Published online Oct 7, 2013. doi: 10.3748/wjg.v19.i37.6199
Revised: April 20, 2013
Accepted: June 8, 2013
Published online: October 7, 2013
Processing time: 213 Days and 11.9 Hours
AIM: To compare clinical success and complications of uncovered self-expanding metal stents (SEMS) vs covered SEMS (cSEMS) in obstruction of the small bowel.
METHODS: Technical success, complications and outcome of endoscopic SEMS or cSEMS placement in tumor related obstruction of the duodenum or jejunum were retrospectively assessed. The primary end points were rates of stent migration and overgrowth. Secondary end points were the effect of concomitant biliary drainage on migration rate and overall survival. The data was analyzed according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
RESULTS: Thirty-two SEMS were implanted in 20 patients. In all patients, endoscopic stent implantation was successful. Stent migration was observed in 9 of 16 cSEMS (56%) in comparison to 0/16 SEMS (0%) implantations (P = 0.002). Stent overgrowth did not significantly differ between the two stent types (SEMS: 3/16, 19%; cSEMS: 2/16, 13%). One cSEMS dislodged and had to be recovered from the jejunum by way of laparotomy. Time until migration between SEMS and cSEMS in patients with and without concomitant biliary stents did not significantly differ (HR = 1.530, 95%CI 0.731-6.306; P = 0.556). The mean follow-up was 57 ± 71 d (range: 1-275 d).
CONCLUSION: SEMS and cSEMS placement is safe in small bowel tumor obstruction. However, cSEMS is accompanied with a high rate of migration in comparison to uncovered SEMS.
Core tip: Gastrointestinal obstruction is a complication of advanced cancer disease. It heavily impacts on patients’ general condition. Endoscopic implantation of self-expanding metal stents (SEMS) is a safe and established procedure for palliative treatment of tumor obstruction. Covered SEMS are considered favorable concerning reobstruction by inhibiting tumor ingrowth. In contrast, uncovered SEMS might harbor a lower risk of migration and dislocation. In the present study covered SEMS were retrospectively compared with uncovered SEMS in patients with small bowel or duodenal obstruction. Significantly higher migration rates were observed in the covered SEMS group without observing significant increase of the rate of patients with tumor ingrowth indicating that uncovered SEMS should be favored for palliative treatment of tumor obstruction of the duodenum or the small bowel.
-
Citation: Waidmann O, Trojan J, Friedrich-Rust M, Sarrazin C, Bechstein WO, Ulrich F, Zeuzem S, Albert JG. SEMS
vs cSEMS in duodenal and small bowel obstruction: High risk of migration in the covered stent group. World J Gastroenterol 2013; 19(37): 6199-6206 - URL: https://www.wjgnet.com/1007-9327/full/v19/i37/6199.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i37.6199
Endoscopic placement of self-expandable metal stents (SEMSs) has become a broadly accepted first line treatment option for patients with advanced malignant intestinal stenosis. Reconstitution of the intestinal transit is paramount in palliation of tumor obstruction, and endoscopic SEMS insertion is weighed against surgical intervention in terms of clinical relief, complication rate, and length of hospital stay of the patient. In the small bowel, duodenal tumor obstruction has increasingly been treated by SEMS placement[1,2], but SEMS insertion in the lower small bowel is less often performed[3,4]. Duodenal SEMS insertion is technically feasible in more than 90% of interventions; it is safe and comes along with a faster start of oral intake, shorter length of hospital stay, lower morbidity and probably reduced in-hospital mortality as compared to surgical treatment[5-13]. SEMS may as well be preferred over surgical treatment in the lower small bowel and at anastomotic small bowel obstructions in case of recurrent malignancy[4].
Tumor in- or over-growth can limit long-term outcome of SEMS in 12% to 21% of cases, but stent occlusion might be reduced by covering the SEMS with silicone or plastic membranes (covered SEMS, cSEMS)[1,2].
Aim of this study was to compare outcome of SEMS vs cSEMS in small bowel tumor obstruction and to identify technical feasibility, safety, clinical impact, complications and patient’s outcome at follow-up.
All patients who underwent endoscopic placement of SEMS or cSEMS for small bowel tumor obstruction (duodenum or jejunum) between August 2009 and September 2012 were retrospectively analyzed. Due to advanced or metastatic disease or comorbidity, none of the patients were considered candidates for curative surgical treatment of the tumor. All patients included into this study were symptomatic and were admitted to the hospital because of their obstructive symptoms including nausea, vomiting, bloating, or abdominal pain. At least for some time all patients were suffering from postprandial vomiting. No patient was treated for non-symptomatic stenosis. Indication for jejunal placement of stents was only given in cases of unilocular stenosis by circumscribed peritoneal carcinomatosis. Histology was obtained by endoscopic biopsy from the intestinal tumor or percutaneous needle biopsy from liver metastasis. The therapeutic procedures were ascertained in an interdisciplinary conference with senior physicians from the departments of surgery, gastroenterology and medical oncology and the recommendation to treat by endoscopic stent placement was given in consent.
We retrospectively reviewed the prospectively collected records on technical success of the procedure, clinical benefit, and the incidence of complications including migration and stent occlusion. The patients’ outcome at follow-up was additionally registered.
Patients were advised to resume oral intake of liquids within 24 h and to advance to a low-residue diet as tolerated. The status of oral food intake was monitored at one-month intervals on an outpatient basis. In case of recurrence of dysphagia, radiographic imaging (iodine or barium upper gastrointestinal series) and/or upper gastrointestinal endoscopy was performed. Patients who had recurrent symptoms caused by tumor overgrowth were treated by placement of a second intestinal stent.
Stents were placed by very experienced gastroenterological endoscopists using a therapeutic gastroscope (GIF-1TQ 160), or a duodenoscope (TJF-Q180V, TJF-160 VR; all Olympus medical Europe, Hamburg, Germany) with a working channel of 3.7 or 4.2 mm, respectively. All stents were inserted through-the-scope (TTS) in combination with over-the-wire technique, and all stents were placed under fluoroscopic guidance. Selection of SEMS vs cSEMS was at the appraisal of the endoscopist and cSEMS was preferably chosen in case of advanced tumors with subtotal or complete obstruction intending to avoid later tumor ingrowth. SEMS was preferred over cSEMS in case that the investigator was in fear of blocking biliary outflow by crossing the duodenal papilla by the stent. If complications (migration or overgrowth) occurred, new stents were placed and the stenting procedure in the patient was switched from a covered to an uncovered stent and vice versa. The stents used in this study were self-expandable Nitinol uncovered SEMS (Niti-S, D-Type, TaeWoong medical, South Korea) and covered SEMS [cSEMS; Niti-S pyloric duodenal covered stent (End Bare Type), TaeWoong medical, South Korea]. A stent diameter from 18 to 28 mm and a stent length from 40 to 120 mm were used. The cSEMS provides a silicone covering and has a retrieval suture for preventing tumor-ingrowth and easy removal. It contains a fixed-cell braided structure. The SEMS is a fine mesh tubular prosthesis that facilitates immediate and continuous wall apposition due to the so-called D-weaving technology, i.e., stent cells are unfixed resulting in a high flexibility and retaining its shape even in bending sections of the intestinal tract. In case that a stent did not cover the entire tumor obstruction, two overlapping stents were implanted to bridge the entire obstructed bowel segment and this was accounted as a single application in this study.
Tumor overgrowth of the stent was defined as deterioration of the patient’s condition (recurrence of dysphagia) and detection of narrowing of the stent lumen within or adjacent to the proximal or distal end of the stent mesh as a result of tumor growth, as shown by endoscopic and/or radiologic findings. Tumor ingrowth was defined as tumor obstruction through the stent mesh as a reason for as deterioration of the patient’s condition (recurrence of dysphagia). Improvement of vomiting or the intake of fluids or food was assessed qualitatively. Postinterventional complication rate was defined as occurrence of complications within 24 h after stent placement, all other complications were observed until the end of the follow-up period.
The retrospective study was approved by the institutional review board (Ethikkomission) of the Johann Wolfgang Goethe-University Hospital.
The present study is a retrospective cohort study. The primary endpoints were complications due to stent implantation including tumor overgrowth and stent migration. The secondary end points were effect of concomitant biliary stenting on migration rates and overall survival. Statistical analyses were performed with SPSS (Version 22.0, IBM, NY, United States). Predictors of survival were determined using a univariate Cox regression hazard model. Death was recorded as event. Survival curves with the estimated hazards where calculated with the Cox regression model. The patients at risk at the individual time points are shown in the figures. The data was analyzed according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines[14].
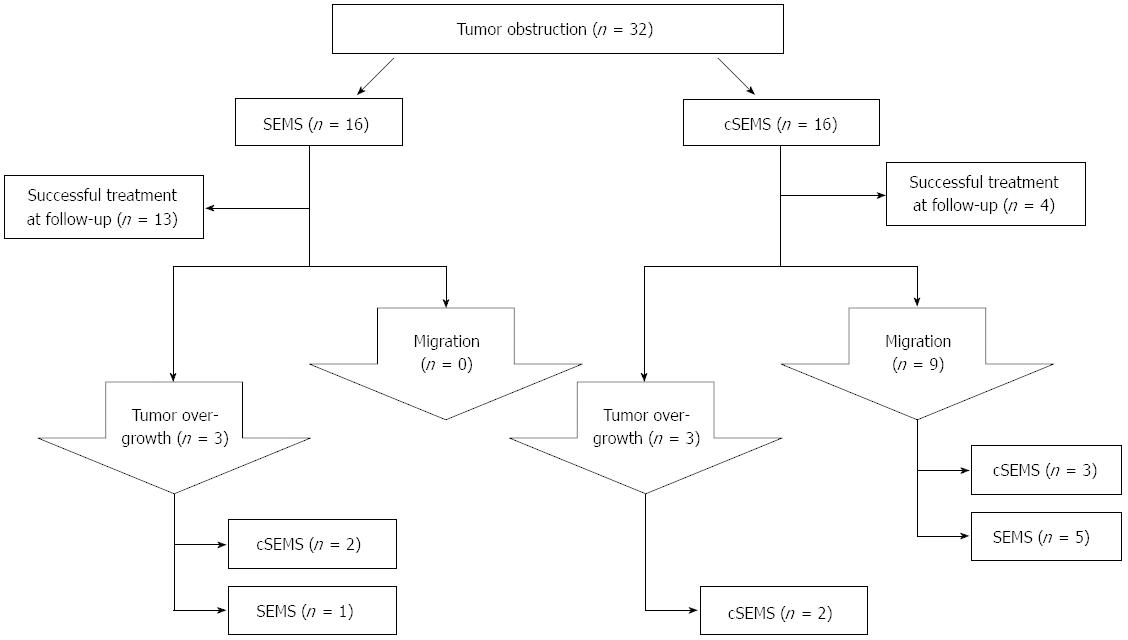
Thirty-two cases of stent insertion were included in this study: 16 cSEMSs and 16 SEMSs were placed in 20 patients. Patient characteristics are shown in Table 1. Five patients received two overlapping stents; in the remaining 27 interventions a singular stent was put in place. Whereas all covered SEMS were implanted in the duodenum, 3 of 16 uncovered SEMS were inserted into the jejunum. The main etiology of gastric outlet obstruction was pancreatic cancer, followed by cholangiocellular carcinoma or gallbladder carcinoma (Table 1). All three jejunal SEMS were placed due to an obstruction that was caused by a circumscribed manifestation of peritoneal carcinomatosis in gastric cancer patients. Nine of the SEMS (56%) and eight of the cSEMS (50%) were placed in patients who presented with concomitant biliary tract stenosis; all these patients were treated with placement of plastic stents or SEMS/cSEMS into the common bile duct (CBD), and plastic endoprosthesis were replaced by biliary SEMS at the time of duodenal SEMS insertion in all patients.
| Characteristics | SEMS | cSEMS |
| Stents | 16 (50) | 16 (50) |
| Male gender | 7 (44) | 10 (63) |
| Age (yr), mean ± SD (range) | 70 ± 11 (50-85) | 71 ± 11 (50-84) |
| Localization | ||
| Jejunum, n | 3 | 0 |
| Duodenum, n | 13 | 16 |
| Disease | ||
| Pancreatic carcinoma, n | 6 | 7 |
| Cholangiocellular carcinoma, n | 3 | 2 |
| Gallbladder carcinoma, n | 1 | 2 |
| Gastric cancer, n | 3 | 2 |
| Colorectal cancer, n | 2 | 0 |
| Breast cancer metastasis, n | 1 | 0 |
| Stenosis due to duodenal ulcer perforation, n | 0 | 3 |
| Balloon dilatation of the stent | 3 (19) | 2 (13) |
| Concomitant biliary drainage | 9 (56) | 8 (50) |
We observed technical success of SEMS placement in all cases without occurrence of peri-interventional complications. In three (SEMS) and two (cSEMS) stent placements, respectively, balloon dilatation was needed for complete expansion of the stents. The duration of endoscopic procedure did not significantly differ between SEMS and cSEMS (Table 2). The clinical condition ameliorated in 14 of 16 (87.5%) cases treated with SEMS and intake of fluids or food improved. In contrast, in patients treated with cSEMS the clinical condition improved in 12 of 16 (75%) cases only. However, this difference was not statistically significant (P = 0.564).
| Complications | SEMS | cSEMS | P value |
| Duration of procedure (min), median (range) | 60 (40-121) | 60 (31-160) | 0.867 |
| Migration | 0 (0) | 9 (56) | 0.002 |
| Time until migration (d), mean ± SD (range) | - | 30 ± 52 (1-161) | NA |
| Tumor overgrowth | 3 (19) | 2 (13) | 0.725 |
| Tumor ingrowth, n | 0 | 0 | NA |
| Time until tumor overgrowth (d), mean ± SD (range) | 143 ± 95 (39-224) | 96 ± 105 (22-170) | 0.572 |
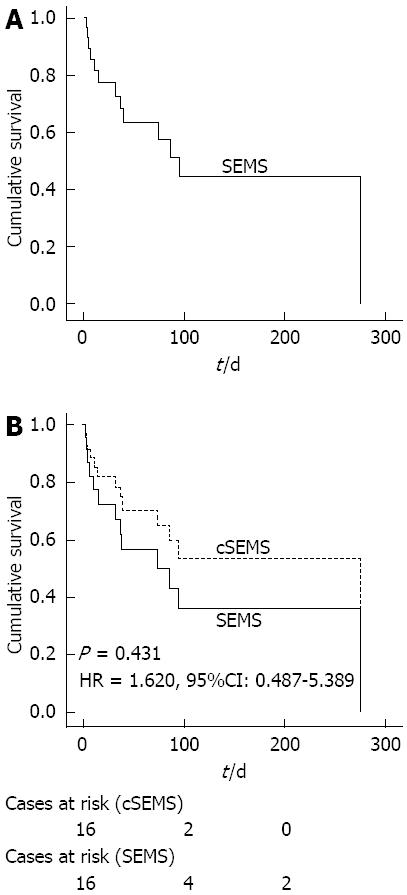
| Overall survival (d), median, range | 40 (3-275) | 75 (11-426) | 0.431 |
The mean follow-up time ± SD was 57 ± 71 d with a range of 1-275 d. In patients with gastric outlet obstruction and concomitant biliary obstruction no migration or occlusion of the bile duct stents was observed. Nine of the 16 cSEMS (56%) migrated within the observation time. In one of the patients the dislodged stent had to be recovered from the jejunum by laparotomy. The patient was dismissed from hospital treatment after recovery from the surgery. On the contrary none of the SEMS dislocated. SEMS placement was superior to cSEMS for duodenal location of obstructions concerning migration rate (0% vs 56%). However, although none of the three SEMS placed in the jejunum migrated within time no further conclusion can be drawn for a superiority of SEMS in the jejunal location as no cSEMS was placed in the jejunum. The mean time until migration ± SD was 30 ± 52 d with a range of 1-161 d.
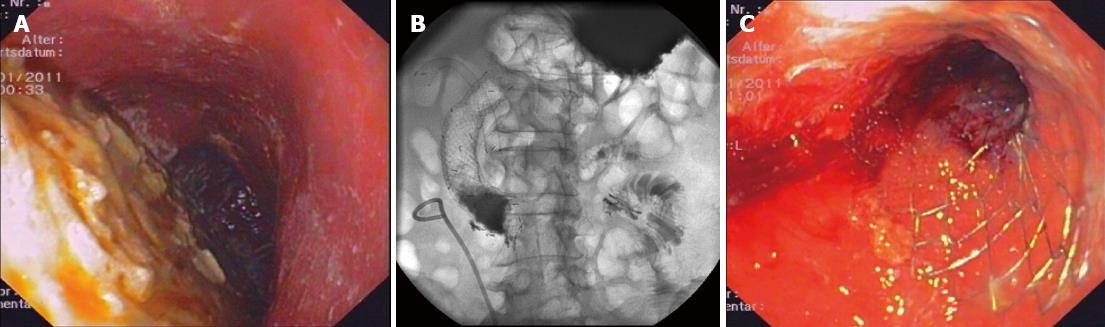
As stents in situ of the CBD might give hold to the duodenal SEMS/cSEMS and might be associated with a reduced rate of stent migration, we analyzed the group of patients with CBD SEMS/cSEMS and concomitant duodenal SEMS/cSEMS placement separately. In 17 of 32 cases, a combined biliary and duodenal SEMS/cSEMS insertion had been undertaken. Comparing the time until migration of stents in patients with and without biliary SEMS/cSEMS, there was no difference in migration of duodenal stents (HR = 1.530, 95%CI: 0.731-6.306; P = 0.556). In those 17 patients in whom a biliary SEMS was in place, all six events of stent migration were observed in patients with duodenal cSEMS, whereas no migration was seen in patients with uncovered duodenal SEMS (P = 0.008). A representative example of duodenal stent with concomitant biliary metal stent implantation is shown in Figure 1.
Overgrowth of a SEMS by progressive cancer occurred in three cases, whereas this complication was seen in two of the cSEMS patients. There was no tumor ingrowth into any of the stents (SEMS or cSEMS) observed. In case of tumor overgrowth or migration, a new stent was placed into the stenosis and migrated cSEMS were replaced by uncovered SEMS. A flow chart demonstrating the algorithm of stent treatment is displayed in Figure 2.
Thirteen patients died within the observation time. The median overall survival was 74 d (Figure 3A). To assess the influence of the stent type on survival, overall survival times for the two kinds of stents were compared. There was no significant difference between SEMS and cSEMS concerning overall survival according to a univariate Cox regression model (Figure 3B).
In this comparative study, we observed a high rate (> 50%) of stent migration in the covered SEMS group in comparison to the non-covered SEMS group (0%) in palliation of duodenal or small bowel obstruction. Concurrently, technical feasibility of stent placement (TTS technique) was similar, and relief of symptoms was equal in both patient groups. Insertion of SEMS was as safe as cSEMS implantation during periinterventional surveillance of the patient. Thereby, biliary SEMS did not prevent migration of duodenal cSEMS migration, and the migration rate in patients with and without concomitant biliary stents was similar. We did not observe clinically significant tumor ingrowth in the SEMS or cSEMS group. However, the overall survival times were quite short and in patients with longer survival times cSEMS might show advantages concerning tumor ingrowth rates.
Randomized trials comparing SEMS vs cSEMS treatment in the small bowel has not been reported up to date, but an increased risk of stent migration in covered SEMS has been reported in colonic tumor palliation: Stent migration was four times as common in the covered SEMS group as in the non-covered stent group in a recent meta-analysis[15]. In patients with inoperable gastric cancer, comparison of cSEMS vs SEMS yielded similar results: Migration was observed in 26.0% of patients, in comparison to 2.8% in non-covered SEMS in a randomized trial[16]. Our results suggest that migration rate in the small bowel is even higher than in the stomach or large bowel. Technical success rate and clinical success was similar in cSEMS vs SEMS in both studies, and immediate complications were near to zero. Migration rates are low (1%-3% of cases) in other studies reporting on SEMS insertion in the duodenum and small bowel[1,17].
As the duration of the endoscopic treatments and the responsible endoscopists did not differ between the two cohorts, we consider two factors to contribute mainly on migration in cSEMS vs SEMS study: First, the stent cover minimizes hyperplastic tissue to get hold of the stent mesh and partial ingrowth of tumorous and hyperplastic tissue is prevented, thus minimizing any anti-migration effect that the stent may provide against the natural motility of the small bowel. Moreover, we think that the fixed-cell braided structure of the SEMS we used might have significantly contributed to migration by causing the stent to straighten itself by use of its axial expansion force. In opposite, the so-called D-weaving technology of the SEMS with unfixed stent cells might have resulted in a higher flexibility and retention of its shape even at bending sections of the intestinal tract, such as the duodenum. The motility of the small bowel contributes to the progression of the stent. Stent migration can be reduced by endoscopic clip fixation of stents in the duodenal position[18]. This procedure might be impracticable and only short-lasting, though, as clips usually dislodge in the short term.
The use of SEMS in duodenal obstruction might be compromised by concomitant biliary obstruction. However, combined endoscopic treatment is safe and successful in the majority of cases with only minor complications (0%-16% of cases)[19-23] even if others report found much higher incidence of complications (up to 52%)[24]. In our cohort of patients there were no treatment related complications of biliary drainage by duodenal stents. Migration rates were also not affected by the existence of biliary stenting. However, all events of migration of duodenal stents in patients receiving combined biliary and duodenal stenting happened in patients who had obtained cSEMS. Therefore, biliary stenting may protect from duodenal stent migration, if the biliary stents are placed in between the meshes of uncovered SEMS. The advantage of SEMS in comparison to cSEMS might be lower rates of tumor occlusion[1,2,19]. In contrast in patients receiving cSEMS recurrence of tumor occlusion is rarely observed[19]. The rate of stent occlusion did not significantly differ between patients with SEMS (19%) and cSEMS (13%) in our study, though. Also in another report no differences were found between SEMS and SEMS concerning necessity of re-interventions[25]. An overview of literature concerning complications of SEMS placements is provided in Table 3.
| Study | Patients, stents | Site of tumor obstruction | Tumor overgrowth | Migration | Bleeding | Perforation |
| Costamagna et al[1] | 202, 212 | Endoscopic duodenal stenting | 12.4% | 1.5% | 3.0% | 0.5% |
| van Hooft et al[2] | 50, 57 | Endoscopic stenting for gastric outlet obstruction | 21.0% | 4.0% | 0.0% | 0.0% |
| Jeong et al[4] | 25, 28 | Gastrojejunostomy, esophagojejunostomy, cSEMS | 17.0% | 4.0% | 0.0% | 1.0% |
| Chandrasegaram et al[7] | 26, 31 | Endoscopic stenting vs operative gastrojejunostomy | 12.0% | 0.0% | 0.0% | 0.0% |
| Jang et al[17] | 583, 603 | Peripyloric region, nonperipyloric region, duodenum alone anastomosis (Billroth I, Billroth II), jejunum | 3.8% | NM | NM | < 1.0% |
| Kim et al[25] | 50, 50 | Endoscopic stenting for malignant gastroduodenal obstructions | 18.0% | 10.0% | 0.0% | 0.0% |
| Wong et al[26] | 6, 6 | Surgical vs endoscopic palliation | NM | NM | NM | NM |
| Mosler et al[27] | 36, 52 | Endoscopic stenting of nonesophageal upper gastrointestinal stenosis | 11.0% | 14.0% | 0.0% | 6.0% |
| Kim et al[28] | 213, 236 | Malignant gastroduodenal obstruction | 7.0% | 4.0% | 1.0% | 0.0% |
| Bang et al[29] | 134, 132 | Endoscopic treatment for malignant antropyloric and duodenal | cSEMS 5.7%SEMS 19.0% | cSEMS 26.4% SEMS 6.3% | 2.2% | < 1.0% |
| Keränen et al[30] | 104, 130 | Endoscopic treatment for malignant gastric outlet obstruction | 18.0% | 0.0% | 0.0% | 1.9% |
| Ahn et al[31] | 47, 52 | Malignant gastroduodenal obstruction, uncovered SEMS | 11.0% | 2.0% | 0.0% | 4.0% |
| Canena et al[32] | 74, 80 | Endoscopic stenting for gastric outlet obstruction | 9.5% | 0.0% | 1.4% | 0.0% |
| Cha et al[33] | 85, 85 | Endoscopic stenting for gastroduodenal obstruction | 29.0% | 4.0% | 4.0% | 4.0% |
| Own data | 20, 32 | Small bowel/duodenum | cSEMS 13.0%SEMS 19.0% | cSEMS 56.0%SEMS 0.0% | 0.0% | 0.0% |
The limitation of the current study is the retrospective non-randomized approach. However, in conclusion, technical feasibility, tumor overgrowth, and overall survival of the patients are comparable in SEMS vs cSEMS, but migration rate is much higher in cSEMS as migration was observed in none of the SEMS group patients.
We prefer SEMS over cSEMS insertion as first choice for malignant duodenal and small bowel obstruction and restrict use of cSEMS in cases with fast tumor ingrowth. Prospective randomized trials are needed to compare SEMS and cSEMS for small bowel obstruction.
Duodenal and small bowel obstructions are complications of advanced cancer disease. Endoscopic implantation of nitinol based self-expanding metal stents (SEMS) is a safe and established procedure for palliative treatment of tumor obstruction. Covered and uncovered SEMS differ in their characteristics concerning risk of tumor ingrowth and overgrowth or migration and dislocation. But up to now, there is no data provided that covered or uncovered SEMS should be favored for treatment of tumor stenosis in the duodenum or small bowel.
Endoscopic treatment of duodenal or small bowel obstruction is an established treatment procedure for malignant stenosis. However, no thorough analysis comparing covered SEMS and uncovered SEMS in for tumor related stenosis has been reported. The authors hypothesized, that covered SEMS and uncovered SEMS differ concerning complications in the indicated localization.
The authors learnt that covered SEMS showed significant higher migrations rates than uncovered SEMS when placed in the duodenal position. In contrast, no significant differences concerning re-obstruction of the lumen or overall survival after SEMS implantation were found.
The authors conclude that uncovered SEMS should be preferred when SEMS implantation in the duodenum is performed.
SEMS are self-expanding metal stents which are often made of nitinol alloyings and are placed by guidewire and under fluoroscopic control in endoscopic procedures. SEMS can be used uncovered or covered with silicone or other materials.
The authors showed in their retrospective analysis for the first time that uncovered SEMS might be preferred for malignant duodenal or small stenosis, as covered SEMS show much higher rates of migration. The results may help to reduce complications raised by migrated SEMS.
P- Reviewers Akyuz F, Guerra I, Murata A S- Editor Gou SX L- Editor A E- Editor Ma S
| 1. | Costamagna G, Tringali A, Spicak J, Mutignani M, Shaw J, Roy A, Johnsson E, De Moura EG, Cheng S, Ponchon T. Treatment of malignant gastroduodenal obstruction with a nitinol self-expanding metal stent: an international prospective multicentre registry. Dig Liver Dis. 2012;44:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | van Hooft JE, van Montfoort ML, Jeurnink SM, Bruno MJ, Dijkgraaf MG, Siersema PD, Fockens P. Safety and efficacy of a new non-foreshortening nitinol stent in malignant gastric outlet obstruction (DUONITI study): a prospective, multicenter study. Endoscopy. 2011;43:671-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Lee H, Park JC, Shin SK, Lee SK, Lee YC. Preliminary study of enteroscopy-guided, self-expandable metal stent placement for malignant small bowel obstruction. J Gastroenterol Hepatol. 2012;27:1181-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Jeong JY, Kim YJ, Han JK, Lee JM, Lee KH, Choi BI, Yang HK, Lee KU. Palliation of anastomotic obstructions in recurrent gastric carcinoma with the use of covered metallic stents: clinical results in 25 patients. Surgery. 2004;135:171-177. [PubMed] |
| 5. | Hosono S, Ohtani H, Arimoto Y, Kanamiya Y. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol. 2007;42:283-290. [PubMed] |
| 6. | Jeurnink SM, Polinder S, Steyerberg EW, Kuipers EJ, Siersema PD. Cost comparison of gastrojejunostomy versus duodenal stent placement for malignant gastric outlet obstruction. J Gastroenterol. 2010;45:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Chandrasegaram MD, Eslick GD, Mansfield CO, Liem H, Richardson M, Ahmed S, Cox MR. Endoscopic stenting versus operative gastrojejunostomy for malignant gastric outlet obstruction. Surg Endosc. 2012;26:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Dormann A, Meisner S, Verin N, Wenk Lang A. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy. 2004;36:543-550. [PubMed] |
| 9. | Fukami N, Anderson MA, Khan K, Harrison ME, Appalaneni V, Ben-Menachem T, Decker GA, Fanelli RD, Fisher L, Ikenberry SO. The role of endoscopy in gastroduodenal obstruction and gastroparesis. Gastrointest Endosc. 2011;74:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Varadarajulu S, Banerjee S, Barth B, Desilets D, Kaul V, Kethu S, Pedrosa M, Pfau P, Tokar J, Wang A. Enteral stents. Gastrointest Endosc. 2011;74:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Piesman M, Kozarek RA, Brandabur JJ, Pleskow DK, Chuttani R, Eysselein VE, Silverman WB, Vargo JJ, Waxman I, Catalano MF. Improved oral intake after palliative duodenal stenting for malignant obstruction: a prospective multicenter clinical trial. Am J Gastroenterol. 2009;104:2404-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | van Hooft JE, Uitdehaag MJ, Bruno MJ, Timmer R, Siersema PD, Dijkgraaf MG, Fockens P. Efficacy and safety of the new WallFlex enteral stent in palliative treatment of malignant gastric outlet obstruction (DUOFLEX study): a prospective multicenter study. Gastrointest Endosc. 2009;69:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Vlavianos P, Zabron A. Clinical outcomes, quality of life, advantages and disadvantages of metal stent placement in the upper gastrointestinal tract. Curr Opin Support Palliat Care. 2012;6:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573-577. [PubMed] |
| 15. | Zhang Y, Shi J, Shi B, Song CY, Xie WF, Chen YX. Comparison of efficacy between uncovered and covered self-expanding metallic stents in malignant large bowel obstruction: a systematic review and meta-analysis. Colorectal Dis. 2012;14:e367-e374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Kim CG, Choi IJ, Lee JY, Cho SJ, Park SR, Lee JH, Ryu KW, Kim YW, Park YI. Covered versus uncovered self-expandable metallic stents for palliation of malignant pyloric obstruction in gastric cancer patients: a randomized, prospective study. Gastrointest Endosc. 2010;72:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Jang JK, Song HY, Kim JH, Song M, Park JH, Kim EY. Tumor overgrowth after expandable metallic stent placement: experience in 583 patients with malignant gastroduodenal obstruction. AJR Am J Roentgenol. 2011;196:W831-W836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Kim ID, Kang DH, Choi CW, Kim HW, Jung WJ, Lee DH, Chung CW, Yoo JJ, Ryu JH. Prevention of covered enteral stent migration in patients with malignant gastric outlet obstruction: a pilot study of anchoring with endoscopic clips. Scand J Gastroenterol. 2010;45:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Katsanos K, Sabharwal T, Adam A. Stenting of the upper gastrointestinal tract: current status. Cardiovasc Intervent Radiol. 2010;33:690-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Baron TH. Management of simultaneous biliary and duodenal obstruction: the endoscopic perspective. Gut Liver. 2010;4 Suppl 1:S50-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Kim KO, Kim TN, Lee HC. Effectiveness of combined biliary and duodenal stenting in patients with malignant biliary and duodenal obstruction. Scand J Gastroenterol. 2012;47:962-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Moon JH, Choi HJ, Ko BM, Koo HC, Hong SJ, Cheon YK, Cho YD, Lee MS, Shim CS. Combined endoscopic stent-in-stent placement for malignant biliary and duodenal obstruction by using a new duodenal metal stent (with videos). Gastrointest Endosc. 2009;70:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Maire F, Hammel P, Ponsot P, Aubert A, O’Toole D, Hentic O, Levy P, Ruszniewski P. Long-term outcome of biliary and duodenal stents in palliative treatment of patients with unresectable adenocarcinoma of the head of pancreas. Am J Gastroenterol. 2006;101:735-742. [PubMed] |
| 24. | Hamada T, Nakai Y, Isayama H, Sasaki T, Kogure H, Kawakubo K, Sasahira N, Yamamoto N, Togawa O, Mizuno S. Duodenal metal stent placement is a risk factor for biliary metal stent dysfunction: an analysis using a time-dependent covariate. Surg Endosc. 2013;27:1243-1248. [PubMed] |
| 25. | Kim YW, Choi CW, Kang DH, Kim HW, Chung CU, Kim DU, Park SB, Park KT, Kim S, Jeung EJ. A double-layered (comvi) self-expandable metal stent for malignant gastroduodenal obstruction: a prospective multicenter study. Dig Dis Sci. 2011;56:2030-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Wong YT, Brams DM, Munson L, Sanders L, Heiss F, Chase M, Birkett DH. Gastric outlet obstruction secondary to pancreatic cancer: surgical vs endoscopic palliation. Surg Endosc. 2002;16:310-312. [PubMed] |
| 27. | Mosler P, Mergener KD, Brandabur JJ, Schembre DB, Kozarek RA. Palliation of gastric outlet obstruction and proximal small bowel obstruction with self-expandable metal stents: a single center series. J Clin Gastroenterol. 2005;39:124-128. [PubMed] |
| 28. | Kim JH, Song HY, Shin JH, Choi E, Kim TW, Jung HY, Lee GH, Lee SK, Kim MH, Ryu MH. Metallic stent placement in the palliative treatment of malignant gastroduodenal obstructions: prospective evaluation of results and factors influencing outcome in 213 patients. Gastrointest Endosc. 2007;66:256-264. [PubMed] |
| 29. | Bang S, Kim HJ, Park JY, Park YS, Kim MH, Park SW, Lee’ YC, Song SY. Effectiveness of self-expanding metal stents for malignant antropyloric and duodenal obstruction with a comparison between covered and uncovered stents. Hepatogastroenterology. 2008;55:2091-2095. [PubMed] |
| 30. | Keränen I, Udd M, Lepistö A, Halttunen J, Kylänpää L. Outcome for self-expandable metal stents in malignant gastroduodenal obstruction: single-center experience with 104 patients. Surg Endosc. 2009;Sep 3; Epub ahead of print. [PubMed] |
| 31. | Ahn HS, Hong SJ, Moon JH, Ko BM, Choi HJ, Han JP, Park JS, Kang MS, Cho JY, Lee JS. Uncovered self-expandable metallic stent placement as a first-line palliative therapy in unresectable malignant duodenal obstruction. J Dig Dis. 2012;13:628-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Canena JM, Lagos AC, Marques IN, Patrocínio SD, Tomé MG, Liberato MA, Romão CM, Coutinho AP, Veiga PM, Neves BC. Oral intake throughout the patients’ lives after palliative metallic stent placement for malignant gastroduodenal obstruction: a retrospective multicentre study. Eur J Gastroenterol Hepatol. 2012;24:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Cha BH, Lee SH, Kim JE, Yoo JY, Park YS, Kim JW, Jeong SH, Kim N, Lee DH, Hwang JH. Endoscopic self-expandable metallic stent placement in malignant pyloric or duodenal obstruction: does chemotherapy affect stent patency? Asia Pac J Clin Oncol. 2013;9:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |