Published online Oct 7, 2013. doi: 10.3748/wjg.v19.i37.6156
Revised: August 13, 2013
Accepted: August 20, 2013
Published online: October 7, 2013
Processing time: 113 Days and 20.9 Hours
Chronic pancreatitis (CP) is a progressive disease with irreversible changes in the pancreas. Patients commonly present with pain and with exocrine or endocrine insufficiency. All therapeutic efforts in CP are directed towards relief of pain as well as the management of associated complications. Endoscopic therapy offers many advantages in patients with CP who present with ductal calculi, strictures, ductal leaks, pseudocyst or associated biliary strictures. Endotherapy offers a high rate of success with low morbidity in properly selected patients. The procedure can be repeated and failed endotherapy is not a hindrance to subsequent surgery. Endoscopic pancreatic sphincterotomy is helpful in patients with CP with minimal ductal changes while minor papilla sphincterotomy provides relief in patients with pancreas divisum and chronic pancreatitis. Extracorporeal shock wave lithotripsy is the standard of care in patients with large pancreatic ductal calculi. Long term follow up has shown pain relief in over 60% of patients. A transpapillary stent placed across the disruption provides relief in over 90% of patients with ductal leaks. Pancreatic ductal strictures are managed by single large bore stents. Multiple stents are placed for refractory strictures. CP associated benign biliary strictures (BBS) are best treated with multiple plastic stents, as the response to a single plastic stent is poor. Covered self expanding metal stents are increasingly being used in the management of BBS though further long term studies are needed. Pseudocysts are best drained endoscopically with a success rate of 80%-95% at most centers. Endosonography (EUS) has added to the therapeutic armamentarium in the management of patients with CP. Drainage of pseudcysts, cannulation of inaccessible pancreatic ducts and celiac ganglion block in patients with intractable pain are all performed using EUS. Endotherapy should be offered as the first line of therapy in properly selected patients with CP who have failed to respond to medical therapy and require intervention.
Core tip: Chronic pancreatitis is a challenge to the therapeutic endoscopist. A patient with chronic pancreatitis can present with ductal calculi, leaks, pseudocysts, strictures, pancreatic malignancy or a biliary obstruction. Endoscopic therapy offers a high rate of success in properly selected patients. It offers many advantages over surgery, which for a long time was considered the gold standard in the treatment of chronic pancreatitis. This chapter deals with the management of chronic pancreatitis associated strictures, calculi, leaks and pseudocysts. The role of endosonography in management of pseudocysts, cannulation of inaccessible ducts and pain relief has also been discussed.
- Citation: Tandan M, Reddy DN. Endotherapy in chronic pancreatitis. World J Gastroenterol 2013; 19(37): 6156-6164
- URL: https://www.wjgnet.com/1007-9327/full/v19/i37/6156.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i37.6156
Chronic pancreatitis (CP) is a disease of varied etiology and characterized by progressive and irreversible damage to the pancreas with resultant loss of both endocrine and exocrine functions. Alcohol, smoking, genetic factors and metabolic disorders are common etiological causes[1]. In our country the non alcoholic type of CP is more prevalent[2,3]. Irrespective of the etiology, the majority of patients with CP present with pain as the dominant symptom.
As the disease is irreversible, almost all therapeutic efforts are directed towards control of pain and management of complications associated with CP. For the therapeutic endoscopist, CP is a challenge as patients can present with ductal strictures, calculi, ductal disruption, pseudocysts, biliary strictures, duodenal narrowing or a pancreatic malignancy. Endotherapy is performed in patients with CP who are unlikely to respond or have failed medical therapy as well as to manage the above mentioned complications. Surgery has often been considered the best therapeutic option for patients with CP[4]. However with advances in technology and techniques, endotherapy is offered as first line management in many patients with CP. Endotherapy offers many distinct advantages over surgery. It has a high success rate and low morbidity in properly selected patients. The procedure can be repeated with no extra risk, unlike the morbidity and difficulty associated with repeat surgery. The results are comparable to surgery and failed endotherapy does not hinder subsequent surgery[5-8] . The endoscopic approach can also predict the response to surgical therapy[9]. The endoscopic techniques used are endoscopic retrograde cholangiopancreatography (ERCP) and endosonography (EUS). Extracorporeal shockwave lithotripsy (ESWL) is a part of the endoscopic armamentarium. Advances in EUS have improved therapeutic options, including pseudocyst drainage and cannulation of inaccessible main pancreatic duct (MPD).
In this review, we will discuss the role of endotherapy in diagnosis and management of CP related pancreatic ductal strictures, stones, common bile duct (CBD) strictures and pseudocyst.
ERCP was earlier used both for diagnosis and management of patients with CP. It has sensitivity of 73%-94% and specificity of 90%-100% in visualizing duct related changes in CP[10]. The emergence of magnetic resonance cholangiopancreatography (MRCP) with secretin stimulation, as well as EUS, has minimized the role of ERCP in diagnosing CP. EUS is a better diagnostic modality, especially in early and less advanced CP, as it identifies both ductal and parenchymal changes[11]. EUS has a sensitivity of close to 100% as compared to 80% with ERCP in patients with early CP[12]. MRCP being non-invasive offers a better alternative to ERCP for visualizing ductal changes.
Painful CP can occasionally present with minimal or no ductal change in absence of ductal strictures or stones. This is classified as type I CP according to Cremer classification or mild CP of Cambridge classification[13,14]. Endoscopic pancreatic sphincterotomy (EPS) is a documented mode of therapy and offers symptomatic relief in some of these patients. Both the standard pull type and the needle knife sphincterotomy over a stent can be performed. A 64% relief in pain on follow up of 6.5 years has been reported following EPS[15]. High success rates of 98% and low complication rates of around 4% have been reported on retrospective analysis[16]. Randomized studies have shown a higher incidence of pancreatitis in high risk patients following pull type sphincterotomy as compared to the needle knife technique[17]. Most workers report an incidence of around 12% for post EPS pancreatitis. Placement of a nasopancreatic tube (NPT) or pancreatic stent can reduce this incidence significantly[18]. Restenosis is reported in around 14% of patients on long term follow up[19]. It is believed that restenosis is less common following the longer incision with the pull type as compared to needle knife technique[20]. The presence of periductal fibrosis seen in patients with CP may lower the incidence of post procedure pancreatitis. An additional biliary sphincterotomy is only indicated in the following conditions[21]: (1) presence of cholangitis; (2) CBD > 12 mm diameter; (3) serum alkaline phosphates > 2 times upper limit of normal; and (4) difficult access to MPD.
Minor papilla sphincterotomy (MiES) was first performed by Cotton[22]. It is indicated in those patients with CP with minimal ductal changes who have a pancreas divisum or a dominant dorsal duct. Both the pull type and needle knife technique can be used. The evidence of any definite benefit from MiES is debatable as studies include small numbers of heterogeneous patients and are not conclusive. Significant pain relief on a 2-year follow up has been reported following MiES and stenting of patients with CP[23]. Relief of pain is also seen in 41% of patients with CP following MiES as compared to 77% with acute recurrent pancreatitis or 33% of patients with CP with no pain[24]. Post ERCP pancreatitis has been reported in up to 15% of patients[25] and restenosis was seen in 20%-24% on a 6-year follow up[26].
Strictures of MPD are frequently seen as a consequence of CP and could be due to inflammation or fibrosis. In our experience of 1000 patients who underwent ESWL, the incidence of strictures was 18%[2]. MPD strictures are defined as a high grade narrowing of MPD with one of the following[9,27]: (1) MPD dilatation > 6 mm beyond the stricture; (2) failure of contrast to flow alongside the stricture or 6 Fr NPT; and (3) presence of pain during continuous perfusion of the NPT with normal saline for 24 h.
Endotherapy is ideal for single strictures in the head while isolated strictures in the tail or multiple strictures with a chain of lake appearance are not amenable to endotherapy[9]. Prior to stent placement tight strictures need to be dilated with Teflon bougies, Sohendra stent retriever or a balloon dilator[9,27]. Large bore stents 7-10 Fr should be deployed as they have longer patency[27]. Delhaye et al[27] followed a protocol where a single stent was placed across a stricture and exchanged every 6 mo or when the patient was symptomatic. Stents were placed for 24 mo. Patients were restented if symptoms recurred. Surgery was considered if patients responded to stent placement but needed frequent or repeated stenting. Cumulative data from several workers revealed pain relief between 70%-94% for a single pancreatic stent on follow up of 14-69 mo[9]. Recurrence of strictures was reported in 38% of patients after 2 years follow up[28]. The concept of multiple plastic stenting for MPD strictures not responding to a single stent placement was advocated by Costamagna et al[29]. In their study, after removal of a single stent, the stricture was dilated and multiple plastic stents 8.5-11.5 Fr diameter were placed. A mean of 3 stents were used. The stents were removed 12 mo later. Stricture resolution was seen in 95% and pain relief in 84% on a 38 mo follow up.
Complications with pancreatic stenting can occur. Occlusion was seen with the passage of time and migration was present in 10% of patients[30]. Distal migration and impaction on the opposite duodenal wall can cause perforation while proximal migration into the pancreas is a technical challenge for the endoscopist. The possibility of stent induced fibrosis has raised concerns[31]. However with the preexisting fibrosis of MPD there has been no significant clinical impact. The search for an ideal pancreatic stent continues and a new “wing stent” to prevent clogging as well as an “S” shaped stent to prevent migration are undergoing evaluation[32,33]. The use of covered metal stents (CSEMS) for pancreatic strictures is also under evaluation. The initially used CSEMS had the disadvantage of stent migration. Subsequently a new “bumpy stent” has been tried for MPD strictures in 32 patients[34,35]. The stent had antimigratory properties and its contours adapted to the MPD. These were extracted at 3 mo and were effective in resolving the MPD strictures. However they were associated with the formation of de novo strictures and further trials are needed to evaluate their long term efficacy and safety.
European Society of Gastrointestinal Endoscopy (ESGE) guidelines state that dominant PD strictures be treated by placing a single 10 Fr stent with stent exchanges planned for 1 year. Multiple plastic stents should be deployed in a stricture which persists after 1 year of single stent placement[36]. Uncovered SEMS should not be placed in MPD. ESGE guidelines also state that temporary placement of fully covered SEMS should only performed in the setting of trials[36].
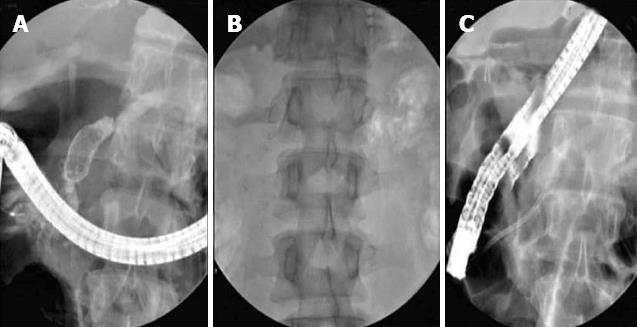
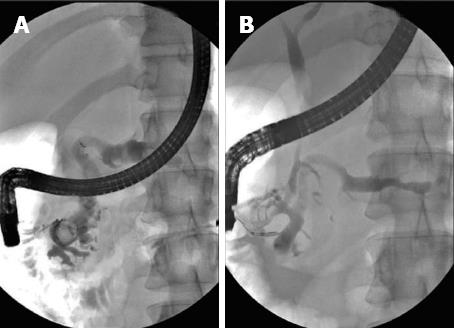
Pancreatic ductal calculi are a consequence of CP and tend to aggravate or produce pain by obstructing pancreatic ducts and producing upstream hypertension. They can occur in 50% of patients with CP[8]. Stones seen in the tropics and of the non-alcoholic type of CP tend to be larger and denser than those seen in the alcoholic variety[37,38]. The large size could also be due to delay in reporting for therapy[2]. Stones > 5 mm in size can usually be extracted with a Dormia basket, or balloon trawl following EPS. However stones > 5 mm in size are often impacted and difficult to extract by the standard techniques[2,37,39]. Large calculi need to be fragmented prior to extraction or spontaneous expulsion from the MPD. ESWL is now accepted as the standard of care in the management of large PD calculi not amenable to routine endotherapy[2,36,37,40-45]. ESWL is very effective in fragmenting both radio-opaque and radio-lucent calculi in the MPD. A meta-analysis of 17 studies with a total of 491 patients revealed a clearance rate between 37%-100% and good pain relief[46]. Another review of 11 studies with over 1100 patients showed successful stone fragmentation in 89%[47]. Our own single center study of over 1000 patients shows complete clearance in 76% patients and partial clearance in another 17% patients following ESWL and endotherapy for large calculi[2] (Figures 1 and 2).
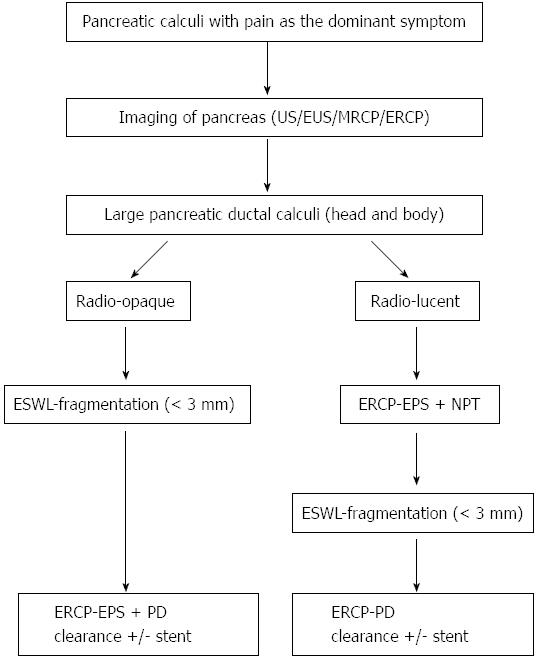
The following protocol is followed at our center for patients with large PD calculi[2]. Patients with large calculi in the head or body and with pain as the main complaint are subjected to ESWL. Patients with isolated calculi in the tail, multiple MPD strictures, extensive calculi in head, body and tail, associated head mass, pseudocysts and pregnancy are excluded from ESWL. The procedure is performed with a III generation electromagnetic lithotripter with bi-dimensional fluoroscopy and ultrasound targeting facility. (Delta compact-Dornier MedTech Wessling Germany). Epidural anesthesia is preferred in most patients[48]. It is effective and offers many advantages as reported in our study of over 1500 patients. Radio-opaque calculi are subjected to ESWL under fluoroscopic guidance. For radio-lucent calculi, a NPT is placed and contrast is passed through this tube to help localize the calculi. The aim of fragmentation is to break the calculi to 3 mm or less to facilitate to their extraction or expulsion[2,49]. An average of 3 sessions is generally required (5000-6000 shocks per session). The protocol is shown in Figure 3. A few studies have advocated use of ESWL alone followed by spontaneous expulsion of fragments[50]. A randomized controlled trial of 55 patients compared results of ESWL and ERCP with ESWL alone. The only difference was higher cost and longer stay in the ESWL and ERCP group[51]. At our center, we prefer to extract the fragments from the MPD by ERCP following the ESWL procedure as fragments tend to be denser and adherent and do not clear spontaneously[2,49].
Short term pain relief following ESWL was seen in 84% of our patients[2] and similar results have been reported by others[39]. Very few long term follow up studies are available. Two-thirds of patients were found to be pain free on long term follow up[8,52]. A recent study showed pain relief in 85%, complete pain relief with no narcotic use in 50% and avoidance of surgery in 84% of 120 patients on long term follow up after ESWL[53]. Our own data on long term follow up is encouraging and over 60% of patients are pain free on follow up of more than 5 years[54]. In conclusion, in properly selected group of patients with large PD calculi, ESWL is a useful tool and provides adequate long term pain relief. A few patients also benefit in exocrine and endocrine dysfunction though the numbers are too small to be significant[54]. ESWL is a safe procedure and well tolerated. Minor side effects such as transient pain and bruising of skin at the site of shock delivery have been described[2,37,49]. The incidence of pancreatitis is not higher following ESWL.
Other techniques for extraction of large PD calculi include intraductal laser or electro hydraulic lithotripsy through a pancreatoscope or spyscope[55,56]. Experience with these modalities is small and success rates are discordant. These procedures are technically difficult and require non standard equipment. At present, they are only to be considered as second line management after failed ESWL[36].
CBD strictures occur in 3%-46% of patients with CP[30]. Strictures can be reversible due to inflammation or compression with a pseudocyst. They are irreversible following fibrosis. ESGE guidelines recommend treating CP related benign biliary strictures (BBS) in cases with symptoms, secondary biliary cirrhosis, biliary stones, asymptomatic elevation of serum alkaline phosphates > 2-3 times upper limit of normal or raised serum bilirubin persisting for over 1 mo[36]. Placement of a single plastic stent in the CBD is associated with poor success rates. Long term results have disappointing and sustained benefit is seen in around 25% of patients on follow up of 46 mo[57]. Single plastic stents are associated with poor resolution and higher relapse rate. The presence of pancreatic head calcification is an important factor for failure of this therapy[58]. Placement of multiple plastic stents in CP related BBS is technically successful in over 95% of patients and offers the best results. Complete therapy requires approximately four ERCP procedures and stents exchanges performed every 3 mo for 1 year. Single stents provided relief in 31% of 350 patients as compared to 62% in 50 patients who received multiple stents[36]. Catalano et al[59] performed a non-randomized study comparing single and multiple plastic stents in CP related BBS. Clinically, success was reported in 92% with multiple stents as compared to 24% with single stents. Uncovered SEMS for BBS are not advocated and partially or fully covered SEMS have been used with a success rate of 50%-80% on follow up for 22-28 mo[60,61]. A recently conducted multicenter trial using fully covered SEMS (FCSEMS) in BBS included 127 patients of CP. It concluded that FCSEMS may be useful for treatment of BBS particularly in patients with CP[62]. There has been no head to head study comparing single or multiple plastic stents and metal stents in BBS due to CP and surgery. The choice and option of surgery depends upon patient preference, expertise at the treating center and the presence of co morbidities such as cirrhosis or collaterals.
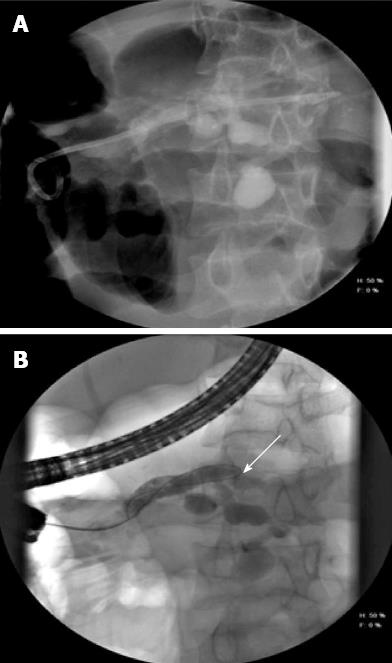
Leaks from the MPD or side branches can occur following blow out of the ducts due to obstruction by stone or strictures. PD leak is defined as extravasation of contrast material from the ductal system at ERCP[63]. Disruption may be partial or complete and leads to fluid collection, pseudocysts, ascites, pleural effusion and external or internal fistulas[9,27]. Placement of transpapillary stents offers the best treatment in patients with PD disruption as it converts the high pressure ductal system into a low pressure one with preferential flow across the stents[27]. Resolution of leak was seen in 92% of patients when the stent bridged the disruption, 50% when placed proximal to the disruption and 44% when a short transpapillary stent was placed[63] (Figure 4). In patients with complete transection where stenting is not feasible a multidisciplinary approach with a help of interventional radiologist or the surgeon may be required.
Pancreatic pseudocyst (PPC) in CP is the result of disruption of the MPD or its side branches and occurs in 20%-40% of patients[64]. Disruption generally follows obstruction by stones or strictures. Treatment is indicated for symptomatic PPC or those which increase in size. Symptoms result due to compression of adjacent structures or due to infection. It has also been suggested that prophylactic treatment be performed in certain specific situations to prevent complications. These include Pancreatic-pleural fistula fistula, cysts > 5 mm lasting for over 6 wk, compression of major vessels or presence of large pancreatic stones in MPD[65]. There is generally a low rate of spontaneous resolution of PPC in patients with CP though small asymptomatic cysts can be followed up[66]. Drainage of pseudocysts can be transmural (transgastric or transduodenal) or transpapillary. Transmural drainage is ideal for PPC which bulge into the lumen of stomach or duodenum. Transduodenal drainage offers the best success when compared to transgastric drainage[67]. This is because cystoduodenal fistulas tend to remain patent longer than cystogastric fistulas. Placement of one or more pig tailed stents is better when compared to straight stents. Straight stents are associated with a higher rate of bleed (around 7%) as well as migration[68]. Stents should be left in place for a longer duration as their removal within 2 mo is associated with a higher incidence of PPC recurrence[36]. Pseudoaneurysm can complicate management of PPC because of the associated haemorrhage and consequent high mortality[69]. Delhaye et al[27] recommend prophylactic embolization of pseudoaneurysms prior to drainage of an adjacent PPC.
Transpapillary drainage is reserved for small cysts (< 6 cm size) and those in communication with the MPD. The role of EUS guided drainage for nonbulging PPC will be discussed in the next section. Comparison of EUS guided drainage with surgery in an RCT revealed that endoscopic drainage was significantly better than surgery in terms of cost and length of stay over a 3 mo follow up[70]. Complications include bleed, infection and leak of around 4% each with a mortality of 0.5%[71]. Infection is more likely with transpapillary drainage and leak is more likely with transmural drainage. Routine antibiotic administration is recommended for drainage of PPC[72]. With a success rate of 80%-95% at most centers, a recurrence rate of 10%-20% and results comparative or better than surgery, endoscopy is the preferred first line of management for patients with PPC in the background of CP[27,36].
EUS is an excellent diagnostic modality especially in patients with early CP. It also has a definite therapeutic role in the following situations and these are discussed briefly.
EUS is ideal for drainage of nonbulging PPC and cysts as far as 4 cm from the stomach or duodenal wall have been drained[73]. Around 44%-53% of PPCs belong to this category. In the presence of collaterals the site of drainage is better identified with EUS, thus making the procedure safer. The complication rate is however similar when PPCs are drained with or without EUS guidance[74]. A recent randomized trial comparing EUS guided and surgical cystogastrostomy for pseudocysts revealed shorter hospital stay, lower cost and better physical and mental health in the endoscopy group. None in the endoscopy group had pseudocyst recurrence and therapy was successful in all the patients[75].
EUS guided access or drainage is indicated following failed conventional drainage of MPD. It can be via the stomach (pancreatogastric) or duodenum (pancreatobulbar). The duodenal route is preferred because of better stability of the EUS scope[9]. A guidewire can be passed into the duodenum for a rendezvous procedure or transmural drainage can be performed. Success rates of 77%-92% have been reported[76,77]. Complications include pain, bleeding, perforation and hematoma and morbidity of 0%-44% has been reported[76-78]. EUS guided access of the MPD is a technically challenging procedure and should always be performed by experts and under radiological guidance[9].
Patients who have failed to respond to intensive medical or endoscopic therapy and are not candidates suitable for surgery can be provided relief from pain by EUS guided celiac block. A combination of corticosteroids (triamcinolone) and anesthetic agents (bupivacaine) is injected in and around the celiac plexus under EUS guidance. A recent meta analysis has reported pain relief in 50%-55% of patients though the pain relief is transient[79,80]. Patients who are younger than 45 years or have previous pancreatic surgery are less likely to benefit[81]. EUS guided celiac block is shown to be superior to fluoroscopy guided celiac block for pain relief and pain preference in our study[82]. EUS guided nerve block can produce diarrhea, hypertension due to sympathetic blockade and unopposed parasympathetic activity[11,80].
In conclusion, management of CP is a multidisciplinary task involving the physician, endoscopist, interventional radiologist and surgeon. Their roles are complementary to each other. As mentioned earlier endotherapy is effective, less invasive than surgery, offers good results and is associated with low morbidity and mortality. It can be repeated and does not interfere with any subsequent surgical procedure. It is therefore advisable to offer endotherapy as the first line treatment in properly selected patients with CP.
P- Reviewers Kawa S, Yen HH S- Editor Wen LL L- Editor O’Neill M E- Editor Ma S
| 1. | Lankisch PG, Banks PA, editors . Pancreatitis, New York. Berlin: Springer-Verlag 1998; 199. [DOI] [Full Text] |
| 2. | Tandan M, Reddy DN, Santosh D, Vinod K, Ramchandani M, Rajesh G, Rama K, Lakhtakia S, Banerjee R, Pratap N. Extracorporeal shock wave lithotripsy and endotherapy for pancreatic calculi-a large single center experience. Indian J Gastroenterol. 2010;29:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Balakrishnan V, Unnikrishnan AG, Thomas V, Choudhuri G, Veeraraju P, Singh SP, Garg P, Pai CG, Devi RN, Bhasin D. Chronic pancreatitis. A prospective nationwide study of 1,086 subjects from India. JOP. 2008;9:593-600. [PubMed] |
| 4. | Cahen DL, Gouma DJ, Nio Y, Rauws EA, Boermeester MA, Busch OR, Stoker J, Laméris JS, Dijkgraaf MG, Huibregtse K. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med. 2007;356:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 507] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 5. | Bradley EL. Long term results of pancreaticojejunostomy in patients with chronic pancreatitis. Am J Surg. 1987;153:207-213. [RCA] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 140] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Cremer M, Devière J, Delhaye M, Baize M, Vandermeeren A. Stenting in severe chronic pancreatitis: results of medium-term follow-up in seventy-six patients. Endoscopy. 1991;23:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 195] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Farnbacher MJ, Schoen C, Rabenstein T, Benninger J, Hahn EG, Schneider HT. Pancreatic duct stones in chronic pancreatitis: criteria for treatment intensity and success. Gastrointest Endosc. 2002;56:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Rösch T, Daniel S, Scholz M, Huibregtse K, Smits M, Schneider T, Ell C, Haber G, Riemann JF, Jakobs R. Endoscopic treatment of chronic pancreatitis: a multicenter study of 1000 patients with long-term follow-up. Endoscopy. 2002;34:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 289] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 9. | Tringali A, Boskoski I, Costamagna G. The role of endoscopy in the therapy of chronic pancreatitis. Best Pract Res Clin Gastroenterol. 2008;22:145-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Enríquez WK. Diagnostic and therapeutic endoscopy of pancreas and biliary tract. Rev Gastroenterol Mex. 2006;71 Suppl 1:36-38. [PubMed] |
| 11. | Attasaranya S, Abdel Aziz AM, Lehman GA. Endoscopic management of acute and chronic pancreatitis. Surg Clin North Am. 2007;87:1379-1402, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Kahl S, Glasbrenner B, Leodolter A, Pross M, Schulz HU, Malfertheiner P. EUS in the diagnosis of early chronic pancreatitis: a prospective follow-up study. Gastrointest Endosc. 2002;55:507-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Cremer M, Toussaint J, Hermanus A, Deltenre M, De Tceuf J, Engelholm L. Primary chronic pancreatitis. A classification based on endoscopic pancreatography (author’s transl). Acta Gastroenterol Belg. 1976;39:522-546. [PubMed] |
| 14. | Sarner M, Cotton PB. Classification of pancreatitis. Gut. 1984;25:756-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 458] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 15. | Gabbrielli A, Mutignani M, Pandolfi M, Perri V, Costamagna G. Endotherapy of early onset idiopathic chronic pancreatitis: results with long-term follow-up. Gastrointest Endosc. 2002;55:488-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Ell C, Rabenstein T, Schneider HT, Ruppert T, Nicklas M, Bulling D. Safety and efficacy of pancreatic sphincterotomy in chronic pancreatitis. Gastrointest Endosc. 1998;48:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Varadarajulu S, Wilcox CM. Randomized trial comparing needle-knife and pull-sphincterotome techniques for pancreatic sphincterotomy in high-risk patients. Gastrointest Endosc. 2006;64:716-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Hookey LC, RioTinto R, Delhaye M, Baize M, Le Moine O, Devière J. Risk factors for pancreatitis after pancreatic sphincterotomy: a review of 572 cases. Endoscopy. 2006;38:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Kozarek RA, Ball TJ, Patterson DJ, Brandabur JJ, Traverso LW, Raltz S. Endoscopic pancreatic duct sphincterotomy: indications, technique, and analysis of results. Gastrointest Endosc. 1994;40:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 77] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Siegel JH, Cohen SA. Pull or push pancreatic sphincterotomy for sphincter of Oddi dysfunction? A conundrum for experts only. Gastrointest Endosc. 2006;64:723-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Kim MH, Myung SJ, Kim YS, Kim HJ, Seo DW, Nam SW, Ahn JH, Lee SK, Min YI. Routine biliary sphincterotomy may not be indispensable for endoscopic pancreatic sphincterotomy. Endoscopy. 1998;30:697-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Cotton PB. Duodenoscopic papillotomy at the minor papilla for recurrent dorsal pancreatitis. Endosc Dig. 1978;3:27-28. |
| 23. | Vitale GC, Vitale M, Vitale DS, Binford JC, Hill B. Long-term follow-up of endoscopic stenting in patients with chronic pancreatitis secondary to pancreas divisum. Surg Endosc. 2007;21:2199-2202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Watkins JL, Lehman GA. Minor Papilla Endscopic Sphincterotomy. ERCP. Amsterdam: Elseiver 2008; 143-157. |
| 25. | Lehman GA, Sherman S, Nisi R, Hawes RH. Pancreas divisum: results of minor papilla sphincterotomy. Gastrointest Endosc. 1993;39:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 140] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Attwell A, Borak G, Hawes R, Cotton P, Romagnuolo J. Endoscopic pancreatic sphincterotomy for pancreas divisum by using a needle-knife or standard pull-type technique: safety and reintervention rates. Gastrointest Endosc. 2006;64:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Delhaye M, Matos C, Devière J. Endoscopic technique for the management of pancreatitis and its complications. Best Pract Res Clin Gastroenterol. 2004;18:155-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Eleftherladis N, Dinu F, Delhaye M, Le Moine O, Baize M, Vandermeeren A, Hookey L, Devière J. Long-term outcome after pancreatic stenting in severe chronic pancreatitis. Endoscopy. 2005;37:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Costamagna G, Bulajic M, Tringali A, Pandolfi M, Gabbrielli A, Spada C, Petruzziello L, Familiari P, Mutignani M. Multiple stenting of refractory pancreatic duct strictures in severe chronic pancreatitis: long-term results. Endoscopy. 2006;38:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Adler DG, Lichtenstein D, Baron TH, Davila R, Egan JV, Gan SL, Qureshi WA, Rajan E, Shen B, Zuckerman MJ. The role of endoscopy in patients with chronic pancreatitis. Gastrointest Endosc. 2006;63:933-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Kozarek RA. Pancreatic stents can induce ductal changes consistent with chronic pancreatitis. Gastrointest Endosc. 1990;36:93-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 186] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Raju GS, Gomez G, Xiao SY, Ahmed I, Brining D, Bhutani MS, Kalloo AN, Pasricha PJ. Effect of a novel pancreatic stent design on short-term pancreatic injury in a canine model. Endoscopy. 2006;38:260-265. [PubMed] |
| 33. | Ishihara T, Yamaguchi T, Seza K, Tadenuma H, Saisho H. Efficacy of s-type stents for the treatment of the main pancreatic duct stricture in patients with chronic pancreatitis. Scand J Gastroenterol. 2006;41:744-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Park do H, Kim MH, Moon SH, Lee SS, Seo DW, Lee SK. Feasibility and safety of placement of a newly designed, fully covered self-expandable metal stent for refractory benign pancreatic ductal strictures: a pilot study (with video). Gastrointest Endosc. 2008;68:1182-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Moon SH, Kim MH, Park do H, Song TJ, Eum J, Lee SS, Seo DW, Lee SK. Modified fully covered self-expandable metal stents with antimigration features for benign pancreatic-duct strictures in advanced chronic pancreatitis, with a focus on the safety profile and reducing migration. Gastrointest Endosc. 2010;72:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Dumonceau JM, Delhaye M, Tringali A, Dominguez-Munoz JE, Poley JW, Arvanitaki M, Costamagna G, Costea F, Devière J, Eisendrath P. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2012;44:784-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 37. | Ong WC, Tandan M, Reddy V, Rao GV, Reddy N. Multiple main pancreatic duct stones in tropical pancreatitis: safe clearance with extracorporeal shockwave lithotripsy. J Gastroenterol Hepatol. 2006;21:1514-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Chari S, Jayanthi V, Mohan V, Malathi S, Madanagopalan N, Viswanathan M. Radiological appearance of pancreatic calculi in tropical versus alcoholic chronic pancreatitis. J Gastroenterol Hepatol. 1992;7:42-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Lehman GA. Role of ERCP and other endoscopic modalities in chronic pancreatitis. Gastrointest Endosc. 2002;56:S237-S240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Sauerbruch T, Holl J, Sackmann M, Paumgartner G. Extracorporeal lithotripsy of pancreatic stones in patients with chronic pancreatitis and pain: a prospective follow up study. Gut. 1992;33:969-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 95] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Delhaye M, Vandermeeren A, Baize M, Cremer M. Extracorporeal shock-wave lithotripsy of pancreatic calculi. Gastroenterology. 1992;102:610-620. [PubMed] |
| 42. | Dumonceau JM, Devière J, Le Moine O, Delhaye M, Vandermeeren A, Baize M, Van Gansbeke D, Cremer M. Endoscopic pancreatic drainage in chronic pancreatitis associated with ductal stones: long-term results. Gastrointest Endosc. 1996;43:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 154] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 43. | Costamagna G, Gabbrielli A, Mutignani M, Perri V, Pandolfi M, Boscaini M, Crucitti F. Extracorporeal shock wave lithotripsy of pancreatic stones in chronic pancreatitis: immediate and medium-term results. Gastrointest Endosc. 1997;46:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 78] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Neuhaus H. Fragmentation of pancreatic stones by extracorporeal shock wave lithotripsy. Endoscopy. 1991;23:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Kozarek RA, Brandabur JJ, Ball TJ, Gluck M, Patterson DJ, Attia F, France R, Traverso LW, Koslowski P, Gibbons RP. Clinical outcomes in patients who undergo extracorporeal shock wave lithotripsy for chronic calcific pancreatitis. Gastrointest Endosc. 2002;56:496-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Guda NM, Partington S, Freeman ML. Extracorporeal shock wave lithotripsy in the management of chronic calcific pancreatitis: a meta-analysis. JOP. 2005;6:6-12. [PubMed] |
| 47. | Nguyen-Tang T, Dumonceau JM. Endoscopic treatment in chronic pancreatitis, timing, duration and type of intervention. Best Pract Res Clin Gastroenterol. 2010;24:281-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Darisetty S, Tandan M, Reddy DN, Kotla R, Gupta R, Ramchandani M, Lakhtakia S, Rao GV, Banerjee R. Epidural anesthesia is effective for extracorporeal shock wave lithotripsy of pancreatic and biliary calculi. World J Gastrointest Surg. 2010;2:165-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Tandan M, Reddy DN. Extracorporeal shock wave lithotripsy for pancreatic and large common bile duct stones. World J Gastroenterol. 2011;17:4365-4371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 50. | Ohara H, Hoshino M, Hayakawa T, Kamiya Y, Miyaji M, Takeuchi T, Okayama Y, Gotoh K. Single application extracorporeal shock wave lithotripsy is the first choice for patients with pancreatic duct stones. Am J Gastroenterol. 1996;91:1388-1394. [PubMed] |
| 51. | Dumonceau JM, Costamagna G, Tringali A, Vahedi K, Delhaye M, Hittelet A, Spera G, Giostra E, Mutignani M, De Maertelaer V. Treatment for painful calcified chronic pancreatitis: extracorporeal shock wave lithotripsy versus endoscopic treatment: a randomised controlled trial. Gut. 2007;56:545-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 197] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 52. | Delhaye M, Arvanitakis M, Verset G, Cremer M, Devière J. Long-term clinical outcome after endoscopic pancreatic ductal drainage for patients with painful chronic pancreatitis. Clin Gastroenterol Hepatol. 2004;2:1096-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 53. | Seven G, Schreiner MA, Ross AS, Lin OS, Gluck M, Gan SI, Irani S, Brandabur JJ, Patterson D, Kuhr C. Long-term outcomes associated with pancreatic extracorporeal shock wave lithotripsy for chronic calcific pancreatitis. Gastrointest Endosc. 2012;75:997-1004.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 54. | Tandan M, Reddy DN, Talukdar R, Vinod K, Santosh D, Lakhtakia S, Gupta R, Ramchandani MJ, Banerjee R, Rakesh K. Long-term clinical outcomes of extracorporeal shockwave lithotripsy in painful chronic calcific pancreatitis. Gastrointest Endosc. 2013;Jul 25; Epub ahead of print. [PubMed] |
| 55. | Hirai T, Goto H, Hirooka Y, Itoh A, Hashimoto S, Niwa Y, Hayakawa T. Pilot study of pancreatoscopic lithotripsy using a 5-fr instrument: selected patients may benefit. Endoscopy. 2004;36:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Howell DA, Dy RM, Hanson BL, Nezhad SF, Broaddus SB. Endoscopic treatment of pancreatic duct stones using a 10F pancreatoscope and electrohydraulic lithotripsy. Gastrointest Endosc. 1999;50:829-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Barthet M, Bernard JP, Duval JL, Affriat C, Sahel J. Biliary stenting in benign biliary stenosis complicating chronic calcifying pancreatitis. Endoscopy. 1994;26:569-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Kahl S, Zimmermann S, Genz I, Glasbrenner B, Pross M, Schulz HU, Mc Namara D, Schmidt U, Malfertheiner P. Risk factors for failure of endoscopic stenting of biliary strictures in chronic pancreatitis: a prospective follow-up study. Am J Gastroenterol. 2003;98:2448-2453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 59. | Catalano MF, Linder JD, George S, Alcocer E, Geenen JE. Treatment of symptomatic distal common bile duct stenosis secondary to chronic pancreatitis: comparison of single vs. multiple simultaneous stents. Gastrointest Endosc. 2004;60:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 60. | Cahen DL, Rauws EA, Gouma DJ, Fockens P, Bruno MJ. Removable fully covered self-expandable metal stents in the treatment of common bile duct strictures due to chronic pancreatitis: a case series. Endoscopy. 2008;40:697-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Behm B, Brock A, Clarke BW, Ellen K, Northup PG, Dumonceau JM, Kahaleh M. Partially covered self-expandable metallic stents for benign biliary strictures due to chronic pancreatitis. Endoscopy. 2009;41:547-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 62. | Deviere JM, Reddy DN, Puspok A, Ponchon T, Bruno MJ, Bourke MJ, Neuhaus H, Roy A, González-Huix F, Barkun AN. 147 Preliminary Results From a 187 Patient Multicenter Prospective Trial Using Metal Stents for Treatment of Benign Biliary Strictures. Gastrointest Endosc. 2012;75:AB123. [DOI] [Full Text] |
| 63. | Telford JJ, Farrell JJ, Saltzman JR, Shields SJ, Banks PA, Lichtenstein DR, Johannes RS, Kelsey PB, Carr-Locke DL. Pancreatic stent placement for duct disruption. Gastrointest Endosc. 2002;56:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 64. | Andrén-Sandberg A, Dervenis C. Pancreatic pseudocysts in the 21st century. Part I: classification, pathophysiology, anatomic considerations and treatment. JOP. 2004;5:8-24. [PubMed] |
| 65. | Lerch MM, Stier A, Wahnschaffe U, Mayerle J. Pancreatic pseudocysts: observation, endoscopic drainage, or resection? Dtsch Arztebl Int. 2009;106:614-621. [PubMed] |
| 66. | Andrén-Sandberg A, Dervenis C. Pancreatic pseudocysts in the 21st century. Part II: natural history. JOP. 2004;5:64-70. [PubMed] |
| 67. | Beckingham IJ, Krige JE, Bornman PC, Terblanche J. Endoscopic management of pancreatic pseudocysts. Br J Surg. 1997;84:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 56] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Cahen D, Rauws E, Fockens P, Weverling G, Huibregtse K, Bruno M. Endoscopic drainage of pancreatic pseudocysts: long-term outcome and procedural factors associated with safe and successful treatment. Endoscopy. 2005;37:977-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 69. | Balachandra S, Siriwardena AK. Systematic appraisal of the management of the major vascular complications of pancreatitis. Am J Surg. 2005;190:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 70. | Varadarajulu S, Trevino J. Wilcox CM, Sutton B, Christein JD. Randomized trial comparing EUS and surgery for pancreatic pseudocyst drainage. Gastrointest Endosc. 2010;71:AB116-AB116. [DOI] [Full Text] |
| 71. | Hookey LC, Debroux S, Delhaye M, Arvanitakis M, Le Moine O, Devière J. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006;63:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 72. | Banerjee S, Shen B, Baron TH, Nelson DB, Anderson MA, Cash BD, Dominitz JA, Gan SI, Harrison ME, Ikenberry SO. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 73. | Sanchez Cortes E, Maalak A, Le Moine O, Baize M, Delhaye M, Matos C, Devière J. Endoscopic cystenterostomy of nonbulging pancreatic fluid collections. Gastrointest Endosc. 2002;56:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 74. | Kahaleh M, Shami VM, Conaway MR, Tokar J, Rockoff T, De La Rue SA, de Lange E, Bassignani M, Gay S, Adams RB. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 75. | Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583-590.e1. [PubMed] |
| 76. | Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. EUS-guided pancreaticogastrostomy: analysis of its efficacy to drain inaccessible pancreatic ducts. Gastrointest Endosc. 2007;65:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 77. | Tessier G, Bories E, Arvanitakis M, Hittelet A, Pesenti C, Le Moine O, Giovannini M, Devière J. EUS-guided pancreatogastrostomy and pancreatobulbostomy for the treatment of pain in patients with pancreatic ductal dilatation inaccessible for transpapillary endoscopic therapy. Gastrointest Endosc. 2007;65:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 159] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 78. | Brauer BC, Chen YK, Fukami N, Shah RJ. Single-operator EUS-guided cholangiopancreatography for difficult pancreaticobiliary access (with video). Gastrointest Endosc. 2009;70:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 79. | Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54:2330-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 198] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 80. | Kaufman M, Singh G, Das S, Concha-Parra R, Erber J, Micames C, Gress F. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 81. | Gress F, Schmitt C, Sherman S, Ciaccia D, Ikenberry S, Lehman G. Endoscopic ultrasound-guided celiac plexus block for managing abdominal pain associated with chronic pancreatitis: a prospective single center experience. Am J Gastroenterol. 2001;96:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 187] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 82. | Santosh D, Lakhtakia S, Gupta R, Reddy DN, Rao GV, Tandan M, Ramchandani M, Guda NM. Clinical trial: a randomized trial comparing fluoroscopy guided percutaneous technique vs. endoscopic ultrasound guided technique of coeliac plexus block for treatment of pain in chronic pancreatitis. Aliment Pharmacol Ther. 2009;29:979-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |