along with a decreased hemoglobin levels of more than 2 g/dL were present after the resection. Perforation was identified by endoscopy during or just after the procedure and/or by the presence of intraperitoneal free air on plain abdominal radiography after the procedure.
All data were recorded on standard forms and computer analyzed. The Student t test was used to compare continuous variables between the groups. Differences between dichotomous variables were evaluated with the χ2 test. The calculations were performed with the SPSS software (SPSS version 12.0, Chicago, IL, United States). Null hypotheses of no difference were rejected if P values were less than 0.05.
A total of 78 patients were enrolled and 76 patients (37 patients of GTS knife group and 39 patients with HKC group) completed this study. Since over 37 patients in each group completed this protocol, additional enrollment was not conducted. One patient dropped out of the study because undifferentiated cancer was found in the resected specimen. Another patient with extensive submucosal infiltration (> 500 μm deep in the submucosa starting from the muscularis mucosae) was also dropped out of the study. Both patients underwent additional surgery after ESD.
The mean age of the GTS group was 62.3 ± 11.4 years and mean age of the HKC group was 65.6 ± 10.1 years (Table 1).There was no significant difference in age and sex ratio between the two groups. Differentiated adenocarcinoma was found in 32.4% in the GTS group and 33.3% in the HKC group. The pathologic distribution and mean size of the resected specimens were not different between the two groups. The area of the resected specimens was 8.30 ± 4.52 cm2 in GTS group and 8.59 ± 4.43 cm2 in HKC group. The depth of tumor distribution was not different between the two groups (94.6% of mucosal layer in the GTS group, 92.3% in the HKC group). With regard to tumor location, 64.9% (24/37 cases) were located at the antrum in the GTS group and 71.8% (28/39 cases) in the HKC group. The locations were not significantly different between the two groups. The total time elapsed during the procedure was 44.54 ± 21.72 min in the GTS group and 43.77 ± 21.84 min in the HKC group (P = 0.88). The time elapsed per square centimeter of the resected lesion was 7.53 ± 6.35 min/cm2 in the GTS group and 6.92 ± 5.93 min/cm2 in the HKC group (P = 0.66). The en bloc resection rate was 100% in both groups. The overall complication rate was 5.41% (2/37 cases) in the GTS group and 7.69% (3/39 cases) in the HKC group (P = 0.68).
| Characteristics | GTS | HKC | P value |
| Number of patients (total) | 37 | 39 | 0.30 |
| Operator 1 (Kim BW) | 10 | 10 | |
| Operator 2 (Chung WC) | 9 | 10 | |
| Operator 3 (Lim CH) | 8 | 9 | |
| Operator 4 (Kim TH) | 10 | 10 | |
| Male:female | 26:11 | 23:16 | 0.19 |
| Age (mean ± SD, yr) | 62.3 ± 11.4 | 65.6 ± 10.1 | 0.58 |
| Final pathologic diagnosis | 0.68 | ||
| Tubular adenoma, low grade dysplasia | 22 | 20 | |
| Tubular adenoma, high grade dysplasia | 3 | 6 | |
| Adenocarcinoma, well differentiated | 6 | 8 | |
| Adenocarcinoma, moderately differentiated | 6 | 5 | |
| Location of the lesion | |||
| Antrum | 24 | 28 | |
| Angle | 5 | 3 | |
| Body | 8 | 8 | |
| Long axis (cm) | 3.59 ± 1.10 | 3.68 ± 0.98 | 0.70 |
| Short axis (cm) | 2.75 ± 0.83 | 2.79 ± 0.84 | 0.83 |
| Area (cm2) | 8.30 ± 4.52 | 8.59 ± 4.43 | 0.78 |
| Total elapsed time (mean ± SD, min) | 44.54 ± 21.72 | 43.77 ± 21.84 | 0.88 |
| Elapsed time/cm2 (mean ± SD, min/cm2) | 7.53 ± 6.35 | 6.92 ± 5.93 | 0.66 |
| En bloc resection | 37/37 (100) | 39/39 (100) | |
| Incomplete resection | 0/37 (0) | 0/39 (0) | |
| Complications | 1/37 (2.7) | 2/39 (5.1) | |
| Perforation | 1 | 1 | |
| > 2.0 g/dL Hb decrease 1 d after procedure | 0 | 1 |
In Korea, the prices of the knives used for ESD are the same. GTS and hook knife cost about 199240 Won (about 185 dollars) and coagrasper cost about 210000 Won (about 191 dollars). HKC are about double in cost compared to GTS alone.
Conventional EMR was previously recommended as a curative local treatment for early gastric cancer[15,16]. To achieve curative resection with adequate margins, the excisions need to be large enough. When piecemeal resection occurs, electro-cautery across the specimens could affect the accuracy of assessment of the lateral margins and this could result in a higher risk of local recurrence. ESD is intended to perform large mucosal resections and improves the rate of successful en bloc resection of an early stage gastrointestinal neoplasia[17,18]. To date, novel devices have been developed for the completion of en bloc resection with adequate margins. However, many endoscopists are eager for the development of devices that allow more effective and faster procedures. In this study, we aimed to introduce and to evaluate the efficacy and technical aspects of the GTS.
Since GSF is ideally designed for both incising the targeted tissue and hemostasis, we tried to improve the device by improving its effect during dissection. Theoretically, the advantage of GSF for ESD is that the device can prevent unexpected incisions. By elevating the lesion during dissection, GSF provides good visualization of the submucosal layer. However, GSF has the disadvantage that it cannot be opened when using a conventional cap because of the small cap diameter. Therefore, a special hood is required when using the GSF. We modified the GSF and developed the GTS, which can accurately grasp and incise the targeted tissue using electrosurgical current. It is smaller than GFS so that it can be used with the conventional transparent cap. The most important difference between GTS and GSF is that the tip of GTS is not insulated and has a thin cutting blade, so that it can facilitate the dissection of the submucosal layer.
Most of the knives are designed for cutting or dissection, but they are not adequate for hemostasis during the ESD procedure. The use of additional instruments increases the cost and requires more procedure time because it takes time to change the instruments. The scissor-type device makes it possible to perform dissection and hemostatic procedures without changing the devices. In this study, we compared the novel GTS, which can be used for both dissection and hemostasis, with HKC for ESD of gastric epithelial neoplasia. We compared these knives because HKC is one of the most commonly used devices for ESD in Korea. In our results, the elapsed time did not differ significantly between GTS and HKC and the procedure time was not saved by using the GTS. This result may have been caused by various factors such as the fact that the endoscopists of this study were not experienced with the new instrument. Furthermore, 2 of the endoscopists had been using the HKC and were familiar with this device which may have affected the procedure time. It is known that there is a learning curve for ESD and that experience of the procedure shortens the procedure time[19,20]. We believe that increased experience with the GTS will reduce the procedure time. Nonetheless all the lesions were removed successfully with the GTS by the 4 endoscopists, which suggest that any experienced endoscopists can complete the whole ESD procedure with the GTS. Feasibility of ESD for gastric epithelial neoplasia with the GTS in beginners should be elucidated in the future.
One case of perforation occurred in the GTS group which might have been prevented if a knife with an insulated body such as GSF was used. The perforation rates of ESD while using different types of knifes were reported to be 4%-10%[21-24], which is similar to the perforation rates of our result. A decrease in hemoglobin level over 2 g/dL per day after the procedure was the same in both groups. Thermal and mechanical tissue damage at the GTS-tissue interface was expected because GTS is larger compared to the hook knife. However, contrary to our expectations, GTS did not interfere with the pathologists’ interpretation of the specimens and complete resections with adequate margins were obtained pathologically with GTS.
There are some limitations in this study. We designed this device to reduce the procedure time, but the sample size was limited and we could not figure it out. Four experienced endoscopits participated in this study and the procedure times by beginners also should be examined. Further studies with larger sample sizes are anticipated to show other benefits of this device.
In conclusion, ESD for gastric epithelial neoplasia can be performed with GTS alone regardless of size and location of the lesion when the endoscopists are experienced. GTS is a safe and effective device for ESD of gastric epithelial neoplasia when compared to HKC. ESD can be performed with GTS alone, which can reduce the costs. Further experiences and large scaled studies would be anticipated to compare the various devices.
Various cutting devices have been developed for endoscopic submucosal dissection (ESD). Most of these knives are designed for dissection of the submucosa and sometimes are not inadequate for control hemorrhages developed during the procedure. The authors recently designed a new device, grasper type scissors (GTS) both for dissection and control hemorrhages.
Grasping-type scissors forceps (GSF) was developed by Akahoshi et al for dissection and control hemorrhages. However, it requires a special hood and rotating the device is frequently difficult because of the size. The safety and effectiveness of GSF was performed in a single center.
The most important difference between GTS and GSF is that the tip of GTS is not insulated and has a thin cutting blade, so that it can facilitate the dissection of the submucosal layer. The safety and effectiveness of GTS was evaluated in multi-centers in this study.
This study suggests that ESD for gastric epithelial neoplasia can be performed with GTS alone regardless of size and location of the lesion when the endoscopists are experienced.
The authors developed a novel device which was modified from GSF, GTS, for ESD of gastric neoplasia. In this study, GTS resolved several disadvantage of GSF and clearly succeeded to reduce the cost of ESD. This study is very interesting, exciting and useful for all endoscopists.
P- Reviewers Yan SL, Yoshida SH S- Editor Qi Y L- Editor A E- Editor Ma S
| 1. | Uedo N, Iishi H, Tatsuta M, Ishihara R, Higashino K, Takeuchi Y, Imanaka K, Yamada T, Yamamoto S, Yamamoto S. Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer. 2006;9:88-92. [PubMed] |
| 2. | Muto M, Miyamoto S, Hosokawa A, Doi T, Ohtsu A, Yoshida S, Endo Y, Hosokawa K, Saito D, Shim CS. Endoscopic mucosal resection in the stomach using the insulated-tip needle-knife. Endoscopy. 2005;37:178-182. [PubMed] |
| 3. | Kim JJ, Lee JH, Jung HY, Lee GH, Cho JY, Ryu CB, Chun HJ, Park JJ, Lee WS, Kim HS. EMR for early gastric cancer in Korea: a multicenter retrospective study. Gastrointest Endosc. 2007;66:693-700. [PubMed] |
| 4. | Hulagu S, Senturk O, Aygun C, Kocaman O, Celebi A, Konduk T, Koc D, Sirin G, Korkmaz U, Duman AE. Endoscopic submucosal dissection for premalignant lesions and noninvasive early gastrointestinal cancers. World J Gastroenterol. 2011;17:1701-1709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Syrén PO, Hult K. Substrate conformations set the rate of enzymatic acrylation by lipases. Chembiochem. 2010;11:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 209] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 6. | Akahoshi K, Akahane H, Murata A, Akiba H, Oya M. Endoscopic submucosal dissection using a novel grasping type scissors forceps. Endoscopy. 2007;39:1103-1105. [PubMed] |
| 7. | Akahoshi K, Honda K, Akahane H, Akiba H, Matsui N, Motomura Y, Kubokawa M, Endo S, Higuchi N, Oya M. Endoscopic submucosal dissection by using a grasping-type scissors forceps: a preliminary clinical study (with video). Gastrointest Endosc. 2008;67:1128-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Uraoka T, Kawahara Y, Ohara N, Kato J, Hori K, Okada H, Yamamoto K. Carbon dioxide submucosal injection cushion: an innovative technique in endoscopic submucosal dissection. Dig Endosc. 2011;23:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Akahoshi K, Okamoto R, Akahane H, Motomura Y, Kubokawa M, Osoegawa T, Nakama N, Chaen T, Oya M, Nakamura K. Endoscopic submucosal dissection of early colorectal tumors using a grasping-type scissors forceps: a preliminary clinical study. Endoscopy. 2010;42:419-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Akahoshi K, Honda K, Kubokawa M, Motomura Y, Matsui N, Endo S, Higuchi N, Taki K, Oya M, Akahane H. Endoscopic resection of a large pedunculated duodenal polyp using a grasping type scissors forceps. Endoscopy. 2008;40 Suppl 2:E74-E75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Akahoshi K, Akahane H. A new breakthrough: ESD using a newly developed grasping type scissor forceps for early gastrointestinal tract neoplasms. World J Gastrointest Endosc. 2010;2:90-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Akahoshi K, Motomura Y, Kubokawa M, Matsui N, Oda M, Okamoto R, Endo S, Higuchi N, Kashiwabara Y, Oya M. Endoscopic submucosal dissection of a rectal carcinoid tumor using grasping type scissors forceps. World J Gastroenterol. 2009;15:2162-2165. [PubMed] |
| 13. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [PubMed] |
| 14. | Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin Gastroenterol Hepatol. 2005;3:S71-S73. [PubMed] |
| 15. | Adachi Y, Shiraishi N, Kitano S. Modern treatment of early gastric cancer: review of the Japanese experience. Dig Surg. 2002;19:333-339. [PubMed] |
| 16. | Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490-4498. [PubMed] |
| 17. | Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc. 2012;76:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 18. | Park YM, Cho E, Kang HY, Kim JM. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc. 2011;25:2666-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 286] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 19. | Kato M, Gromski M, Jung Y, Chuttani R, Matthes K. The learning curve for endoscopic submucosal dissection in an established experimental setting. Surg Endosc. 2013;27:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Yamamoto S, Uedo N, Ishihara R, Kajimoto N, Ogiyama H, Fukushima Y, Yamamoto S, Takeuchi Y, Higashino K, Iishi H. Endoscopic submucosal dissection for early gastric cancer performed by supervised residents: assessment of feasibility and learning curve. Endoscopy. 2009;41:923-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Kato M. Endoscopic submucosal dissection (ESD) is being accepted as a new procedure of endoscopic treatment of early gastric cancer. Intern Med. 2005;44:85-86. [PubMed] |
| 22. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [PubMed] |
| 23. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [PubMed] |
| 24. | Lee H, Yun WK, Min BH, Lee JH, Rhee PL, Kim KM, Rhee JC, Kim JJ. A feasibility study on the expanded indication for endoscopic submucosal dissection of early gastric cancer. Surg Endosc. 2011;25:1985-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |